Left ventricular solid body rotation in non-compaction cardiomyopathy: A potential new objective and quantitative functional diagnostic criterion?
Abstract
Background:
Left ventricular (LV) twist originates from the interaction between myocardial fibre helices that are formed during the formation of compact myocardium in the final stages of the development of myocardial architecture. Since non-compaction cardiomyopathy (NCCM) is probably caused by intrauterine arrest of this final stage, it may be anticipated that LV twist characteristics are altered in NCCM patients, beyond that seen in patients with impaired LV function and normal compaction.
Aims:
The purpose of this study was to assess LV twist characteristics in NCCM patients compared to patients with non-ischaemic dilated cardiomyopathy (DCM) and normal subjects.
Methods and results:
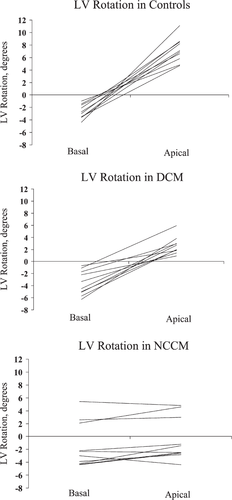
The study population consisted of 10 patients with NCCM, 10 patients with DCM, and 10 healthy controls. LV twist was determined by speckle tracking echocardiography. In all controls and DCM patients, rotation was clockwise at the basal level and counterclockwise at the apical level. In contrast, in all NCCM patients the LV base and apex rotated in the same direction.
Conclusions:
These findings suggest that ‘LV solid body rotation’, with near absent LV twist, may be a new sensitive and specific, objective and quantitative, functional diagnostic criterion for NCCM.
1. Introduction
Left ventricular (LV) twist, defined as the wringing motion of the heart as the apex rotates with respect to the base around the LV long-axis, has an important role in LV ejection and filling 1,2. The final stage of the development of myocardial architecture is characterized by the formation of compact myocardium and development of oppositely wound epicardial and endocardial myocardial fibre helices 3,4. LV twist originates from the dynamic interaction between these helices. Non-compaction cardiomyopathy (NCCM) is a heterogeneous disorder probably caused by intrauterine arrest of the final stage of cardiac embryogenesis 5. It may be anticipated that LV twist characteristics are altered in NCCM patients, beyond that seen in patients with impaired LV function and normal compaction.
Recently, speckle tracking echocardiography (STE) has been introduced as a new method for angle-independent quantification of LV twist 6. Speckles are natural acoustic markers that occur as small and bright elements in conventional grayscale ultrasound images. The speckles are the result of constructive and destructive interference of ultrasound, back-scattered from structures smaller than a wavelength of ultrasound 7. This gives each small area a rather unique speckle pattern that remains relatively constant from one frame to the next. Therefore, a suitable pattern-matching algorithm can identify the frame-to-frame displacement of a speckle pattern, allowing myocardial motion to be followed in two dimensions.
This study sought to assess LV twist characteristics by STE in NCCM patients compared to patients with non-ischaemic dilated cardiomyopathy (DCM) and normal subjects.
2. Methods
2.1. Study participants
The study population consisted of 10 patients with NCCM (mean age 41±16 years, 6 men), 10 patients with DCM (mean age 47±13 years, 5 men), and 10 healthy controls (mean age 43±8 years, 5 men) without hypertension or diabetes, and with normal left atrial dimensions, LV dimensions, and LV function. Only subjects in sinus rhythm with good two-dimensional image quality were enrolled. Informed consent was obtained from all subjects and the institutional review board approved the study.
2.2. Diagnostic criteria for NCCM and non-ischaemic DCM
NCCM patients strictly fulfilled all 4 echocardiographic diagnostic criteria for NCCM according to Jenni et al. 8: [1] absence of co-existing cardiac abnormalities (including coronary stenoses); [2] a 2-layered structure of the LV wall, with the end-systolic ratio of non-compacted to compacted layer >2; [3] finding this structure predominantly in the apical and mid-ventricular areas; and [4] blood flow directly from the ventricular cavity into the deep intertrabecular recesses as assessed by Doppler and contrast echocardiography 9. Hypertensive heart disease was excluded by clinical and echocardiographic examinations (septal thickness <13 mm). DCM was characterized by ventricular chamber enlargement and systolic dysfunction, based on current guidelines 10. All NCCM and DCM patients had undergone coronary angiography to exclude coronary artery disease.
2.3. Echocardiography
Two-dimensional grayscale harmonic images at a frame rate of 60 to 80 frames/s were obtained in the left lateral decubitus position using a commercially available ultrasound system (iE33, Philips, Best, The Netherlands), equipped with a broadband (1-5 MHz) S5-1 transducer (frequency transmitted 1.7 MHz, received 3.4 MHz). Measurements of LV dimensions, volumes, fractional shortening, and ejection fraction were obtained in accordance with the recommendations of the American Society of Echocardiography 11. According to the recommendations of the American Heart Association on standardized myocardial segmentation and nomenclature for tomographic imaging of the heart, a 17-segment model was used for the assessment of regional LV wall motion 12. Parasternal short-axis images at the basal level (showing the tips of the mitral valve leaflets), with the cross section as circular as possible, were obtained from the standard parasternal window, in which the LV and aorta were most in-line with the mitral valve tips in the middle of the sector. To obtain a short-axis image at the apical level (just proximal to the level with LV luminal obliteration at the end-systolic period) the transducer was positioned 1 or 2 intercostal spaces more caudal as previously described by us 13. From each short-axis image, three consecutive end-expiratory cardiac cycles were acquired and transferred to a QLAB workstation (Philips, Best, The Netherlands) for off-line analysis.
2.4. Data analysis
Analysis of the datasets was performed using QLAB Advanced Quantification Software (version 6.0, Philips, Best, The Netherlands) that was recently validated against MRI for assessment of LV twist by speckle tracking 14. To assess LV rotation, six tracking points were placed manually (after gain correction) on an end-diastolic frame in each parasternal short-axis image on the midmyocardium. In NCCM patients the tracking points were placed in the inner to midsection of the compacted part of the muscle. Tracking points were separated about 60° from each other and placed on 1 (anteroseptal insertion into the LV of the right ventricle), 3, 5, 7, 9 (inferoseptal insertion into the LV of the right ventricle) and 11 o'clock to fit the total LV circumference. LV rotation was estimated as the average angular displacement of all six tracking points relative to the center of a best-fit circle through the same tracking points. Rotation data were exported to a spreadsheet program (Excel, Microsoft Corporation, Redmond, WA) to determine LV peak rotation and time-to-peak LV rotation at the different short-axis planes, instantaneous peak LV twist (apical LV peak rotation-basal LV peak rotation), and time-to-peak LV twist. Counterclockwise rotation and twist as viewed from the apex was expressed as a positive value, clockwise rotation and twist was expressed as a negative value. To adjust for intersubject differences in heart rate, the time sequence was normalized to a percentage of systolic duration. The end of systole was defined as the point of aortic valve closure.
2.5. Statistical analysis
Continuous variables were presented as mean±SD, and tested for normality. Categorical data were expressed as percentages. Variables were compared using the Student's t test, the Chi-square test or ANOVA when appropriate. A P value <0.05 was considered statistically significant. To test the intraobserver variability, measurements were repeated 4 weeks apart by the same observer (BVD) on the same echocardiographic loop for 10 randomly selected subjects. To test inter-observer variability, a second observer (MLG) who was unaware of the results of the first measurements, performed repeated measurements on the same randomly selected subjects. Variability was calculated as the mean percent error, derived as the absolute difference between the two sets of measurements, divided by the mean of the measurements. Intra- and inter-observer variability for all parameters varied from 2.1% to 6.3% and 4.2% to 8.7% respectively.
3. Results
3.1. Subject characteristics
All clinical and traditional echocardiographic characteristics in controls, DCM and NCCM patients are shown in Table 1. Controls had a significantly shorter QRS duration, smaller LV dimensions and volumes, and higher LV fractional shortening and ejection fraction compared to NCCM and DCM patients. Regional wall motion was normal in all segments in controls (P<0.001 vs. DCM and NCCM), none of the segments in DCM patients (P<0.001 vs. NCCM), and in 27% of the compacted, and 11% of the non-compacted segments in patients with NCCM.
| NCCM | DCM | Controls | ||
|---|---|---|---|---|
| (n=10) | (n=10) | (n=10) | ||
| Clinical data | ||||
| Age, years | 41±16 | 47±13 | 43±8 | |
| Men, n (%) | 6 (60) | 5 (50) | 5 (50) | |
| QRS duration, ms | 116±38 | 117±34 | 89±8* | |
| Bundle branch block (left/right/aspecific), n | 2/0/1 | 3/0/0 | 0/0/0 | |
| Echocardiographic data | ||||
| LV-EDD, mm | 56±8 | 67±12 | 50±6* | |
| LV-ESD, mm | 44±9 | 55±14 | 34±6* | |
| LV fractional shortening, % | 23±6 | 18±9 | 32±7* | |
| LV-EDV, ml | 152±52 | 167±55 | 115±23* | |
| LV-ESV, ml | 92±43 | 117±44 | 44±15 † | |
| LV ejection fraction, % | 38±13 | 30±9 | 62±7 † | |
| Regional wall motion | Compacted | Non-compacted | ||
| Segments, n (%) | 94 (55) | 76 (45) | 170 (100) | 170 (100) |
| Normal, n (%) | 25 (27) †† | 8 (11) †† | 0 (0) | 170 (100) ‡ |
| Hypokinesis, n (%) | 49 (52) | 46 (61) | 100 (59) | 0 (0) ‡ |
| Akinesis, n (%) | 20 (21)** | 18 (24)** | 64 (38) | 0 (0) ‡ |
| Dyskinesis, n (%) | 0 (0) | 4 (5) | 6 (3) | 0 (0) |
- a Data are presented as mean±SD. NCCM=non-compaction cardiomyopathy, DCM=dilated cardiomyopathy, LV=left ventricular, EDD=end-diastolic dimension, ESD=end-systolic dimension, EDV=end-diastolic volume, ESV=end-systolic volume.
- * P<0.05
- † P<0.01
- ‡ P<0.001 vs. NCCM and DCM
- ** P<0.05
- †† P<0.001 vs. DCM.
3.2. LV rotation in NCCM
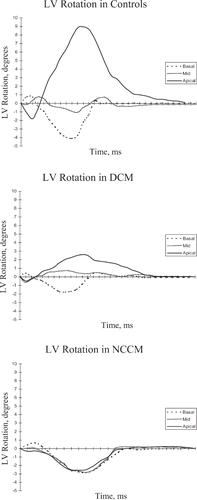
In all controls and DCM patients, LV rotation was clockwise at the basal level and counterclockwise at the apical level. In contrast, in all NCCM patients the LV base and apex rotated in the same direction. The LV rotated as a solid body in a clockwise direction in 7 NCCM patients, and in a counterclockwise direction in 3 NCCM patients (Fig. 1). LV basal rotation (−2.7°±1.1° vs. −3.6°±2.0° vs. −3.5°±1.0°, P=NS) was comparable in controls, DCM patients, and the 7 NCCM patients with clockwise solid body rotation. In the 3 NCCM patients with counterclockwise solid body rotation, LV basal rotation (3.4°±1.8°) was significantly different from LV basal rotation in controls and DCM patients (both P<0.001). LV apical rotation was significantly lower in both NCCM patients with clockwise (−2.5°±1.1°, P<0.001) and counterclockwise (4.2°±1.0°, P<0.05) solid body rotation, and DCM patients (2.6°±1.4°, P<0.001) as compared to controls (7.2°±2.0°). There were no differences in basal and apical time-to-peak rotation between controls, DCM, and NCCM (Table 2). Typical examples of rotation-time curves in controls, DCM and NCCM are shown in Fig. 2.
| NCCM (n=10) | DCM (n=10) | Controls (n=10) | ||||
|---|---|---|---|---|---|---|
| Clockwise | Counterclockwise | |||||
| LV rotation | n | n | ||||
| Basal, degrees | −3.5±1.0 | 7 | 3.4±1.8††‡ | 3 | −3.6±2.0 | −2.7±1.1 |
| Apical, degrees | −2.5±1.1††‡ | 7 | 4.2±1.0** | 3 | 2.6±1.4‡ | 7.2±2.0 |
| LV twist, degrees | −2.0±0.9*† | 2 | 2.5±1.0*‡ | 8 | 5.4±2.5** | 9.4±3.7 |
| Time-to-peak LV rotation | ||||||
| Basal, % | 82±28 | 83±30 | 91±18 | 89±19 | ||
| Apical, % | 93±29 | 93±30 | 89±23 | 94±8 | ||
| Time-to-peak LV twist, % | 94±12 | 97±15 | 93±17 | 93±5 | ||
- a Data presented as mean±SD. Time-to-peak LV rotation and time-to-peak LV twist as a percentage of duration of systole. Abbreviations are as in Table 1.
- * P<0.01
- †† P<0.001 vs. DCM
- ** P<0.05
- † P<0.01
- ‡ P<0.001 vs. controls.
3.3. LV twist in NCCM
Even though rotation at the basal and apical level was in the same direction in NCCM, there was still a small instantaneous LV twist because of differences in the degree and timing of peak rotation at the LV basal and apical level. Nevertheless, LV twist in both the NCCM patients with clockwise (−2.0°±0.9°) and counterclockwise solid body rotation (2.5°±1.0°) was significantly lower compared to DCM patients (5.4°±2.5°, both P<0.01) and controls (9.4°±3.7°, P<0.001 and <0.01, respectively).
3.4. Counterclockwise vs. clockwise rotation in NCCM
No significant differences in clinical or traditional echocardiographic data between NCCM patients with LV rotation in a clockwise or counterclockwise direction could be identified, although patients with counterclockwise LV rotation tended to have a shorter QRS duration (90±12 ms vs. 127±41 ms). In both NCCM patients with a left bundle branch block and the NCCM patient with aspecific intraventricular conduction delay, solid body rotation was in a clockwise direction.
4. Discussion
The main findings of our study are 1) in patients with DCM LV basal rotation is clockwise and LV apical rotation is counterclockwise as in normal controls but LV apical rotation is of a lesser magnitude (LV twist is less), and 2) in NCCM patients LV apical rotation is also of a lesser magnitude but in contrast to normal controls and DCM, LV basal and LV apical rotation are in the same direction (‘LV solid body rotation’). The development of the myocardial architecture of the heart wall passes through several distinct steps 15. In the early tubular heart, the myocardium has an epithelial nature with just a few layers of cells. The next step is the cavity-specific formation of sheet-like myocardial protrusions into the lumen, so-called trabeculations. These early trabeculations effectively increase the myocardial surface area, enabling the myocardial mass to increase in the absence of a coronary circulation. Currently, there is no consensus on what happens to this trabecular layer. Although some state that the trabeculations become compacted to form the compact wall of the ventricular mass 15, others claim that this is most unlikely 16, supported by a lack of proof for the former theory. Anyway, the final stage of the development of myocardial architecture is characterized by the development of a multilayered spiral system in the compact myocardium, coinciding with invasion of the coronary vascular system from the epicardium 3,4. The different layers of the spiral system can be revealed by the technique of peeling. It can be seen that there is an ordered structure for the ventricular mass, albeit that the aggregated myocytes do not form clearly separable fibres, nor are the layers isolated by supporting scaffolds of connective tissue 17. In the matured heart, the ventricular mass is arranged in the form of a modified blood vessel, with each myocyte anchored to its neighbor within a three-dimensional myocardial mesh 18. Streeter et al. 19 introduced the myocyte helix angle, representing the angle between the myocytes, as projected onto the circumferential-longitudinal plane, and the circumferential axis. The myocyte helix angle changes continuously from the subendocardium to the subepicardium, typically ranging from +60° at the subendocardium to −60° at the subepicardium 20. LV twist originates from the dynamic interaction between the oppositely wound subepicardial and subendocardial myocyte helices 21. Furthermore, transmural oriented myocytes may be necessary to ensure stability of the shape of the ventricular walls throughout this twisting deformation 17. The direction of LV twist is governed by the epicardial myocytes, mainly owing to their longer arm of movement 22. Mathematical models have shown that this counterdirectional helical arrangement of muscle fibres in the heart is energetically efficient and is important for equal redistribution of stresses and strain in the heart 23. NCCM is a heterogeneous disorder probably caused by intra-uterine arrest of compaction of the myocardial fibres during embryogenesis 5. Due to this arrest of myocardial compaction it may be anticipated that the characteristic spiral helix will also not develop. Absence of the endocardial helix would lead to increased clockwise basal and counterclockwise apical LV rotation, due to loss of the counteracting activity. On the other hand, absence of the epicardial helix would lead to counterclockwise basal and clockwise apical LV rotation. Therefore, based on our results, the assumption has to be made that both helices must be involved to a similar extent in NCCM. LV solid body rotation with near absent LV twist may be one of the main mechanisms of impaired LV function in NCCM patients. In healthy neonates with an immature heart LV solid body rotation has also been described with basal and apical rotation being in a counterclockwise direction 24. Why some of our patients show clockwise and others counterclockwise LV solid body rotation remains unclear at this moment. Nevertheless, it is striking that all patients who showed the neonatal form of LV solid body (counterclockwise) rotation had no evidence for abnormal LV conduction, evidenced by a normal QRS duration. At present, there is no consensus on how to precisely define NCCM. Recently, Kohli et al. 25 studied 199 patients referred to a dedicated heart failure clinic. There was an unexpectedly high percentage of patients that could be identified as having NCCM: 23.6% of the patients fulfilled one or more of the echocardiographic criteria currently used for the identification of NCCM 8,26,27. This high percentage suggests that current diagnostic criteria may be too sensitive. Furthermore, there was a poor correlation between the echocardiographic definitions, with only 29.8% of the identified NCCM patients fulfilling all three criteria. We propose ‘LV solid body rotation’ as a new sensitive and specific, objective and quantitative, functional criterion, supplementing the classic subjective morphologic NCCM criteria 8,26,27. It should be noticed that others, in contrast to our findings, have occasionally described LV solid body rotation in DCM patients 28. Although it cannot be excluded that in these patients the diagnosis NCCM was overlooked, the true specificity of LV solid body rotation for the diagnosis of NCCM may be lower than that in our study. Other studies should confirm our data before this new criterion should be used clinically.




