The use of E/Em and the time interval difference of isovolumic relaxation (TIVRT−IVRTm) in estimating left ventricular filling pressures
Abstract
Background and aims:
The ratio of the transmitral and myocardial early diastolic velocities (E/Em) can be used to estimate LV filling pressures (LVFP). Additionally, the time difference between the onset of E and Em also correlates to LVFP. The aim of this study was to evaluate which of these two indices is the best marker of LVFP in a heterogeneous group of patients during a simultaneous invasive procedure.
Methods and results:
Thirty two patients were studied. Em and the isovolumic relaxation time (IVRTm) at four segments of the LV were measured using pulsed tissue Doppler echocardiography. Pulsed Doppler echocardiography was used to measure E and IVRT. E/Em and IVRT−IVRTm (TIVRT−IVRTm) were then calculated. Highly significant correlations were found between TIVRT−IVRTm and PCWP at the lateral (r=−0.80, p<0.001) and posterior (r=−0.71, p<0.001) segments whereas only a weak relationship was found between PCWP and E/Em (p<0.05). The sensitivity and specificity of using a negative TIVRT−IVRTm for identifying patients with PCWP >12 mm Hg were 89 and 90%, respectively.
Conclusion:
We found a highly significant correlation between TIVRT−IVRTm and PCWP, which was not seen for E/Em. We propose TIVRT−IVRTm as a stronger predictor of LVFP. TIVRT−IVRTm also seems to correlate to LVFP for many different clinical aetiologies of elevated LVFP.
1. Introduction
A large number of patients report functional limitation due to dyspnoea and fatigue.[1] In many of these cases, signs of “pure” diastolic left ventricular dysfunction have been reported. [2] Further, in registry studies the number of patients with acute heart failure and normal ejection fraction (EF) varies from 30 to more than 50% [3].
Since a close relationship between diastolic function and systolic long axis function is recognised [4] this syndrome has been referred to as heart failure with normal ejection fraction (HFNEF) [5].
Doppler echocardiography is a rapid and accurate non-invasive method for the evaluation of cardiac function. However, invasive measurements of left ventricular end-diastolic pressure (LVEDP) and/or pulmonary capillary wedge pressure (PCWP) still remain the gold standard, although invasive measures are not suitable for all patients due to cost and the risk of complications [5]. The increase in LVEDP might precede the increase in PCWP due to elevated LA booster pump function during atrial systole without increasing left atrial pressure [6]. It has been suggested that Doppler measurements of transmitral and pulmonary vein blood flow velocities may be useful for assessing left ventricular diastolic function, and these parameters have been used to estimate ventricular relaxation, chamber compliance and increased filling pressures [7]. These Doppler velocity profiles can identify a restrictive LV filling pattern that predicts an adverse outcome in heart failure [8].
Pulmonary vein and transmitral flow velocities however, are both load and heart rate dependent [9] and are not always useful in discriminating cardiac dysfunction from normal cardiac function [10]. Tissue Doppler echocardiography (TDE), measuring regional myocardial function, has been perceived to be a relatively load-independent measurement [11] and therefore useful for assessment of LV relaxation in patients with HFNEF [7]. The relationship between blood flow derived velocities and regional myocardial wall motion derived velocities, expressed as the ratio of peak early diastolic velocities E/Em, has been shown in a number of studies to be useful in estimating PCWP in different cardiac diseases [12,13]. However, E/Em has limitations in discriminating patients with mildly to moderately elevated left ventricular filling pressures, [13] is not sensitive in acute coronary syndromes, [14] is influenced by the preload in normal LV relaxation [15] and by the level of LVEF and the sampling site for TDE. [16] Furthermore, the interpretation of E/Em is disturbed if primary mitral valve disease is present [17], and the ratio has also been shown to be increased in asymptomatic healthy subjects older than 65 years [18].
The time difference between the R on ECG to the onset of blood flow in early diastole (T−E) and myocardial motion (T−Em), respectively, expressed as TE−Em, has also been shown to be useful for predicting PCWP and for determining impaired diastolic function [19]. This index has also been shown to be useful in patients with a “grey zone” E/Em ratio of between 8 and 15 [20]. TE−Em is dependent on LV relaxation and LA pressure, where the onset of Em is delayed when LV relaxation is impaired resulting in a prolonged myocardial isovolumic relaxation time (IVRTm) while there is a shortening of blood flow derived IVRT which is explained by the increased driving force of elevated left atrial pressure. This relative time difference in IVRT and IVRTm can easily be calculated, as both IVRT and IVRTm are commonly measured in clinical practice, unlike the time difference between R and the onset of E or Em (T−E or T−Em) [21].
The aim of the present study was to find out whether TIVRT−IVRTm, as a surrogate for TE−Em, is a useful indicator for increased LVEDP and PCWP in an unselected group of patients undergoing simultaneous invasive pressure measurement.
2. Methods
Thirty two patients were studied to assess the relationship between the time difference of onset and the peak velocity ratio of blood flow and myocardial motion of early diastole with simultaneous invasive pulmonary capillary wedge and LV end-diastolic pressures recordings. We investigated patients who were consecutively referred for diagnostic cardiac catheterisation.
Clinical and anthropometric data for all patients are shown in Table 1. All the patients with atrial fibrillation or flutter were excluded from the main analysis because of differences in the RR time interval of more than 100 ms between the transmitral and TDE based diastolic function measurements. To ascertain that inclusion of patients with atrial fibrillation/flutter does not negatively influence the main results, a second analysis was performed, which included 4 patients with atrial fibrillation/flutter. All patients gave their written consent to participate in the study, which was approved by the local ethics committee at Uppsala University.
| Mean±SD | Range | |
|---|---|---|
| Patient anthropometric data | ||
| Age (years) | 53±13 | 20–75 |
| Female/male | 10/22 | |
| Height (cm) | 176±10 | 152–193 |
| Weight (kg) | 84±18 | 52–117 |
| Systolic blood pressure (mm Hg) | 126±27 | 90–190 |
| Diastolic blood pressure (mm Hg) | 78±13 | 50–95 |
| Heart rate (beats/min) | 75±15 | 47–122 |
| NYHA 1/2/3/4 | 0/17/13/2 | |
| Cardiac diseases | ||
| Dilated cardiomyopathy | 12 | |
| Hypertrophic cardiomyopathy | 4 | |
| Heart transplantation | 4 | |
| Primary pulmonary hypertension | 4 | |
| Systemic hypertension | 1 | |
| Mitral valve disease | 2 | |
| Aortic valve stenosis | 1 | |
| Atrial septal defect | 1 | |
| Ventricular septal defect | 1 | |
| Sarcoidosis and aortic valve prosthesis | 1 | |
| Constrictive pericarditis | 1 | |
| Medications | ||
| ACE-inhibitors | 19 | |
| Beta blockers | 15 | |
| Diuretics | 20 | |
| Calcium antagonists | 13 | |
| Immunosuppressants | 4 | |
| Amiodarone | 4 | |
| Drugs for pulmonary hypertension | 5 | |
- a NYHA = New York Heart Association Classification.
2.1. Echocardiographic examination
Doppler echocardiography examination was performed simultaneously with the cardiac catheterisation procedure with patients lying in the supine position. All patients were in stable haemodynamic condition with no drug administration during the periods of data collection. A commercially available ultrasound system (HP, Sonos 5500, Philips, Andover, Mass. U.S.) with a multi frequency phased array transducer and pulsed Doppler tissue imaging technique was used. Parasternal and apical views were obtained according to the recommendations of the American Society of Echocardiography. Recordings were made with a simultaneous superimposed ECG and phonocardiogram (PCG) and second heart sound (S2) used if applicable as acoustic signal for the aortic valve closure. The ultrasound system used (HP) has not previously been found to present time delays between Doppler signal and ECG or PCG [22]. Recordings were made at a sweep speed of 100 mm/s and stored on magnetic optical discs. The sample volume from pulsed TDE was defaulted to 5.7 mm and the acoustic power and filter frequencies were adjusted and optimized for detecting myocardial velocities.
Separate measurements were made for each subject with 2D, M-mode and Doppler echocardiography recordings. Left ventricular (LV) internal diameter at end-diastole and end-systole were measured and fractional shortening was calculated as recommended by the American Society of Echocardiography [23]. Ejection fraction was derived from Simpson's modified single plane method using the apical 4-chamber view. Peak blood flow velocities from transmitral and pulmonary venous flow were registered as previously recommended [24]. From the transmitral flow, the peak early (E) and late atrial (A) diastolic velocities, E-deceleration time (DT) and isovolumic relaxation time (IVRT) were all measured. IVRT was measured as the time difference between aortic valve closure from subaortic valve pulsed Doppler flow, and/or S2 from PCG, to onset of E from transmitral flow, Fig. 1. The R-R time interval was also measured at the transmitral flow velocities. The peak systolic and diastolic velocities were measured from pulmonary venous flow.
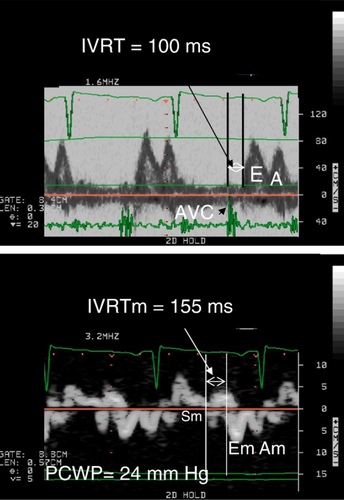
TDE was used to measure left ventricular longitudinal myocardial wall motion from the apical 4- and 2-chamber views. The myocardial isovolumic relaxation time (IVRTm) was measured from end of systolic myocardial velocity during ejection (Sm) to onset of the early diastolic myocardial velocity (Em) as previous described, Fig. 1 [9]. This was done at the left lateral, septal, anterior and posterior basal walls respectively and the time difference IVRT−IVRTm was further calculated. R-R time interval was measured in all segments. Care was taken to identify the wall motion during IVRTm to determine the onset of Em, as previously described [25]. All patients with a time difference >100 ms in the RR interval between measurements of myocardial motion and transmitral tracings were excluded. To adjust for the differences in RR between measurements, IVRT measured from transmitral and myocardial segments were both further adjusted to RR by dividing the respective IVRT with √RR. Furthermore, peak Em and Am were measured and E/Em and Em/Am ratios were calculated for the four segments.
2.2. Cardiac catheterisation
One experienced investigator (GW) performed all the cardiac catheterisations. Briefly, the right ventricular catheterisation was performed by catheter insertion through the internal jugular vein or right brachial vein and guided to the pulmonary artery where capillary wedge pressures (PCWP) were obtained. Retrograde left ventricular catheterisation was performed via the brachial or radial artery to measure the left ventricular end-diastolic pressures. (Becton Dickinson Criticath SP5 107 HTD catheter). All values are taken from a mean of 5 consecutive tracings after being controlled and judged as stable by the investigator. Pressures were registered with a Cathcor® system 3.3 (Siemens Elema AB, Electromedical systems divisions, Solna, Sweden).
2.3. Statistical analysis
A commercially available statistical program, Statistical Package of Social Sciences (SPSS 14.0, Chicago, Ill. USA), was used. All data are presented as the mean±SD.
Pearson's and when relevant (n<20) additional Spearman's correlation was applied and linear regression plot was used to show relationships. A p-value less then 0.05 was considered significant. Inter- and intraobserver variability analysis was performed by calculating the coefficient of variation in 10 patients (standard deviation of difference between two measurements divided by the mean).
3. Results
PCWPs were measured using cardiac catheterisation in all 32 patients. LVEDP was measured in 16 patients. LV fractional shortening ranged between 7 and 49% and was >26% in 53% of the patients. LVEF ranged between 6 and 78%, and was >50% in 36% of the cases. LVEDP was >15 mm Hg in 63% of the patients and PCWP was >12 mm Hg in 59% of the patients. Invasive and non-invasive data are shown in Table 2. The interobserver coefficient of variation for analysing fluid dynamic IVRT was 8.2% whereas myocardial IVRTm from the 4 segments varied from 9.8–14.3%. The intraobserver coefficient of variation for IVRT was 6.9% and for myocardial IVRTm ranged between 8.4% and 14.2%.
| Mean±SD | Range | |
|---|---|---|
| LA and LV dimensions and function | ||
| Left atrial diameter (mm) | 42±9 | 27–57 |
| LV septal wall thickness, diastole (mm) | 11.6±3.8 | 5.0–19.0 |
| LV posterior wall, diastole (mm) | 9.6±3.4 | 4–7 |
| LV diastolic diameter (mm) | 56±16 | 28–89 |
| LV systolic diameter (mm) | 42±17 | 17–80 |
| LV fractional shortening (%) | 27±11 | 7–49 |
| LV ejection fraction (%) | 46±18 | 6–78 |
| Mitral E/A ratio | 1.6±1.0 | 0.2–4.1 |
| Mitral IVRT (ms) | 93±49 | 20–220 |
| Mitral DT (ms) | 167±65 | 80–350 |
| Invasive data | ||
| LVEDP (mm Hg) | 19±6 | 10–30 |
| PCWP (mm Hg) | 17±8 | 6–33 |
- a LV = left ventricular; E = early diastolic; A = atrial diastolic; IVRT = isovolumic relaxation; DT = deceleration time; EDP = end-diastolic pressure; PCWP = pulmonary capillary wedge pressure.
3.1. The relation between TIVRT−IVRTm and pulmonary capillary wedge pressure
A highly significant correlation was found between TIVRT−IVRTm and PCWP at all four longitudinal segments of the left ventricle (r=−0.80, p<0.001, n=28 for lateral, r=−0.39, p<0.05, n=28 for septal, r=−0.58, p<0.01, n=24 for anterior, and r=−0.71, p<0.001 n=23 for posterior), Table 3. The sensitivity and specificity for detecting an elevated PCWP of more than 12 mm Hg, using a cut off value of TIVRT−IVRTm(lateral) being negative or less than 0, were 89% and 90% and the positive predictive and negative predictive values were 94% and 82%, respectively, Fig. 1A.
| Mean±SD | Range | Correlation to LVEDP | Correlation to PCWP | |
|---|---|---|---|---|
| Invasive pressures | ||||
| PCWP, mm Hg | 17±8 | 6–33 | r=0.32, p=0.233 | - |
| LVEDP, mm Hg | 19±6 | 10–30 | - | r=0.32, p=0.233 |
| Tissue Doppler echocardiography | ||||
| TIVRT−IVRTm Lat, ms | −20±51 | −112–67 | r=−0.41, p=0.15 | r=−0.80, p<0.001 |
| TIVRT−IVRTm Sep, ms | −34±53 | −126–105 | r=−0.34, p=0.24 | r=−0.39, p<0.05 |
| TIVRT−IVRTm Ant, ms | −21±42 | −175–70 | r=−0.72, p<0.01 | r=−0.58, p<0.01 |
| TIVRT−IVRTm Post, ms | −30±45 | −130–49 | r=−0.79, p<0.01 | r=−0.71, p<0.001 |
| E/Em, Lat | 9.7±4.9 | 2.1–21.6 | r=0.58, p<0.05 | r=0.43, p<0.05 |
| E/Em, Sep | 11.5±5.4 | 5.8–27.3 | r=0.64, p<0.05 | r=0.44, p<0.05 |
| E/Em, Ant | 11.6±6.6 | 3.0–27.6 | r=0.57, p=0.069 | r=0.43, p<0.05 |
| E/Em, Post | 11.9±6.1 | 3.2–30.7 | r=0.64, p<0.05 | r=0.35, p=0.076 |
| Em/Am, Lat | 1.6±1.1 | 0.3–5.6 | r=0.08, p=0.814 | r=0.14, p=0.501 |
| Em/Am, Sep | 1.1±0.6 | 0.5–3.0 | r=0.09, p=0.784 | r=0.52, p<0.05 |
| Em/Am, Ant | 1.2±0.7 | 0.3–3.0 | r=−0.15, p=0.645 | r=0.19, p=0.358 |
| Em/Am, Post | 1.1±0.8 | 0.3–2.7 | r=−0.22, p=0.502 | r=0.47, p=0.018 |
| Spectral Doppler | ||||
| MF E, cm/s | 71±47 | 25–266 | r=0.14, p=0.631 | r=0.61, p<0.001 |
| MF A, cm/s | 65±66 | 15–335 | r=0.67, p=0.593 | r=−0.04, p=0.850 |
| MF IVRT, ms | 93±49 | 20–220 | r=−0.35, p=0.178 | r=−0.60, p<0.001 |
| MF DT, ms | 167±65 | 80–350 | r=−0.06, p=0.842 | r=−0.18, p=0.344 |
| PVF systole cm/s | 34±16 | 13–76 | r=−0.02, p=0.966 | r=−0.47, p<0.05 |
| PVF diastole cm/s | 48±14 | 25–79 | r=0.47, p=0.201 | r=0.47, p<0.05 |
- a LV = left ventricular; E = early diastolic; A = atrial diastolic; IVRT = isovolumic relaxation; DT = deceleration time; EDP = end-diastolic pressure; PCWP = pulmonary capillary wedge pressure; m = myocardial; MF = mitral flow; PVF = pulmonary venous flow; TDE = tissue Doppler echocardiography.
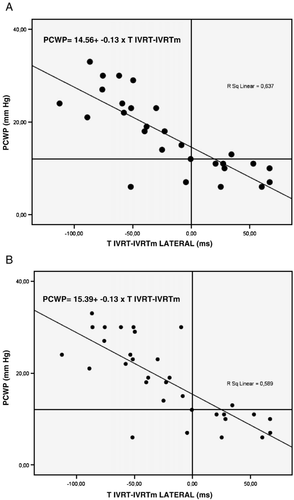
When the 3 patients with atrial fibrillation and 1 with atrial flutter were included there was still a good correlation between TIVRT−IVRTm and PCWP at all four longitudinal segments of the left ventricle (r=−0.78, p<0.001, n=32 for lateral, r=−0.45, p<0.01, n=32 for septal, r=−0.62, p<0.001, n=28 for anterior, and r=−0.69, p<0.001 n=24 for posterior), Fig. 2B. The sensitivity and specificity for detecting an elevated PCWP of more than 12 mm Hg using a cut off value of TIVRT−IVRTm(lateral) being negative or less than 0 were 95% and 82% respectively, the positive predictive and negative predictive values were 91% and 90%, respectively, Fig. 2B.
We found a weak correlation between E/Em and PCWP at lateral, septal and anterior walls (r=0.43–0.44, p<0.05, n=25–27). There was also a significant correlation between transmitral IVRT, (r=−0.60, p<0.001, n=29) peak E velocity (r=0.61, p<0.001, n=30), pulmonary venous peak systolic (r=−0.47, p<0.05, n=21) and diastolic flows (r=0.47 p<0.05, n=21) to PCWP, Table 3. No relation was found between PCWP and myocardial IVRTm (n=22–28) at any segment of the left ventricle.
3.2. The relation between TIVRT−IVRTm and LV end-diastolic pressure
We found that the TIVRT−IVRTm at the anterior (r=−0.72, p<0.01, n=13) and posterior (r=−0.79, p<0.01, n=11) segments of the LV was correlated to LVEDP; however, no correlation was found to the lateral or septal segments. The correlation between E/Em and LVEDP at different segments of the LV was for lateral; r=0.58, p<0.05, n=12, for septal; r=0.64, p<0.05, n=12 and for posterior; 0.64, p<0.05, n=12. We found no relation between traditional transmitral or pulmonary venous flow indices of LV diastolic function and LVEDP, Table 3.
4. Discussion
4.1. Main findings
We have for the first time demonstrated that the time difference between onset of early diastolic blood flow (fluid dynamics) and myocardial motion (mechanics) of the longitudinal early relaxation wall motion, measured as TIVRT−IVRTm, is strongly correlated to PCWP and LVEDP, a relation that was not found for the E/Em. Importantly, this study was performed in a consecutive but not diagnosis-specific group of patients during a simultaneous cardiac catheterisation procedure with a wide range of LVFP.
4.2. Data interpretation
Traditional Doppler echocardiography studies of the transmitral and pulmonary flow are used to assess left ventricular (LV) diastolic function [7]. However, the technique has a number of limitations [9] including differentiating diastolic dysfunction from normals [10]. E peak velocity is strongly dependent on both preload and relaxation [26]. With alterations in left atrial pressure and relaxation, E is positively correlated with left atrial v-wave and LVEDP, but negatively and weakly correlated with relaxation, measured as time constant of relaxation (tau). Results from our present study are in agreement with this, as a positive relation between E and LVFP was found. However, a combined measurement using the peak E velocity as a indicator of LV filling pressure and being a preload-dependent variable, with a parameter being relaxation dependent but relatively pressure and preload independent would be preferable. Such a marker has been proposed, using myocardial longitudinal E myocardial (m) early relaxation motion from TDE [11].
Measurement of the longitudinal myocardial velocities with TDE from apical 4- and 2-chamber views is easily obtainable, and previous studies have shown an inter- and intraobserver variability when measuring velocities and time intervals of less than 4%, in addition the time required for assessment is relatively short [27,28]. Despite non-optimal examination conditions during simultaneous cardiac catheterisation (as was the case in our present study), Em and IVRTm were measurable in 70–90% of cases and the coefficient of variation of measuring IVRT and IVRTm ranged from 8–14%. Therefore, we used the longitudinal Em as the relaxation dependent variable, and calculated the ratio of E and Em (E/Em) as proposed. The E/Em has been found to be elevated but also correlated to LVFP in patients with different cardiac diseases [29,30,17].
In normal subjects, the Em gradually declines with increasing age concordant with Doppler indices of LV relaxation, i.e. transmitral E velocity, [31] and in these subjects the peak Em is preload dependent [32]. This dependence disappears when LV relaxation is impaired, thus Em can be used as a preload-independent measurement of myocardial relaxation [12]. A ratio of E/Em less than 8 has been shown to be useful in identifying patients with normal LVFP, an E/Em above 15 indicating elevated LVFP. However, for E/Em ratios in the range 8–15, elevated LVFP cannot be determined accurately [13]. Furthermore, limitations in using the E/Em ratio have been shown in patients with primary mitral regurgitation [17] and in constrictive pericarditis [33]. In our study in patients with different cardiac diseases there was only a modest relationship between E/Em and LVFP, as has previously been shown [16,34].
Recently, a new index for measuring the time onset relation between early diastolic velocities from Doppler echocardiography and TDE has been shown to be useful in different stages of diastolic dysfunction [35] and of incremental value in HFNEF patients with E/Em between 8 and 15 [20]. This index can be calculated by measuring the time difference between the two components of blood flow (E) and myocardial motion (Em) using the R-wave on ECG as a reference point, respectively, i.e. TE−Em or TEm−E. This index has been shown to correlate to LV filling pressures [21,19]. In normal subjects, the LV isovolumic relaxation phase is short and the diastolic suction results in simultaneous onset of mitral E, or slightly after the Em [35]. With normal aging, IVRT increases both concerning blood flow and myocardial motion. However, IVRT from blood flow is found to be 20–30 ms longer than IVRTm throughout the age range of 20–81 years in healthy subjects [27]. Generally, LV filling is controlled by LV compliance, LV elastic recoil, myocardial viscoelasticity, and left atrial pressure. When LV relaxation is hampered, for example in normal ageing, LV filling is maintained by increased atrial contraction seen in both blood flow and myocardial motion derived velocities [36]. However, when atrial contraction is not sufficient to maintain adequate stroke volume, left atrial pressure rises and the relations between E and Em and IVRT and IVRTm disappear. Thus, with an increasing decline in LV relaxation and increasing LVFP, Em falls and the onset of Em is delayed concomitant with an increase in E velocity and shortening of IVRT [21]. This was demonstrated in our study, as a negative correlation between PCWP and IVRT was found without a correlation with IVRTm.
4.3. Clinical implications
An important reason for the differences between our results and data from other studies is based on the technical methods used for measuring myocardial velocities. Commonly, the mean of two (septal and lateral) [13,12] or 4 segments (anterior, lateral, septal and posterior) [37] has been routinely used to determine Em values. Ommen et al. showed that the septal segment had a slightly higher correlation to LV mean diastolic pressure than the lateral site [13] and the authors proposed the use of a combination of lateral and septal sites as this appeared to be the most accurate method to use. They also found a higher cut off value in the septal site in patients with a LVEF <50%. Thus, the grade of systolic function, measured as LVEF, might be another reason for the divergent results using E/Em [16].
The higher cut off level for the septal segment measurement could be related to abnormal septal motion which is commonly seen in patients with heart failure, but is not always related to an elevated LV filling pressure.
In our present study, the weak relation between E/Em and LVFP was similar in the different segments and to both PCWP and LVEDP. It was not possible to investigate the relation of LVEF (i.e. < or >50%) to E/Em, due to the small number of patients. However, the clinical implications of using only E/Em as a marker of elevated LVFP in a wider range of heart failure patients is questionable [5].
Both for TIVRT−IVRTm and for E/Em, the choice of the measured segment seems to be of importance and previous results have indicated that the lateral segment is the most useful to use for determining PCWP from E/Em [16] and also is less preload dependent [15]. Explanations might be that the lateral segment is, a) less influenced by the right ventricle through common septum, and b) less altered in myocardial function that might be related to cardiac surgery. However, we found the lateral wall to be the most accurate segment for identifying patients with elevated LVFP. In terms of the influence of arrhythmias on TIVRT−IVRTm, when we included patients with atrial fibrillation or atrial flutter, there was no difference in the correlation or the reliability in detecting elevated LVFP, despite the small number of patients.
4.4. Limitations
The present study is limited by the sample size of 32 patients, but this was sufficient to draw conclusions on pertinent relationships. The studied group had a broad range of cardiac diseases with variable degrees of left ventricular systolic function. However, this could be considered to be a strength making our results applicable in a wider clinical setting.
TIVRT−IVRTm and E/Em cannot be measured simultaneously with current technology. Due to the time differences between blood flow and myocardial diastolic measurements, we excluded patients with a difference of more than 100 ms between paired measured beats and we further corrected the time intervals using a well established method. Evaluation of LVEDP by Doppler echocardiography and TDE was performed for only a limited number of patients, and thus no specific conclusions concerning these parameters could be reached.
5. Conclusion
E/Em has been proposed to be a clinically important tool as an indirect measure of LVFP; however, the method has recently been brought into question. In the present study we found only a weak correlation between E/Em and LVFP. However, we found a highly significant correlation between TIVRT−IVRTm, especially when using the LV lateral wall segment, and LVFP. TIVRT−IVRTm is a reliable and easily measured index of LVFP. Importantly, the method seems to be useful in a wide range of cardiac diseases.
6 Acknowledgements
This study was supported by the Swedish Heart and Lung Foundation, The Heart Foundation of Northern Sweden and Uppsala Academic Hospital Research Fund. Ulla-Marie Andersson, Mona Andrén, Karin Fagerbrink, Kjell Karlström, Berit Lowén and Elisabeth Lindström are acknowledged for skilful assistance during the catheterisation procedure. Johan Landelius was of invaluable support. We acknowledge Dr Michael Haney for helpful contribution in the preparation of the manuscript.




