Carvedilol reduces exercise-induced hyperventilation: A benefit in normoxia and a problem with hypoxia
Abstract
Aims:
To evaluate whether carvedilol influences exercise hyperventilation and the ventilatory response to hypoxia in heart failure (HF).
Methods and results:
Fifteen HF patients participated to this double blind, randomised, placebo controlled, cross-over study. Patients were evaluated by quality of life questionnaire, echocardiography, pulmonary function and cardiopulmonary exercise tests (ramp and constant workload) both in normoxia (FiO2=21%) and hypoxia (FiO2=16%, equivalent to a simulated altitude of 2000m). Carvedilol improved clinical condition and reduced left ventricle size, but had no effect on lung mechanics. In normoxia during exercise, ventilation was lower, VCO2 unchanged and PaCO2 (constant workload) or PetCO2 (ramp) higher with carvedilol, exercise capacity was unchanged (peak workload 92±22 and 90±22W for placebo and carvedilol, respectively). Abnormal VE/VCO2 slope was reduced by carvedilol. Hypoxia increased ventilation but less with carvedilol; exercise capacity decreased to 87±21W (placebo) and to 80±11W (carvedilol, p<0.01). With hypoxia, carvedilol decreased VE/VCO2 slope. At constant workload exercise with hypoxia, PaO2 decreased to 69±6 mm Hg (placebo) and to 64±5 (carvedilol, p<0.01).
Conclusion:
Carvedilol reduced hyperventilation possibly by reducing peripheral chemoreflex sensitivity as suggested by PaCO2 increase with normoxia and PaO2 decrease with hypoxia without VCO2 and VD/VT changes. Lessening hyperventilation is beneficial when breathing normally, but detrimental when hyperventilation is needed for exercise at high altitude.
Abnormal hyperventilation is a striking characteristic of the integrated response to exercise in heart failure (HF) patients 123. Indeed, for a given work-rate, HF patients have a higher ventilation rate than normal subjects 1. Several studies have shown a relationship between HF severity and hyperventilation. Hyperventilation is associated with dyspnoea, one of the most common symptoms of HF 2,4, and hyperventilation generates a low ventilatory efficiency, defined as a high ventilation relative to CO2 production, which is a strong predictor of negative prognosis 5,6. In addition, abnormal ventilation is a limiting factor for exercise capacity 789. Hyperventilation can be due to several causes, including overactive chemoreceptor reflexes 101112, one of several abnormalities in cardiovascular reflex control related to increased sympathetic tone in HF 6.
Beta-blocker therapy is an effective treatment for chronic HF 1314151617. To date, only a few reports have evaluated the effects of beta-blockers on exercise induced hyperventilation 18,19. Furthermore, there are no data on the effects of beta-blockers on exercise under hypoxic conditions, when arterial hypoxia increases chemoreceptor activity 20. This information is important because exposure to hypoxia occurs in normal life, for example when travelling by plane or while ascending to a high altitude. The present study was therefore undertaken to assess the effects of hypoxia, simulating an altitude of 2000m, on exercise performance in chronic HF patients with and without beta-blocker therapy.
1. Methods
1.1. Study population
Fifteen patients with chronic HF due to idiopathic dilated cardiomyopathy (male=9, female=6, age 61.8±8.0years) participated in the study. All patients belonged to a cohort of subjects who were regularly followed at our heart failure unit and had previously undergone cardiopulmonary exercise testing (CPET) in our laboratory. All patients had been in a stable clinical condition for at least 3months. Twelve patients were in regular sinus rhythm and three in atrial fibrillation. Drug treatment was stable, optimized and individually tailored and included ACE-inhibitors (n=11), AT1 blockers (n=3), diuretics (n=10), aldosterone antagonists (n=7), amiodarone (n=1), and digitalis (n=5). Study inclusion criteria were: NYHA class II or III, no previous beta-blocker therapy, left ventricle ejection fraction <40%, and able to perform a cardiopulmonary exercise test. Study exclusion criteria were: history and/or clinical documentation of primary valvular heart disease, pericardial disease, severe obstructive lung disease, pulmonary embolism, primitive pulmonary hypertension or occupational lung disease, asthma, renal failure (serum creatinine >2.5mg/dl), significant peripheral vascular disease, cardiac pace-maker, atrio-ventricular block (PQ≥0.24s) or exercise induced angina, significant ST changes or severe arrhythmias.
1.2. Study design
This was a double blind, randomised, placebo controlled, cross-over study (Fig. 1). All subjects who participated in the study underwent a run-in period of 2weeks, during which clinical stability was assessed and patients performed at least two cardiopulmonary exercise tests to familiarize them with the procedure. Patients were randomised into two groups: A (n=8) and B (n=7). In group A, placebo treatment was given first, followed by carvedilol titration and treatment; and in group B, placebo followed carvedilol. The study protocol lasted 8months. The carvedilol titration period lasted 2months 15,18 and was performed by an investigator who did not participate in any other part of the investigation. The full carvedilol dosage was administered for 4months. Standard labelled carvedilol tablets were used. Carvedilol was administered twice a day. Placebo treatment was given for 2months.

During the study, patients underwent clinical evaluation and ECG every 15days or more often if required or wished by the patients. For research purposes, patients were evaluated twice, once at the end of the carvedilol treatment period and again at the end of the placebo period. Over 3days patients underwent: (A) evaluation of quality of life; (B) pulmonary function test and lung diffusion evaluation; (C) echocardiographic evaluation; (D) two ramp protocol CPETs with inspiratory O2 fraction of 0.21 and 0.16; (E) two constant workload CPETs with inspiratory O2 fraction of 0.21 and 0.16. The two constant workload exercise protocols were done on the same day, 3h apart in random order. The ramp protocol CPETs were done on two separate days in random order. The maximal workload measured in the second familiarization CPET performed during the run-in period was used for all other CPETs. Treatment was kept constant throughout the study period. Research personnel involved in the randomisation procedure and the personnel who evaluated patients were blinded to the study protocol, treatment and time course of the study. Our institutional ethics and scientific committees approved the protocol and each patient gave written informed consent to the study and to each procedure.
1.3. Echocardiography and quality of life evaluations
Standard echocardiographic evaluation was performed to measure left ventricle dimensions and ejection fraction. Quality of life was evaluated by means of the Minnesota Living with Heart Failure Quality-of-Life Questionnaire 21.
1.4. Pulmonary function and lung diffusion
All subjects were evaluated by standard pulmonary function tests, which included lung diffusion for carbon monoxide (DLCO). Forced expiratory volume in 1s (FEV1) and vital capacity (VC) were measured according to the American Thoracic Society standard criteria 22 using the predicted values of Quanjer et al. 23. DLCO was measured with the single breath—constant expiratory flow technique (Sensor Medics 2200, Yorba Linda, CA). Diffusion subcomponents, capillary volume and membrane conductance (DM), were also measured applying the Roughton and Forster method 24. For this purpose, patients inspired gas mixtures containing 0.3% CH4, 0.3% CO, with 3 different oxygen fractions equal to 20%, 40% and 60%, respectively, and balanced with nitrogen.
1.5. Cardiopulmonary exercise test (CPET): ramp protocol
CPET (V-Max 2900 metabolic cart, Sensor Medics, Yorba Linda, CA) was performed on a cycle ergometer (Ergo 800S Sensor Medics, Yorba Linda, CA); work rate was increased in a ramp pattern after 3min of rest and 3min of unloaded cycling. The workload applied was personalized and chosen to achieve peak exercise in about 10min 25. Once chosen the workload applied was kept constant for a given individual in all the CPETs. Inspiratory O2, expiratory O2, CO2 and ventilation (VE) were measured breath-by-breath. A 12-lead ECG was also recorded from which heart rate was obtained. The test was self-ended by patients who were strongly encouraged to perform a maximal test. Peak exercise was considered the highest achieved. Anaerobic threshold was measured with the V-slope analysis from the plot of vs. 26. The anaerobic threshold value was confirmed by ventilatory equivalents (increase of VE/ with a constant VE/) and end-tidal pressures (increase of end-tidal with constant end-tidal ). The /work rate relationship was evaluated throughout the entire exercise, during the ramping period. The VE vs. slope was calculated as the slope of the linear relationship between VE and from the beginning of loaded exercise to the end of the isocapnic buffering period. Two experts independently read each test and the results were averaged. O2 inspiratory fraction was either 0.21 or 0.16, equivalent to sea level and ~2000m, respectively. Gas mixture was obtained in a 25-L low pressure rubber balloon inflated with oxygen and nitrogen with two flow-meters running in parallel. The balloon was also connected to a T-shape respiratory valve with an inspiratory line which was open only during inspiration (2700 L Hans Rudolf, Kansas City, MO). Twenty minutes of quiet breathing were allowed with both gas mixtures before the tests began.
1.6. Cardiopulmonary exercise test (CPET): constant workload
Constant workload CPETs used the same instruments as the ramp protocol. Inspiratory and expiratory gases were measured breath by breath. The workload applied was the mean of workload at anaerobic threshold and workload at the end of the isocapnic buffering period. The exercise duration was 6min preceded by 3min of unloading pedaling. Before the test a small arterial catheter was placed in the radial artery to obtain blood samples for gas analysis (IL, Synthesis 25, Milano, IT). Blood samples were withdrawn at rest and after 4min of constant workload exercise. VD/VT was measured as (PaCO2−PeCO2)/PaCO2−VDapp/VT; where PaCO2 is arterial pressure for carbon dioxide, PeCO2 is mean expiratory CO2 pressure, VDapp is valve and mouthpiece dead space and VT is tidal volume 27. The Δ6–3 was measured as the difference between the 6th and the 3rd minute of exercise 28. Oxygen inspiratory fraction was either 0.21 or 0.16. As for the ramp protocol test, 20min of quiet breathing were allowed with both gas mixtures before the tests began.
1.7. Statistical analysis
Data reported are mean±SD. The mean values of the cardiopulmonary exercise test at a specific moment of the test are means over 30s. The sample size was calculated assuming a reduction of average (calculated throughout the exercise, see Methods) VE=3.0L/min with treatment group with a standard deviation=2.5L/min, a two-sided test, a value for alpha=0.05 and a desired power=0.90. Statistical analysis was performed by paired or unpaired t-test as appropriate. Differences between groups were assessed by comparing increments. In case of multiple comparisons the Bonferroni correction was applied as needed. Differences with p<0.05 were considered significant. /work and VE/ relationships were built by linear regression analysis.
2. Results
All 15 patients completed the 8-month trial with no untoward effects. No difference was observed between the two groups regarding patients' characteristics, heart failure severity, carvedilol dose or underlying treatment. Mean carvedilol daily dose at the end of the drug titration period was 30±16mg. Carvedilol dose was kept constant afterwards. Pulmonary function, echocardiographic, quality of life and CPETs data were similar in the 2 groups during placebo treatment, therefore data from groups A and B were pooled; furthermore the changes induced by carvedilol were also similar in both groups. The Minnesota Living with Heart Failure Quality-of-Life Questionnaire scored 35±30 and 23±18 (p<0.05) with placebo and carvedilol, respectively. Echocardiographic and pulmonary function measurements are reported in Table 1. After 6months of active treatment left ventricular diastolic volume was reduced. Pulmonary function was not significantly affected by carvedilol while DLCO was decreased mainly due to a reduction of DM (Table 1).
| Placebo | Carvedilol | |
|---|---|---|
| LVEF (%) | 35±7 | 38±10 |
| LVDV (ml) | 181±76 | 159±60* |
| LVSV (ml) | 122±74 | 106±58 |
| FEV1 (L) | 2.48±0.68 | 2.40±0.61 |
| FEV1 (%) | 94±20 | 91±19 |
| VC (L) | 3.24±0.77 | 3.20±0.78 |
| VC (%) | 96±16 | 94±20 |
| DLCO (ml/mm Hg/min) | 21.1±3.8 | 17.7±3.6a |
| DLCO (%) | 88±15 | 74±13a |
| DM (ml/mm Hg/min) | 37±11 | 29±9b |
| Capillary volume (ml) | 83±36 | 73±25 |
- a LV=left ventricular; EF=ejection fraction; DV=diastolic volume; SV=systolic volume; FEV1=forced expiratory volume (1 s); VC=ventilatory capacity; DLCO=lung diffusion for carbon dioxide; DM=membrane conductance.
- a p<0.001 vs. placebo.
- b p<0.02 vs. placebo.
- * p<0.05 vs. placebo.
2.1. Cardiopulmonary exercise test (CPET): ramp protocol
The mean workload of the ramp protocol was 10.1±1.6W/min. Exercise capacity was not affected by carvedilol in normoxia (Table 2). In normoxia, comparing placebo with carvedilol, VE was lower, was unchanged while PetCO2 was higher. To show this, we calculated the mean of VE, and PetCO2 in each patient measured at rest, 2nd, 4th, 6th, 8th minute and peak exercise. Mean exercise VE was reduced by carvedilol (Fig. 2). Mean exercise was 748±411 and 694±423ml/min, for placebo and carvedilol, respectively (p=NS); PetCO2 was 34.7±3.1 and 35.9±4.5mm Hg, for placebo and carvedilol, respectively (p<0.005). As an average in control conditions (normoxia, placebo) the /work and VE/ slopes were abnormal (Table 2) 3,5,12,30,31. In normoxia, carvedilol tends to decrease the VE/ slope; excluding the 4 patients with normal VE/ slope (<34) 5,12,18 VE/ slope was reduced from 42.3±5.9 to 38.9±5.0 (p<0.05) by carvedilol.
| Normoxia | Hypoxia | |||
|---|---|---|---|---|
| Placebo | Carvedilol | Placebo | Carvedilol | |
| Watt (peak) | 92±22 | 90±22 | 87±21* | 80±19*,*** |
| p (ml/kg/min) | 16.31±2.49 | 15.82±3.96 | 15.96±2.85 | 14.57±4.02 |
| p (%) | 72.4±24.5 | 74.3±33.6 | 71.9±21.5 | 69.7±32.0 |
| HRp (bpm) | 145±27 | 122±22** | 147±34 | 118±24** |
| RQ (peak) | 1.11±0.08 | 1.09±0.06 | 1.17±0.08 | 1.27±0.34 |
| AT (ml) | 708±156 | 678±168 | 851±145 | 863±173 |
| Watt AT | 44±15 | 45±16 | 24±13* | 26±10* |
| /work | 8.81±0.93 | 9.09±0.96 | 8.41±3.73 | 7.70±2.68 |
| VE/ | 40±7 | 38±7 | 39±10 | 32±14** |
- a =oxygen consumption; p=peak exercise; HR=heart rate; RQ=respiratory quotient (/); AT=anaerobic threshold.
- * p<0.01 vs. normoxia.
- ** p<0.01 vs. placebo.
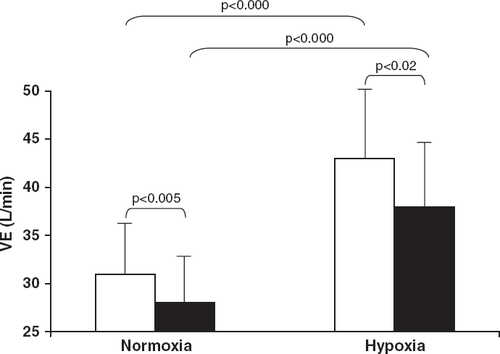
In hypoxia, exercise tolerance decreased with placebo 29 and decreased further with carvedilol. Compared to normoxia, in hypoxia average VE (measured throughout the exercise during the ramp protocol test, Fig. 2) was higher both with placebo and carvedilol. In hypoxia with carvedilol average VE was lower compared to placebo (Fig. 2). In hypoxia mean average was 942±355 and 950±332ml/min and mean average PetCO2 was 32.3±3.6 and 32.7±4.51mm Hg, for placebo and carvedilol, respectively, (p<0.000 vs. normoxia for both placebo and carvedilol, p=NS between carvedilol and placebo). The VE/ slope was reduced by carvedilol in hypoxia (Table 2).
2.2. Cardiopulmonary exercise test (CPET): constant workload
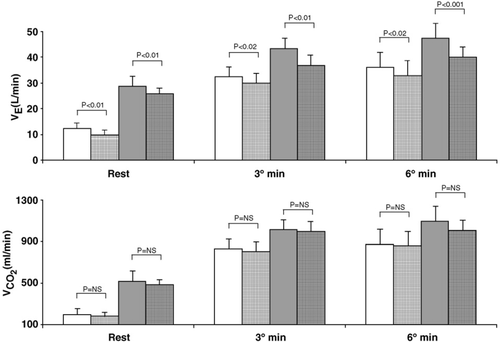
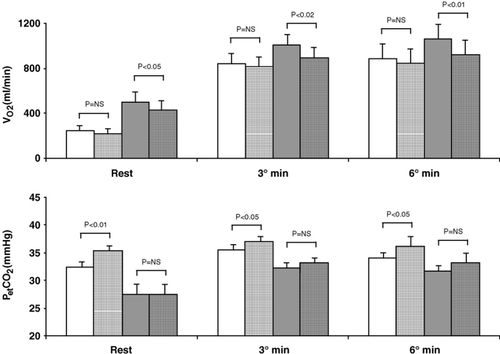
Constant workload exercise was performed at 48±13W. Results are reported in Figs. 3 and 4. Ventilation was lower with carvedilol both in normoxia and hypoxia at rest, and at the 3rd and 6th minute of exercise. Δ6–3 increment on placebo was 45±53ml/min for normoxia and 51±118ml/min for hypoxia, and, on carvedilol, values were 31±74ml/min for normoxia and 31±93ml/min for hypoxia. Measured VD/VT was: (a) normoxia rest 0.59±0.05 and 0.60±0.06, (b) normoxia exercise: 0.36±0.06 and 0.38±0.055, (c) hypoxia rest: 0.46±0.08 and 0.49±0.11, (d) hypoxia exercise: 0.34±0.06 and 0.36±0.06, for placebo and carvedilol, respectively. Blood gas data are reported in Table 3; with carvedilol PaCO2 was higher during exercise both in normoxia and hypoxia. In normoxia increased from rest to exercise. In hypoxia decreased with exercise and to a greater extent with carvedilol. The Δ between the 4th minute of exercise and rest in normoxia was 4±7mm Hg and 5±9mm Hg, for placebo and carvedilol, respectively (p=NS) and in hypoxia −8±6mm Hg, and −14±9mm Hg, for placebo and carvedilol, respectively (p<0.05).
| (mm Hg) | (mm Hg) | pH | HCO3− (mmol/l) | |||||
|---|---|---|---|---|---|---|---|---|
| R | 4′ | R | 4′ | R | 4′ | R | 4′ | |
| Normoxia | ||||||||
| Placebo | 97.2±7.1 | 101.5±6.3a | 35.0±2.9 | 34.6±2.4 | 7.44±0.03 | 7.41±0.02b | 23.3±1.3 | 21.7±1.3b |
| Carvedilol | 92.4±10.3* | 97.9±9.0a,** | 37.5±2.8** | 36.5±3.4** | 7.44±0.02 | 7.42±0.02b,* | 25.2±1.5** | 23.6±1.9b,** |
| Hypoxia | ||||||||
| Placebo | 79.0±7.6 | 68.5±5.7b | 28.9±4.4 | 31.5±2.0b | 7.49±0.04 | 7.44±0.02b | 22.1±1.6 | 21.1±1.2b |
| Carvedilol | 77.7±10.7 | 64.2±5.2**,b | 30.9±4.5 | 33.5±3.5** | 7.50±0.06 | 7.45±0.02b | 23.7±2.1** | 23.4±2.2** |
- a R=Rest, 4′=4th minute of exercise.
- a p<0.05 vs. rest.
- b p<0.01 vs. rest.
- * p<0.05 vs. placebo.
- ** p<0.01 vs. placebo.
3. Discussion
This study shows that in chronic HF at sea level, carvedilol reduces exercise induced hyperventilation through an up-shifting of the CO2 set-point, without affecting exercise performance 1,32. Lowering of hyperventilation is a positive event because hyperventilation is related to an increase in the work of breathing 1,9,33, HF symptoms such as dyspnoea 4, and to poor prognosis 5,6,12. However, when an increase in ventilation is needed, such as when exercising in hypoxia, the reduced ventilatory response negatively affects exercise capacity.
In this study, as in several previous reports 13,14,15, chronic treatment of HF with carvedilol improves clinical status and reduces left ventricle dimensions without affecting exercise performance. Indeed, peak , at anaerobic threshold and 6–3Δ at constant workload were unaffected by carvedilol. Carvedilol does not affect lung mechanics (Table 1) 18,34. However, DLCO is reduced by carvedilol due to a reduction of DM. The present observation confirms previous data obtained in HF patients, showing a reduction in DLCO with carvedilol 18,34. Although the finding of a lower DLCO with beta-blockers is an old one 35 the physiological mechanism remains elusive.
At sea level, ventilation depends on , arterial and VD/VT according to the following formula: VE=×863/[PaCO2×(1−VD/VT)] where VE=ventilation, PaCO2=CO2 arterial pressure and VD/VT is tidal volume/dead space ratio 1. In HF, during exercise, hyperventilation is associated with an increased VD/VT and and a lower arterial 1,32. Carvedilol reduces ventilation during exercise acting mainly on arterial 18. Indeed during constant workload exercise, arterial was increased but and measured VD/VT remained unchanged. During the ramp protocol, in order to avoid multiple systemic artery catheterisations, instead of measuring arterial , we measured its non-invasive equivalent, the end-tidal CO2 pressure (PetCO2) 27. As in the constant workload exercise in the ramp protocol, ventilation was lower, unchanged and PetCO2 increased. Together, these data suggest that carvedilol reduces hyperventilation by acting mainly on the arterial , i.e. affecting the “so-called” CO2 set-point which is related to chemoreceptor response 32. Furthermore, the VE/ slope, which is both the best index of the efficiency of ventilation and a strong prognostic indicator independent of peak 5,6,12,36, is reduced with carvedilol in patients with an abnormal VE/ slope 18. The value we chose to define an abnormal VE/ slope was 34, which is the mean±2 S.D. of the VE/ slope in normal subjects; and which has been previously used to define abnormal VE/ slope 18. Therefore, our observations in normoxia suggest that carvedilol restores or tends to restore a normal pattern for ventilation during exercise in HF through an up-shifting of the CO2 set-point which is possibly due to a reduction in chemoreflex activity.
Our results in hypoxia were obtained after acute exposure to hypoxia without any adaptation to simulated high altitude. Results of the present study are in line with previously published data showing a progressive reduction in exercise capacity with altitude increase in HF patients 29. The greater reduction in exercise capacity, evaluated as the workload reached at peak exercise, vs. peak is due to an increase in the work of breathing with hypoxia 29. With hypoxia, adding carvedilol further reduces exercise capacity (Table 2). In normoxia, the major trigger of chemoreflex induced increase in ventilation is arterial . However, the major adaptive response to acute exposure to hypoxia is the peripheral chemoreceptors response to low arterial which increases ventilation 20. As in normoxia, with hypoxia carvedilol reduces exercise ventilation. Our results show that the reduced ventilation during exercise with carvedilol is associated with a greater reduction in arterial and an increase in . In other words, the blunted-by-carvedilol response to low is unable to increase ventilation adequately so that systemic falls even at sub-maximal exercise levels. It should be noted that carvedilol reduces ventilation in hypoxia even though carvedilol lowers resting DLCO which is another factor influencing exercise adaptation to high altitude 37. The blunting of the enhanced ventilatory response by carvedilol should be taken into consideration when allowing occasional exposure of HF patients treated with beta-blockers to high altitude, as may happen when travelling or flying. Indeed, in hypoxia during constant workload exercise, arterial reduces 37 but this reduction is greater when patients are treated with carvedilol (Table 3).
This study has several limitations. First of all, we did not test chemoreflex activity, so we cannot say whether the chemoreflex response was affected by carvedilol therapy or whether carvedilol acts on central or peripheral chemoreceptors, or both. We tested a more practical question: does chronic carvedilol treatment affect exercise capacity and the ventilatory response at a simulated altitude of 2.000m? Second, we did not test the effects of carvedilol on the long-term response to hypoxia; therefore our data cannot be extrapolated to this condition. Finally, we did not investigate whether the reduced ventilatory response with hypoxia in patients treated with beta-blockers is peculiar to heart failure patients or whether it is also present in normal subjects. Indeed, in normal subjects who live at high altitude, the chemoreflex response to hypoxia seems to be unaffected by beta-blockers 38.




