Effects of nebivolol on left ventricular function in elderly patients with chronic heart failure: results of the ENECA study*
Abstract
Aim
To examine the effect of the beta1-selective beta-blocker nebivolol, administered as add-on therapy, on left ventricular function in 260 elderly patients (>65 years) with chronic heart failure (CHF).
Methods
The principal inclusion criteria were (1) NYHA class II–IV CHF and (2) a left ventricular ejection fraction (LVEF) ≤35%. The primary end-point was the change in LVEF in response to nebivolol treatment for 8 months.
Results
Baseline LVEF values in the two groups were as follows: nebivolol 25.41±7.09% and control 26.41±5.55%. LVEF improved significantly (p=0.027) more in the nebivolol group (6.51±9.15%) than in the control group (3.97±9.20%), the relative improvement (percentage increase in the initial value) being 35.70±57.62% in the nebivolol group and 19.19±40.96% (p=0.008) in the placebo group. Examination of different subgroups did not reveal any heterogeneity in the effects of nebivolol treatment vs. placebo treatment. There were no significant differences between the nebivolol and placebo groups as concerns the changes in clinical status, quality of life, or safety parameters.
Conclusion
The findings of the ENECA study confirmed that nebivolol significantly improved cardiac function and proved to be safe and well tolerated in elderly patients with signs of CHF and an impaired LVEF.
1. Introduction
Chronic heart failure (CHF) is one of the most common and constantly increasing health problems in developed countries 1 2 3. CHF is the only significant cardiovascular disease in which both the incidence and prevalence are increasing. The 1-year mortality rate is 5–10% in patients with mild symptoms, but 30–50% in patients with a more advanced stage of the illness. A relatively recent therapeutic treatment strategy, which is now highly recommended in all recent therapeutic guidelines, includes the administration of beta-blockers in addition to basic therapy with ACE inhibitors, diuretics, and digitalis 1. In more than 20 placebo-controlled clinical studies, involving more than 10,000 patients with CHF, combined therapy with ACE inhibitors and beta blockers has been demonstrated to improve clinical symptoms of CHF and to reduce the risk of hospitalization or mortality 4 5 6 7 8 9.
Beta-adrenergic receptor antagonists are widely used in cardiovascular therapy 10. Recent pharmacological development has been aimed at improving the beta1-selectivity of the agents, and agents with additional vasodilator activities were recently introduced. Nebivolol is a highly beta1-selective blocker used in clinical practice 11. Further clinical studies have demonstrated that nebivolol possesses an additional nitric oxide (NO)-mediated vasodilatory effect which is not observed for other beta-blockers (e.g., atenolol and metoprolol) 12 13 14.
In recent clinical studies of beta-blockers in the treatment of CHF, the patient populations have been relatively young 15. A large proportion of CHF patients, however, are over the age of 65. For this reason, the ENECA (Efficacy of Nebivolol in the treatment of Elderly patients with Chronic heart failure as Add-on therapy to ACE inhibitors or angiotensin II receptor blockers, diuretics, and/or digitalis) study has been designed to include CHF patients older than 65 years. The study design was based on the following assumptions: (1) the highly beta1-selective blocker nebivolol completely blocks the cardiac beta1-receptors and thereby improves the left ventricular ejection fraction (LVEF) by inhibiting the deleterious neurohumoral response in CHF; (2) nebivolol additionally has the ability (stimulation of NO release) to reduce the vascular resistance of both veins and arteries (preload and afterload) 16; and (3) nebivolol possesses antioxidative properties. The primary end-point of the ENECA study was change in LVEF following nebivolol treatment. LVEF is regarded as an important surrogate end-point for clinical outcome in CHF, and a hyperbolic inverse relationship has been shown between LVEF and survival rate 2. Specifically, the aims of the ENECA study were to evaluate (1) the efficacy of nebivolol treatment as compared with placebo in improving LVEF; and (2) the safety and tolerability of nebivolol treatment in elderly patients.
2. Patients and methods
2.1. Criteria for evaluation
The primary objective of the ENECA trial was to evaluate the efficacy of nebivolol, administered as add-on therapy (in comparison with placebo) in improving LVEF in the treatment of elderly CHF (NYHA class II–IV) patients. The secondary objective was an investigation of other efficacy end-points and of the tolerability and safety of nebivolol, as follows:
- Change in clinical status (NYHA functional classification)
- Quality of life (the Minnesota Living with Heart Failure Questionnaire)
- Hospitalization rate (following randomization)
- Survival rate (related to the time of randomization)
- Safety parameters (adverse events, vital signs, and laboratory parameters)
2.2. Study plan
The study was performed as a prospective, multicenter, double-blind, randomized, placebo-controlled, parallel group study in 70 centers which fulfilled all quality requirements. All of the patients had to fulfill the following inclusion criteria: (1) hospitalized patients or outpatients aged more than 65 years; (2) NYHA class II, III, or IV CHF; (3) a stable clinical course; (4) an LVEF ≤35%; and (5) stable basic medication for CHF with ACE inhibitors and/or ARBs, diuretics, and/or digitalis for a minimum of 2 weeks prior to inclusion.
Important exclusion criteria were as follows: (1) acute coronary syndrome; (2) a myocardial infarction within the last 3 months; (3) PTCA or coronary artery bypass surgery within the last month; (4) obstructive or hypertrophic cardiomyopathy; (5) hemodynamically relevant congenital or valvular heart disease; (6) tachyarrhythmia resistant to therapy (>100/min); and (7) bradycardia (heart rate <50/min). Moreover, patients were excluded from the study if they had received beta-blocker therapy in the 4 weeks prior to the beginning of the trial, or if they had known intolerance or hypersensitivity to nebivolol. Approval for the trial was obtained both from the ethics committee at each center and from the various national health authorities, and all patients gave written informed consent.
2.3. Overall study design
The total study period of 12 months per patient was divided into the following periods.
2.3.1. A screening period of 14 days before randomization
During the screening period, the inclusion and exclusion criteria were checked, and the initial clinical and echocardiographic examinations were performed (Table 1).
| Assessment | Screening | Titration period | Treatment period | Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Week | −2 | 0 | 2 | 4 | 6 | 8 | 12 | 16 | 24 | 32 | 40 | 42 | 48 |
| Explanation and informed consent | X | ||||||||||||
| Demography, medical history | X | ||||||||||||
| Cardiovascular history | X | ||||||||||||
| Physical examination | X | X | X | X | |||||||||
| Brief examination | X | X | X | X | X | X | X | X | X | ||||
| Effectiveness: | |||||||||||||
| Determination of LVEF | X | X | X | X | |||||||||
| Change in clinical status (NYHA classification) | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Quality of life (Minnesota Questionnaire) | X | X | X | X | X | ||||||||
| Hospitalization rate | X | X | X | X | X | X | X | X | X | X | X | X | |
| Death | X | X | X | X | X | X | X | X | X | X | X | X | |
| Blood pressure/heart rate | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Concomitant medication | X | X | X | X | X | X | X | X | X | X | X | X | |
| Compliance control | X | X | X | X | X | X | X | X | X | X | |||
| Safety: | |||||||||||||
| Adverse events | X | X | X | X | X | X | X | X | X | X | X | X | |
| ECG at rest with 12 leads | X | X | X | X | X | X | |||||||
| 24-h Holter ECG | X | X | X | X | |||||||||
| Routine laboratory tests | X | X | X | X | |||||||||
- a LVEF—left ventricular ejection fraction; NYHA—New York Heart Association.
2.3.2. A titration period of 8 weeks (including randomization)
Patients who met the inclusion and exclusion criteria were randomized and were allocated a patient number in ascending order. This number determined whether their treatment was to be performed with nebivolol or placebo. Starting with 1.25 mg of nebivolol (Nebilet®) or placebo, the dose of study medication was doubled every 14 days until the highest tolerated dose was reached or a maximum of 10 mg/day (after 8 weeks). Criteria for increasing the dose or for the highest dose were a systolic blood pressure ≥100 mm/Hg and a heart rate ≥50/min.
2.3.3. The treatment period
The patients received the highest tolerable dose or a maximum of 10 mg/day of nebivolol or placebo for a period of 8 months.
2.3.4. The follow-up
The end of the treatment period was followed by an observation period of 2 months. The study medication was gradually discontinued during the first 14 days of this observation period.
2.4. Measurement of efficacy and safety variables
LVEF was determined by means of two-dimensional echocardiography (visits 1, 7, 9, and 11; see Table 1) in accordance with the recommendations of the American Society of Echocardiography. The findings were analyzed at a central site (ECHO laboratory), and blind conditions for data evaluation were ensured.
The effect of nebivolol on LVEF was also evaluated in different predefined subgroups. The subgroup analysis included examination of the roles of gender, heart rate, and the etiology of CHF, since numerous publications have indicated that these factors may influence beta-blocker-mediated effects on LVEF 17 18 19 20 21 22.
The Minnesota Living with Heart Failure Questionnaire comprised 21 questions to evaluate the quality of life of the patients. A six-point response format ranging from 0 (“not at all”) to 5 (“very highly”) was used to quantify the patient's impression concerning the degree of disability relating to each of the 21 items.
The hospitalization rate was measured by documenting the number of visits to an emergency department, the number of hospitalizations, the time to the first hospitalization due to cardiovascular or noncardiovascular events or death, and the total number of hospitalizations for any reason.
Blood pressure (Korotkoff phase V) and heart rate were measured at each of the visits. Systolic and diastolic blood pressure values were recorded with a standard sphygmomanometer after 5 min of rest in a supine position and after 1 min standing, measurements were always taken on the same arm. The heart rate was taken via the radial pulse, in both supine and sitting positions.
Blood samples for laboratory examinations were taken on visits 1, 6, 9, and 11 (Table 1). Different parameters were analyzed to check the hemostatus and blood chemistry of the patient. Values outside the normal range were checked for their clinical relevance and noted in the case report form.
All adverse events (AE) were documented and followed up until the event was either resolved or adequately explained, even after the subject had completed the trial. Preexisting conditions that worsened during the study were reported as AEs. Any AE classified as serious was reported to Berlin-Chemie by the investigator within 24 h.
2.5. Statistical methods
The statistical evaluation was performed with the statistical software package SAS, Version 8.0.0. The changes in the primary and secondary efficacy outcome criteria were analyzed by intention to treat (ITT) and per protocol (PP). Patients with a relevant protocol deviation (poor echo quality or missing echocardiography, compliance problems concerning study medication, deaths, etc.) were excluded from the PP analysis. The primary target variable was the change in LVEF after 8 months of treatment as compared with the baseline value. The difference in the LVEF (post–pre difference) between visits 1 and 11 was checked for statistical significance by the t-test. The sample size calculation was based on the assumptions of (1) conceiving the percentage change in the LEVF as continuous factor; (2) with a power of 80% and a sigma value of 13%; and (3) assuming a 20% drop-out rate. The total sample size in a 1:1 allocation ratio according to these statistical criteria was 270.
Moreover, the effects of nebivolol treatment were examined in different subgroups, and the differences in the LVEF improvements between the nebivolol and placebo-treated groups were calculated. All secondary efficacy parameters were analyzed in detail. Depending on the type of variable (qualitative or quantitative parameters), the following descriptive methods were used: calculation of absolute and relative frequencies, or calculation of the mean, standard deviation, median, minimum, maximum, first and third quartiles, and 95% confidence intervals for the mean. For dichotomous parameters, the comparison was performed with Fisher's Exact Test at an α-level of 5%. The parameters that are at least ordinal were compared by means of the Wilcoxon rank-sum test at an α-level of 5%.
3. Results
Of the 354 patients who were screened, 94 were excluded from the study; thus, the ITT population comprised 260 patients (fig. Fig. 1). The most important reasons for exclusion were (1) the patients did not meet the inclusion/exclusion criteria (n=48) and (2) the quality of the echo was not acceptable (n=42). Further exclusion reasons were as follows: patient withdrawal (n=5), nonattendance (n=1), noncompliance (n=2), and initial protocol violation (n=3). For some patients, there was more than one reason for exclusion from the ITT population. The 260 patients in the ITT population (who met the inclusion and exclusion criteria) were randomized and treated either with nebivolol (n=134) or placebo (n=126).
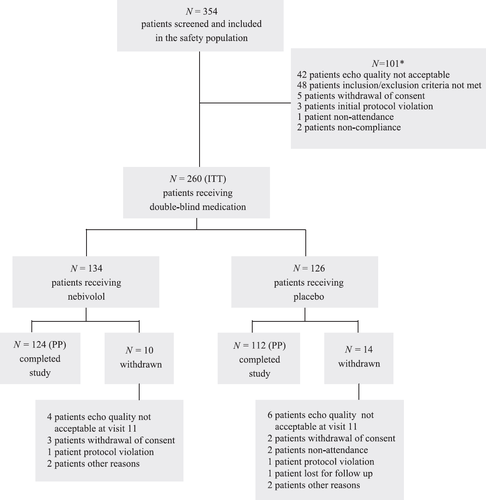
The baseline LVEF values in the two groups were as follows: nebivolol, 25.41±7.09%; and control, 26.41±5.55%. During the study, major deviations from the protocol occurred in 24 patients, 10 in the nebivolol group, and 14 in the control group. These patients were therefore excluded from the PP analysis. The patient characteristics at entry (demographic variables, clinical data, symptoms, and concomitant medication) were similar in the two groups (nebivolol vs. placebo-treated; Table 2).
| Variables | Nebivolol group (n=134) | Control group (n=126) | p-value |
|---|---|---|---|
| Demographic data | |||
| Gender (male) | 70.15% | 76.98% | 0.261 |
| Race (caucasian) | 99.25% | 98.41% | 0.612 |
| Age (years), mean±S.D. | 71.97±5.02 | 72.19±5.20 | 0.717 |
| Height (cm), mean±S.D. | 168.73±8.69 | 170.30±7.37 | 0.130 |
| Weight (kg), mean±S.D. | 74.56±13.17 | 75.59±12.01 | 0.422 |
| BMI (kg/m2), mean±S.D. | 26.11±3.71 | 26.02±3.50 | 0.854 |
| Clinical data | |||
| Hypertension (%) | 60.90 | 53.60 | 0.286 |
| Previous myocardial infarction (%) | 59.70 | 57.14 | 0.707 |
| Atrial fibrillation (%) | 26.52 | 25.40 | 0.142 |
| Previous cardiac surgery (%) | 25.56 | 26.19 | 0.888 |
| Diabetes (%) | 24.63 | 26.98 | 0.673 |
| Heart rate at baseline (beats/min), mean±S.D. | 76.90±10.88 | 75.29±9.96 | 0.274 |
| Blood pressure at baseline (mm Hg), mean±S.D. | 136.22±17.51/80.46±9.35 | 134.64±16.57/80.76±9.82 | SBP: 0.287 DBP: 0.826 |
| NYHA functional class II (%) | 52.24 | 45.24 | |
| NYHA functional class III (%) | 45.52 | 47.62 | 0.128 |
| NYHA functional class IV (%) | 2.24 | 7.14 | |
| LVEF (mean±S.D.) | 25.41±7.09 | 26.41±5.55 | 0.204 |
| Quality of life (Minnesota Questionnaire) score, mean±S.D. | 32.33±19.73 | 35.63±21.30 | 0.272 |
| Sign and symptoms | |||
| Peripheral edema (%) | 29.85 | 31.75 | 0.789 |
| Hepatomegaly (%) | 23.88 | 34.13 | 0.076 |
| Dyspnea on exertion (%) | 97.01 | 96.83 | 1.000 |
| Pulmonary rale (%) | 23.88 | 30.16 | 0.266 |
| Third heart sound (%) | 14.18 | 20.00 | 0.499 |
| Concomitant medication at baseline | |||
| ACE inhibitor | 91.04 | 90.48 | 1.000 |
| ARB | 5.22 | 7.14 | 0.610 |
| Combination therapy in the form of ACE inhibitor+diuretic or ARB+diuretic | 82.84 | 84.92 | 0.737 |
| Digitalis | 59.70 | 53.17 | 0.318 |
| Diuretics (any kind) | 87.31 | 88.10 | 1.000 |
- a S.D.—standard deviation; BMI—body mass index; LVEF—left ventricular ejection fraction; NYHA—New York Heart Association; ACE—angiotensin converting enzyme; ARB—angiotensin II receptor blocker; SBP—systolic blood pressure; DBP—diastolic blood pressure. The p-value reflects the difference between the nebivolol and control groups.
The treatment dose was up-titrated in four stages, starting with 1.25 mg, over a titration period of 8 weeks. The maximum possible dose was 10 mg/day. The titration phase was terminated at visit 6, when the patients received their individual maximum tolerated dose for the following 8 months of treatment. Altogether, 64.2% of the nebivolol-treated patients tolerated the maximum possible dose of 10 mg daily (the average dose was 7.4 mg). There was a significant negative correlation (p=0.007) in the ITT population between the functional class (NYHA) of the patients and the maximum tolerated dose, i.e., the lower the functional status, the higher was the maximum tolerated dose.
3.1. Primary efficacy outcome (primary criterion)
The primary target variable was the absolute change in LVEF relative to baseline after 8 months (between visit 1 and visit 11) of treatment (Table 3). The LVEF improved significantly (p=0.027) more in the nebivolol group (6.51±9.15%) than in the control group (3.97±9.20%), the relative improvement (the percentage increase in the initial value) being 35.70±57.62% in the nebivolol group and 19.19±40.96% (p=0.008) in the placebo group.
| Nebivolol group | Control group | p-value | ||||
|---|---|---|---|---|---|---|
| ITT (N=134) | PP (N=124) | ITT (N=126) | PP (N=112) | ITT | PP | |
| LVEF baseline values [%], mean±S.D. | 25.41±7.09 | 25.56±7.14 | 26.41±5.55 | 26.59±5.62 | 0.2042 | 0.2197 |
| LVEF values at Visit 11 [%], mean±S.D. | 31.92±8.85 | 32.11±8.34 | 30.38±8.93 | 30.94±9.07 | 0.1647 | 0.3008 |
| LVEF post–pre difference, improvement in absolute values, mean±S.D. | 6.51±9.15 | 6.55±8.49 | 3.97±9.20 | 4.35±9.30 | 0.027 | 0.058 |
| LVEF post–pre difference [%], relative improvement, compared to the initial value, mean±S.D. | 35.70±57.62 | 35.94±56.87 | 19.19±40.96 | 20.70±42.02 | 0.008 | 0.021 |
- a LVEF—left ventricular ejection fraction; ITT—intention to treat; PP—per protocol; S.D.—standard deviation. The p-value reflects the significance of the difference between the nebivolol and control groups.
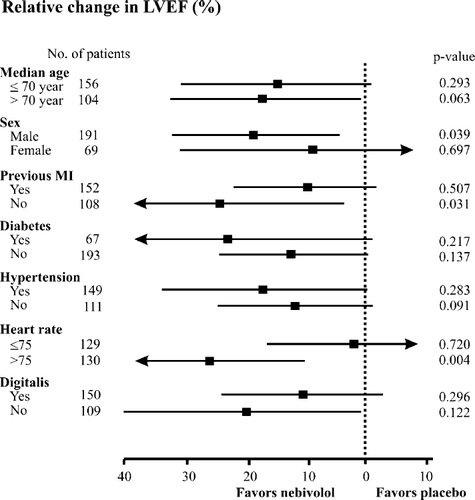
Examination of the different subgroups did not reveal any heterogeneity in the effects of nebivolol treatment vs. placebo treatment (fig. Fig. 2), and the improvement in LVEF was consistent in all the subgroups. The superiority of nebivolol treatment was particularly apparent in male patients (37.15% relative improvement) without a previous myocardial infarction (53.42% relative improvement) or with a heart rate >75/min (41.44% relative improvement).
3.2. Secondary efficacy outcomes (secondary criteria)
The changes in functional clinical status (NYHA classification) are presented in Table 4. During the study, 33 patients in the nebivolol group and 34 patients in the control group improved by one class, while 2 patients in the nebivolol group and 3 patients in the placebo group improved by two classes (the overall difference in functional status between nebivolol and placebo was not significant; p=0.835).
| Nebivolol group | Control group | |||
|---|---|---|---|---|
| ITT N=134 | PP N=124 | ITT N=126 | PP N=112 | |
| QOL sum score (mean±S.D.) | ||||
| Visit 1 | 32.33±19.73 | 32.44±19.82 | 35.63±21.30 | 35.95±21.95 |
| Visit 11 | 23.04±15.94 | 23.17±15.99 | 25.12±17.73 | 25.42± 17.76 |
| Difference between visits 1 and 11 | −9.13±13.78 | −9.23±13.77 | −11.01±14.66 | −10.78±14.28 |
| NYHA functional class (n) | ||||
| Improvement by one class | 33 | 30 | 34 | 31 |
| Improvement by two classes | 2 | 2 | 3 | 3 |
| Remained stable | 81 | 80 | 67 | 63 |
| Worsened by one class | 2 | 2 | 1 | 1 |
- a ITT—intention to treat; PP—per protocol; QOL—quality of life; S.D.—standard deviation; NYHA—New York Heart Association. There were no significant statistical differences in the changes in quality of life (p=0.342) or functional class (p=0.835) between the nebivolol and control groups.
As regards quality of life (the Minnesota Living with Heart Failure Questionnaire), after 8 months of treatment (at visit 11), the mean value of the sum score had decreased by 9.13%±13.78% in the nebivolol group and by 11.01%±14.66% in the control group (p=0.342) as compared with the baseline scores (Table 4).
All of the patients had at least one visit to an emergency physician, were hospitalized at least once, and had at least one unscheduled contact for any reason. The mean time to the first hospitalization was nearly the same in the two treatment groups: 15.92 days in the nebivolol group and 15.77 days in the placebo group (p=0.507). Altogether, 1535 hospitalizations for any reason were registered in the nebivolol group and 1411 in the control group. A total of 14 patients experienced an AE with a fatal outcome, 7 in each group. Due to insufficient data, recording/collecting it was not possible to determine the exact reasons for deaths (sudden cardiac death and worsening of heart failure were the frequently assumed reasons for death). The total mortality rate (including the withdrawn patients) amounted to 14 patients (ITT: 5.38%; PP: 5.93%), 7 in the nebivolol group (ITT: 5.22%; PP: 5.65%) and 7 in the placebo group (ITT: 5.56%; PP: 6.25%). The survival rate according to Kaplan–Meier was 67.47% in the nebivolol group and 62.89% in the control group, the difference not being significant (p=0.696).
3.3. Safety results
During the study, 159 of the 260 patients (61.15%) experienced at least one AE: 81 (60.45%) in the nebivolol group and 78 (61.90%) in the control group (Table 5). These patients suffered 152 different AEs, which occurred 360 times in total: 186 in the nebivolol group and 174 in the control group. Of the 186 AEs in the nebivolol group, 22 (12.09%) were rated as severe (Table 5), while of the 174 AEs in the control group, 26 (15.03%) were classified as severe. The AEs with the highest frequencies were worsening of CHF (nebivolol group: 14 vs. placebo group: 16), ventricular tachycardia (nebivolol group: 5 vs. placebo group: 7), and atrial fibrillation (nebivolol group: 4 vs. placebo group: 8).
| Nebivolol group | Control group | p-value | ||||
|---|---|---|---|---|---|---|
| ITT N=134 | PP N=124 | ITT N=126 | PP N=112 | ITT | PP | |
| Patients with adverse events, N (%) | 81 (60.45) | 75 (60.48) | 78 (61.90) | 70 (62.50) | 0.899 | 0.790 |
| Total number of adverse events, N | 186 | 166 | 174 | 162 | 0.695 | 0.090 |
| Percentage of severe adverse events (%) | 12.09 | 11.73 | 15.03 | 14.29 | 0.084 | 0.038 |
- a ITT—intention to treat; PP—per protocol. The p-value reflects the difference between the nebivolol and control groups.
Altogether, 54 AEs were reported to be drug-related (nebivolol group: 40 vs. placebo group: 14; p<0.0001). The most frequent drug-related AEs were as follows: bradycardia (nebivolol group: 9 vs. placebo group: 2), hypotension (nebivolol group: 8 vs. placebo group: 4), and dizziness (nebivolol group: 5 vs. placebo group: 2).
General blood tests (glucose, total protein, sodium, potassium, calcium, uric acid, total cholesterol, erythrocyte sedimentation rate, and hemoglobin A1c), liver function [total bilirubin, transaminases (GOT and GPT), alkaline phosphatase, and gamma GT], kidney function (serum urea and creatinine), and hematology (hemoglobin, hematocrit, counts of red blood cells, white blood cells, and platelets) were performed at visit 1 to ensure that patients with a disturbed hepatic function, renal diseases, and/or hematological disorders were not randomized to medication. Additional laboratory tests were carried out at visits 6, 9, and 11. There were no clinically significant changes in the parameters investigated, indicating that there was no risk of the induction of liver, kidney, or hematological disorders in either treatment group.
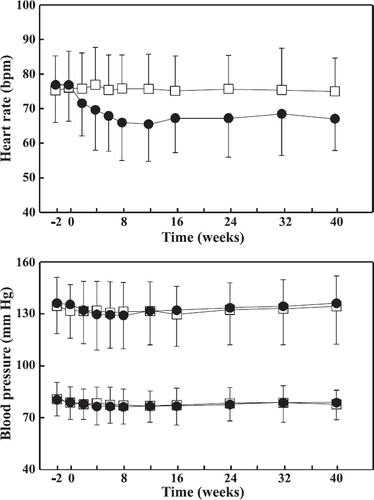
Vital signs including heart rate and systolic and diastolic blood pressures after 5 min of rest were measured at all visits (fig. Fig. 3). By visit 11 (after 40 weeks of titration/treatment), the heart rate was significantly (p<0.0001) lowered in the nebivolol group (baseline: 76.90±10.88/min vs. visit 11: 67.08±9.21/min), whereas no significant change was noted for the placebo group (baseline: 75.29±9.96/min vs. visit 11: 75.00±9.62/min). The systolic and diastolic blood pressures did not change relevantly from the baseline values in either treatment group (fig. Fig. 3).
4. Discussion
4.1. Effect on the primary end-point
The objective of this study was to evaluate the efficacy of nebivolol, administered as add-on therapy, in comparison with placebo in the treatment of CHF (NYHA II–IV) in elderly patients. Efficacy was primarily assessed via the absolute and relative increases in LVEF. An improvement in left ventricular function is regarded as an important surrogate end-point for clinical outcome in CHF, since a hyperbolic inverse relationship exists between LVEF and the annual survival rate 2,23. Several studies have concluded that small differences in LVEF are of prognostic importance in patients with CHF, and the effect of treatment on the LVEF (even if small) can predict survival 22 23 24.
On the basis of the relative increase in LVEF, nebivolol proved to be superior to placebo (nebivolol 35.70±57.62% vs. placebo 19.19±40.96%). This clinical trial confirms the previously reported effect of nebivolol in improving left ventricular function 25 26 27 28 29. Moreover, the increase in LVEF in absolute units (6.5%) in response to nebivolol treatment is in agreement with previously published data on CHF patients following beta-blocker therapy 20,22. However, the mean age (72 years) of the patients in this study was significantly higher than the mean age of the patients in previous beta-blocker trials with carvedilol, metoprolol, or bisoprolol. The improvement in LVEF was consistent both in males and in females, in all age groups, among patients with or without hypertension, or with or without diabetes mellitus, and in patients taking digitalis or not (fig. Fig. 2). It is important to note that the improvement in LVEF in response to nebivolol treatment occurred when it was administered on top of the recommended therapy for CHF (ACE inhibitors and/or ARBs, digitalis, and diuretics).
Nebivolol is a highly selective beta-1 receptor antagonist with an additional vasodilatory effect. Besides its function as a highly selective beta-blocker, nebivolol also has the ability to modulate the release of NO and consequently to reduce the vascular resistance of the veins and arteries. In this way, both the preload and the afterload of the heart are reduced 14,16. In positive comparator studies of CHF and/or hypertensive patients, the effects of nebivolol and other beta-blockers on both the systolic and diastolic left ventricular function were characterized 30,31. Unlike traditional beta-blockers (atenolol), nebivolol reduced both the peripheral resistance and the pulmonary wedge pressure, increased the stroke volume, and preserved cardiac output in these patients 30 31 32. The nebivolol-related specific effects on the cardiac function were explained by the unique mechanism of action of the compound (peripheral vasodilation and increased NO release). Consequently, the main results of the ENECA study [significant improvement in cardiac function in a relatively old CHF population and the good tolerability of the compound (>64% of the patients received the maximum intended dose of nebivolol)] are in line with those of previous studies on the profile of this beta-blocker.
Nebivolol was generally well tolerated. Disturbing side effects of nonselective beta-blocker therapy, such as excess fatigue, a reduction in physical performance, and cold extremities, attributed to a reduction in cardiac output and vasoconstriction were not encountered during the present trial. The numbers of AEs in the two treatment groups were nearly identical.
4.2. Subgroup analysis
The subgroup analysis of the effect of nebivolol on the LVEF revealed that various covariates (gender, heart rate, and etiology of CHF) significantly influenced the effect of the compound within the various subgroups. Nebivolol was particularly effective in patients with a heart rate >75/min as compared with patients with a heart rate of ≤75/min. The relationship between the LVEF (myocardial contractility) and the heart rate is multifactorial and complex 17 18 19. In isolated tissue samples from failing human hearts, an inverse relationship has been reported between force and frequency (the tension was found to be maximum at a pacing rate of 60/min and gradually decreased at higher frequencies 18). Consequently, patients with a higher heart rate (>75/min) in the ENECA trial may have profited more from the negative chronotropic effect of nebivolol in consequence of this inverse relationship.
Another important determinant of the nebivolol-mediated effect on the myocardium is the etiology of CHF. Large clinical trials with metoprolol or carvedilol have demonstrated a negative relationship between the proportion of patients with coronary artery disease (a previous myocardial infarction) and the change in LVEF during treatment 20 21 22. The greater the proportion of patients with a previous myocardial infarction, the less marked was the increase in LVEF. It has been suggested that the reason for the poorer response in ischemic cardiomyopathy is that myocardial fibrosis (akinetic regions) may cause unresponsiveness to beta-blockers 9,20. Our data on the effects of nebivolol on LVEF are consistent with this observation, since nebivolol was particularly effective in patients who had not had a previous myocardial infarction.
The number of patients in the ENECA trial (ITT population n=260) was large enough for the changes in the primary end-point (LVEF) to be examined, but it was not adjusted for the investigation of other important end-points (total mortality, cardiovascular mortality, or survival rate). Consequently, these end-points need to be addressed in a larger trial adjusted to these variables, such as the SENIORS study 33.
In summary, the findings of the ENECA study confirmed that in elderly patients (>65 years) with signs of CHF and an impaired left ventricular function, (1) nebivolol as add-on therapy significantly improved cardiac function; (2) the effects of nebivolol were consistent in all examined subgroups of patients; (3) the administration of nebivolol was safe and well tolerated; and (4) the cardiac response to nebivolol was similar to those found in previous large clinical trials with different beta-blockers (metoprolol or carvedilol) in younger patient populations.
Appendix A
ENECA investigators:
Poland O. Kruszelnicka-Kwiatkowska, R. Zymlinski, W. Ruzyllo, J. Grzybowski, D. Wojciechowski, P. Sionek, W. Piwowarska, O. Kruszelnicka, P. Podolec, A. Cieslinski, S. Grajek, W. Banasiak, P. Ponikowski; Hungary R. De Chatel, K. Keresztes, A. Janosi, J. Tenczer, P. Karpati; Czech Republic M. Witowec, J. Bultas, V. Stanek, T. Marek, B. Semrad, J. Schildberger; Romania M. Muraru, A. Gurghean, I.V. Bruckner, C. Homentcovschi, M. Cinteza, D. Vinereanu; Lithuania A. Laucevic̆ius, Z̆. Petrulionienė, R. Vidugirienė, A. Dailytkienė, G. Uz̆davinys, E. Uz̆davinytė-Gatelienė, R. Sudikienė, J. Treideris, P. Zabiela, R. Zaliunas, R. S̆lapikas, B. S̆lapikienè, V. Grinius, G. S̆akalytè; Kirgizia T.M. Murataliev; Uzbekistan R. Kurbanov; Germany K-F. Appel, M. Baar, C. Bergmeier, A. Bernasowski, K-O. Bischoff, G. Böhm, H-J. Busse, U. Desaga, S. Fischer, B. Gysan, G. Hasenfuss, A. Henrichs, P. Krupp, M. Müser, W. Oldenburg, W. Pfleiderer, F. Richard, G. Sabin, R. Schaupp, W. von Scheidt, W. Schlag, W. Schön, M. Schumacher, W. Türk, A. Zorn.




