Carvedilol improves left ventricular function in murine coxsackievirus-induced acute myocarditis Association with reduced myocardial interleukin-1β and MMP-8 expression and a modulated immune response
Abstract
Background
Proinflammatory cytokines induce the expression of matrix metalloproteinases that play a crucial role in myocardial remodeling. Beta-adrenergic receptor stimulation influences the production of cytokines heralding the possibility of modulating cytokine production by beta-adrenergic blockers.
Methods and results
In a coxsackievirus B3 murine myocarditis model (BALB/c), effects of carvedilol and metoprolol on myocardial cytokine expression, inflammatory cell infiltration and MMP/TIMP profiles were investigated. In carvedilol-treated mice, a significant improvement in left ventricular function was documented 10 days post infection. In infected mice (n=10), IL-1β, TNF-α, TGF-β1 and IL-10 myocardial mRNA abundance were increased significantly (240%, 200%, 161%, and 230%) compared to controls (n=10), while IL-15 mRNA was markedly reduced (70%). Infected mice showed significantly increased infiltrations with CD3-, CD4- and CD8-T-lymphocytes (730%, 1110%, 380%). In the infected mice, myocardial MMP/TIMP profiles presented a significant upregulation of membrane type-1 MMP, MMP-9, MMP-8 and MMP-3 (150%, 160%, 340%, and 270%) and a significant decrease in TIMP-4 levels (75%). Carvedilol attenuated over-expression of myocardial TGF-β1, IL-1β and MMP-8 mRNA expression significantly and induced a relevant IL-10 mRNA expression in the infected mice (n=10). By an unchanged infiltration with CD3-T-lymphocytes, carvedilol showed a representative reduction in CD4-T-lymphocytes.
Conclusion
Carvedilol treatment in experimental myocarditis leads to reduced expression of proinflammatory cytokines and MMPs, which contributes to reduced matrix degradation and ultimately to improved structural integrity of the heart. Besides the antiadrenergic potential, carvedilol is beneficial due to a wide range of biological activities (antiinflammatory, antifibrotic, antioxidative and immunomodulatory).
Abbreviations
-
- CVB-3,
-
- coxsackievirus B3
-
- Col,
-
- collagen
-
- ECM,
-
- extracellular matrix
-
- EMMPRIN,
-
- extracellular-MMP-inhibitor
-
- FN,
-
- fibronectin
-
- HR,
-
- heart rate
-
- IL,
-
- interleukin
-
- INF,
-
- interferon
-
- LVP,
-
- left ventricular pressure
-
- LV,
-
- left ventricle
-
- MMP,
-
- matrix metalloproteinase
-
- MT-MMP,
-
- membrane type MMP
-
- PCR,
-
- polymerase chain reaction
-
- SE,
-
- standard error of mean
-
- TGF,
-
- transforming growth factor
-
- TIMP,
-
- tissue inhibitor of MMP
-
- TNF,
-
- tumor necrosis factor.
1. Introduction
Viral myocarditis is a common cause of acute heart failure, especially in young patients 1. Viral infection of the myocardium and the ensuing inflammatory reaction constitute the major pathogenic mechanisms, which ultimately lead to left ventricular dysfunction 2.
The balance between the protective and deleterious immune mechanisms may eventually determine the course of the disease 3.
Cytokines play a critical role in maintaining this balance as they are known to modulate lymphocyte and myocardial cell functions 4,5. Since proinflammatory cytokines promote myocyte hypertrophy in vitro 6 and regulate cardiac fibroblast function 7 including expression of extracellular matrix components like collagen type I, type III, and fibronectin, they also play an important role in left ventricular remodeling. Proinflammatory cytokines disturb the balance between matrix metalloproteinases (MMPs), a family of zinc-dependent proteases which degrade various extracellular matrix proteins and their derivative peptides and tissue inhibitors of MMPs (TIMPs) 8,9, thereby contributing to development of left ventricular dysfunction 8,10. Immunomodulation, by targeting the cytokines, can influence prognosis. Interleukin-2 (IL-2) treatment in a murine model of viral myocarditis aggravated myocardial inflammation and the severity of disease 11. Recently, local expression of interleukin-1 (IL-1) receptor antagonist was found to improve mortality and decrease myocardial inflammation in experimental coxsackieviral myocarditis 12.
Carvedilol is a non-selective β-blocker with additional properties including α1-adrenergic-blockade, antioxidative effects, and antifibrotic properties, which may facilitate the overall clinical benefit observed in heart failure. Moreover, carvedilol improves ventricular function, reduces ventricular dilatation, and improves survival in patients with left ventricular dysfunction 13. Carvedilol improves left ventricular function in dilated cardiomyopathy patients 14 and alters myocardial gene expression in post-myocardial ischemia 15 by influencing the circulating levels of cytokines (IL-6, TNF-α and IL-1β). These findings are important, since cytokines are associated with progressive heart failure, in parts due to negative inotropic effects 16.
However, the precise mechanisms of the beneficial effects of carvedilol, especially the possible influence on the remodeling of the extracellular matrix (ECM), are still elusive. In the present study, we compared the effects of carvedilol and metoprolol treatment in a murine model of acute viral myocarditis, on left ventricular function and myocardial gene expression profiles, focusing on extracellular matrix remodeling (ECM-components, MMPs and TIMPs) and the myocardial expression of cytokines.
2. Methods
2.1. Murine viral myocarditis
Specific pathogen-free inbred, 8-week-old, male Balb/c (H-2d) mice obtained from Robert von Ostertag Institute, Berlin, Germany, were used for the experiments described in this study (n=60). Animals were infected as described previously (n=30) 17. Briefly, 5×105 plaque forming units (p.f.u.) of coxsackievirus B3 (Strain Nancy, ATCC VR-30) were diluted in phosphate-buffered saline to a final volume of 0.2 ml and injected intraperitoneally. Sham-infected animals (0.2 ml PBS intraperitoneally) were used as control (n=30). Suitable controls and infected animals were maintained and sacrificed in accordance with German Animal Protection Law 1993.
2.2. Drug administration
Carvedilol (5 mg/kg/12 h) and metoprolol (15 mg/kg/12 h) were administered orally (5 mg/kg/12 h) for 9 days, starting 24 h after infection (n=20) and sham infection (n=20) 18.
2.3. Hemodynamic characterization of LV function
Ten days after infection, hemodynamic parameters were measured in anesthetized, artificially ventilated and closed-chest animals. Due to the invasive procedure, the hemodynamic parameters were analyzed in a subgroup of animals; controls n=8, controls/carvedilol n=7, controls/metoprolol n=8, infected n=6, infected/carvedilol n=9, infected/metoprolol n=6. Left ventricular pressure (LVP), maximal rate of pressure increase over time (dP/dtmax) as a parameter of left ventricular (LV) contractility, the minimal rate of pressure decrease over time (dP/dtmin) as an indicator of the LV relaxation and heart rate were determined via a 1.2 F Milar-Tip catheter introduced into the LV chamber. Immediately after the hemodynamic parameters were obtained, the hearts were rapidly removed and transversely dissected.
2.4. RNA isolation and reverse transcription
Following hemodynamic evaluation, mice were sacrificed and the myocardial tissue was collected for different measurements. Total RNA, frozen in liquid nitrogen, was extracted from the myocardial samples by the Trizol method (GIBCO BRL) as per the manufacturer's instructions. Following treatment with RNase-free DNase I (Roche, Mannheim, Germany), the reverse transcription was performed using first-strand cDNA synthesis kit (Promega, Mannheim, Germany) 17.
2.5. Enteroviral RNA detection
Nested polymerase chain reaction was employed with the reverse transcripted RNA to detect the presence of viral genome in the infected myocardium as described earlier 17.
2.6. Reverse transcriptase-polymerase chain reaction
Semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR) was used to detect the mRNA abundance of MMP-3, MMP-8, MMP-9, MT-1MMP, EMMPRIN (extracellular-MMP-inducer), TIMP-4, IL-1β, TGF-β1 TNF-α, IL-10, IL-15, collagen type I (Col I), collagen type III (Col III), and fibronectin, based on the methods described earlier (Table 1) 17. Additionally, RT-PCR was also used to detect the CVB-3 RNA abundance in the infected myocardium. The mRNA abundance was quantified as optical densities (OD) equalized with β-actin mRNA levels using a software program (Scion Image. Maryland, USA). Nucleic acid sequences of all PCR products were confirmed to be identical to published GenBank data.
| mRNA | 5′ Region | 3′ Region | Annealing temperature (°C) | Product (bp) |
|---|---|---|---|---|
| MT-1 MMP | tgtcccccttttaccagtg | gccatccttcctttcatag | 55 | 362 |
| EMMPRIN | aaggtcggaaagaaatcagag | cgtaggtgccagggtcaacat | 55 | 241 |
| MMP-3 | ggcttcagtaccttcccagg | gcagcaaccaggaataggtt | 60.0 | 361 |
| MMP-8 | gcaggactccttcttcctcttcttcagtg | aaggtagtaaacggcttgctc | 62.0 | 350 |
| MMP-9 | cgacagcacctcccactatg | cccaacttatccagactcct | 62.0 | 481 |
| TIMP-4 | ctcttccctctgtggtgtga | gtggttcctggtccctacta | 60.0 | 399 |
| IL-1β | gggatgatgatgataacctg | ccactttgctcttgacttct | 60.0 | 363 |
| TGF-β1 | cggaagcgcatcgaagccatcc | gcaagcgcagctctgcacgg | 59.0 | 346 |
| TNF-α | caaagggagagtggtcaggtt | cactgaacctctgctccccac | 65.5 | 425 |
| FN | tccagccccaccctacaagt | ccagaccaaaccataagaac | 60.0 | 281 |
| Coll I | ctggaagagcggagagta | taaactccctccaccacccca | 62.2 | 607 |
| Coll III | cccagaacattacataccact | cttcaaaatgtctcaatggtg | 59.0 | 548 |
| IL-10 | acatactgctaaccgactcct | actcttcacctgctccactgc | 61.0 | 245 |
| IL-15 | ggcactgtattccccttctgt | atcccgtcttcgtccaactct | 61.0 | 212 |
| β-actin | agggaaatcgtgcgtgacat | catctgctggaaggtggaca | 55.0 | 450 |
2.7. Immunohistochemistry
Immunohistochemical analysis was performed as described earlier 17. To analyze T-lymphocyte infiltration, we used rat anti-mouse-CD4 antibody (Pharmingen 1:50), rat anti-mouse-CD8a antibody (Pharmingen 1:50) and goat anti-mouse-CD3 antibody (Santa Cruz 1:25). Biotinylated rabbit anti-rat IgG (Dako 1:100) and biotinylated rabbit anti-goat IgG (Vector 1:200) were used as secondary antibodies and VECTASTAIN® (Vector) as ABC-reagent, respectively. Immunocompetent infiltrates were quantified by digital image analysis as described in detail elsewhere 19.
2.8. Statistical analysis
All data are expressed as mean±S.E. Statistical significance between multiple groups was determined using Kruskal–Wallis test followed by Mann–Whitney U-test. Values of P<0.05 were considered as significant. Correlations between two parameters were performed using Pearson's correlation. Values of r2<0.5 were considered as significant.
3. Results
3.1. Hemodynamics of CVB3-infected mice
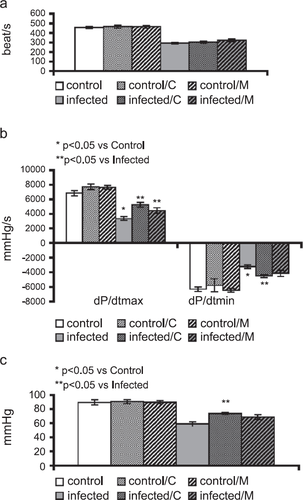
Coxsackievirus infection caused left ventricular dysfunction at 10 days post-infection. This was evidenced by significantly reduced left ventricular pressure (LVP), dP/dtmax, dP/dtmin and heart rate (HR) (fig. Fig. 1a–c). This impairment of left ventricular function was significantly improved with carvedilol treatment for all parameters. Moreover, metoprolol treatment only produced a significant improvement in LV-contractility.
3.2. Presence of viral genome in the myocardium
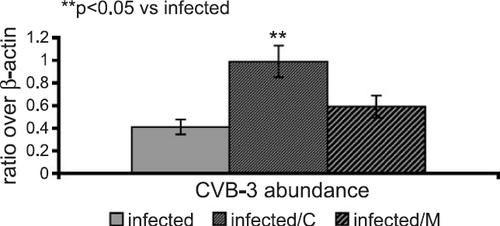
Nested PCR analysis confirmed the presence of the viral genome in the myocardium of infected mice. In contrast, all sham-infected myocards were virus negative. Additionally, semiquantitative RT-PCR-analysis showed a significantly higher CVB-3-RNA abundance (240%, P<0.001) in the infected myocardium treated with carvedilol compared to the infected animals (fig. Fig. 2).
3.3. Myocardial cytokine profiles
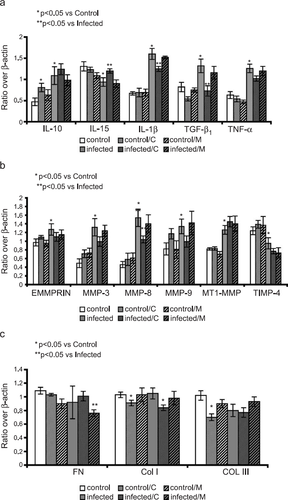
In the myocardium of the infected mice semiquantitative RT-PCR revealed a significant up-regulation of the mRNA abundances of IL-1β by 240% (P<0.0001), TNF-α by 200% (P<0.001) TGF-β1 by 160% (P<0.001) and IL-10 by 230% (P<0.002) compared to controls (fig. Fig. 3a). In addition, cardiac IL-15 levels were significantly down-regulated by 70% (P<0.023). In the infected mice, carvedilol administration attenuated the increase in IL-1β and TGF-β1 significantly (p<0.05), while IL-10 levels were relevantly increased in the uninfected mice and the infected mice. Furthermore, carvedilol treatment maintained the baseline levels of IL-15 in the infected group. Metoprolol treatment had no effect on the myocardial cytokine profile.
3.4. Myocardial MMP/TIMP profiles
fig. Fig. 3b illustrates the myocardial MMP/TIMP profiles in the experimental animals. mRNA analysis of the infected myocardium showed a significant increase in EMMPRIN by 130%, MT-1MMP by 150%, MMP-9 by 160%, MMP-3 by 270%, and MMP-8 by 340% (P<0.01, P<0.0001, P<0.019, P<0.0001, P<0.01, respectively). TIMP-4 was down-regulated to 75% (P<0.04). In the carvedilol-treated myocarditic mice, the MMP-8 mRNA abundance was significantly reduced with a trend to a reduction in the MMP-3 and the MMP-9 mRNA abundance. The mRNA abundance of MT-1MMP and TIMP-4 was unaffected. In contrast, the mRNA abundance of the MMP/TIMP-system was not influenced by metoprolol treatment.
3.5. Myocardial ECM-component profiles
In line with our previous report 17, mRNA analysis of the infected myocardium did not reveal any significant changes in the levels of fibronectin, collagen type I, and collagen type III (fig. Fig. 3c). Carvedilol treatment produced a significant reduction in collagen type I and collagen type III mRNA abundance in the uninfected mice as well as collagen type I in the infected mice (P<0.05). However, treatment with metoprolol showed a significant reduction of fibronectin in the uninfected mice, with no effect on myocardial collagen levels.
3.6. Myocardial T-lymphocyte infiltration
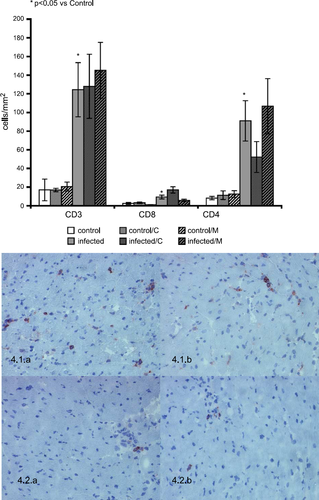
The infected mice showed a significant infiltration of the CD3+, CD4+ and CD8+ T-lymphocytes (P<0.05; Fig Fig. 4). In addition, carvedilol treatment produced a relevant reduction in CD4+ T-lymphocytes in parallel with an induction of the CD8+ T-lymphocytes. However, the infiltration of CD3+ T-lymphocytes was not significantly affected by either carvedilol or metoprolol treatment.
3.7. Link between cytokine expression, MMP expression, myocardial cell infiltration and hemodynamic function
The significant correlations between mRNA abundance of IL-1β, TNF-α and hemodynamic parameters such as dP/dtmax (r2>0.5; p<0.001), and dP/dtmin (r2>0.5; p<0.001) emphasize the relevance of these cytokines in left ventricular dysfunction in this treatment trial. In addition, this significant interaction of IL-1β and TNF-α with myocardial remodeling and myocardial cell infiltration is demonstrated by a significant correlation between the mRNA abundance of these cytokines (IL-1β, TNF-α) and MMP-8 and MMP-9, as well as to CD4+ T-lymphocytes infiltration (fig. Fig. 5 and Table 2).
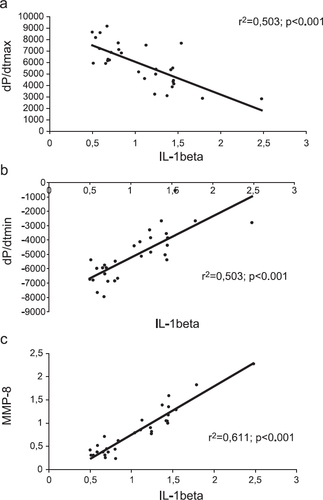
| IL-1β | TNF-α | CD4 | CD8 | HR | LVESP | dP/dtmax | dP/dtmin | MMP-8 | MMP-9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β | 1.000 | 0.469** | 0.429** | 0.162 | 0.409** | 0.484** | 0.503** | 0.503** | 0.611** | 0.380** |
| TNF-α | 0.469** | 1.000 | 0.453** | 0.251* | 0.501** | 0.513** | 0.504** | 0.387** | 0.240** | 0.072* |
- a Expressed by r2
- * P<0.05,
- ** P<0.001.
4. Discussion
Impairment of left ventricular function in the coxsackievirus B3 infected Balb/c mice was significantly improved by treatment with the non-selective adrenoreceptor antagonist, carvedilol, for 10 days post infection. In addition, carvedilol treatment showed significant effects on altered myocardial cytokine and MMP/TIMP mRNA abundance: The increased myocardial IL-1β and TGF-β1 mRNA abundance in the infected mice was significantly attenuated in the carvedilol treatment group. Furthermore, carvedilol treatment restored the IL-15 mRNA abundance to baseline levels and induced the IL-10 mRNA abundance in the uninfected mice as well as in the infected mice. In addition, increased mRNA levels of MMP-8 in the infected mice were significantly down regulated in response to carvedilol administration. Moreover, a relevant down-regulation of CD4+ T-lymphocytes and an up-regulation of CD8+ T-lymphocytes was produced by carvedilol treatment. The significant correlations between IL-1β and MMP-8 mRNA abundance and CD4+ T-lymphocytes, as well as the correlation between IL-1β mRNA abundance and hemodynamic parameters emphasize the importance of this cytokine with respect to the MMP/TIMP ratio and hemodynamic function. Therefore, the overall beneficial effect of carvedilol on the left ventricular function observed in the acute phase of coxsackieviral murine myocarditis may be a result of a concerted action of carvedilol on hemodynamics, myocardial inflammation and alteration of the ECM. These effects were not produced by metoprolol treatment, which indicates that these carvedilol specific effects cannot be attributed to β1-selective blocking agents.
4.1. Myocardial inflammation and alteration of the ECM
In this study, we showed a highly induced infiltration of myocardial T-lymphocytes, with a predominance of CD4+ T-lymphocytes. In the context of viral myocarditis, a close association between the extent of the myocardial inflammation and the alteration of the ECM is possible, since cytokines modulate the expression of MMPs and TIMPs. It is thought that proinflammatory cytokines (IL-1β and TNF-α) play an important role in influencing ECM protein expression. IL-1β may activate fibroblasts 20, which are involved in the remodeling process after myocardial injury via production of extracellular matrix proteins 21. Moreover, over-expression of IL-1β and TNF-α correlates with the increase in MMP and the reduction in TIMP expression. Thomas et al. 22 showed that the increase in myocardial MMP-3 and MMP-9 in human heart failure is associated with abnormal LV-remodeling. Furthermore, the inhibition of TNF-α contributes to a reduced level of myocardial MMP-9 expression 23. These observations are in line with this study, which demonstrates an association between proinflammatory cytokine expression (IL-1β and TNF-α) and the expression of all MMP-groups, as well as the decreased expression of TIMP-4.
In contrast to the proinflammatory IL-1β, IL-10 reduces the severity of the disease by attenuating the immune response. IL-10 also has the ability to inhibit the production of proinflammatory cytokines, including IL-1 and TNF-α 24. Furthermore, Santin et al. 25 showed that IL-10 has antiviral effects by acting as a differentiation factor for the CD8+ T-lymphocytes. IL-15 is a cytokine with anti-viral properties, which acts as a growth factor and stimulates IFN-γ production by CD8+ T-lymphocytes 26, 27. IL-10 reduces the IL-15-mediated expression of IFN-γ, without affecting T-lymphocyte proliferation and viral clearance 28. This differential expression of IL-10 and IL-15 is also seen in this study, with a significant up-regulation of IL-10 mRNA abundance accompanied by a significant down-regulation of IL-15 mRNA abundance in the infected animals compared to controls.
4.2. Actions of carvedilol on inflammation and alteration of the ECM
Several reports have suggested that carvedilol may have an effect on myocardial inflammation and remodeling. Recently, in an experimental coxsackieviral myocarditis model, Lim et al. 12 showed that local expression of interleukin-1 receptor antagonist by plasmid DNA reduces mortality and myocardial inflammation. Barth et al. 29 reported that the increase in left ventricular IL-6 and IL-1β in norepinephrine-induced cardiac hypertrophy is completely abolished by inhibition of α- and β-adrenergic receptors with carvedilol. In support of these findings, treatment with carvedilol in coxsackievirus B3-infected mice caused a significant improvement in left ventricular function, combined with significant changes in cytokine expression especially IL-1β suppression. These changes in the cytokine profile were linked to a relevant shift in the infiltrating T-lymphocytes combined with an increased CVB-3 RNA abundance in the carvedilol-treated animals 10 days post infection. The CVB-3 RNA abundance was performed using semiquantitative RT-PCR. The increased replication of CVB-3 in the heart was confirmed by RNA/RNA in situ hybridization (data not shown). Noticeably, these different immunomodulatory effects on cytokine expression, inflammatory cell infiltration and CVB-3 RNA abundance by carvedilol were not reproduced by β1 selective blockade with metoprolol. However, this increased CVB-3 RNA abundance was not linked to an impairment in left ventricular function. Therefore, the degree of myocardial inflammation seems to be more important than CVB-3 RNA abundance for the depression of left ventricular dysfunction in this murine model of acute viral myocarditis. Nonetheless, due to the dynamic course of viral myocarditis, this increased CVB-3 RNA abundance as well as the modulation of the CD8+ T-lymphocytes at 10 days post infection, provides no definite information about the virus load in the myocardium over the whole infection period. Therefore, further studies with sequential analysis of virus load would enable the evaluation of the immunomodulatory effects of carvedilol on the virus load in this model.
In addition, treatment with carvedilol contributes to the reduction in MMP mRNA abundance, especially MMP-8. This link between myocardial cytokine expression and the imbalance of the MMP/TIMP profile is emphasized by the significant correlation between the IL-1β, TNF-α and MMP-8 mRNA abundance as well as parameters of hemodynamic function and CD4+ T-lymphocytes infiltration. Therefore, these effects on the MMP system can partially explain the restored myocardial collagen network with reduced myocardial collagen turnover, which attenuates LV-dysfunction. These effects were not achieved by β1-selective blockade with metoprolol in our murine myocarditis model.
In addition to the significant suppression of the proinflammatory cytokine IL-1β by carvedilol, the antiinflammatory cytokine IL-10 was up-regulated in both the uninfected and infected mice, which might be due to a predominance of the TH2 immune response.
Grimm et al. 30 have shown the antifibrotic properties of carvedilol by reducing levels of collagen type I, type III and fibronectin in hearts with chronic pressure overload. In this murine model of acute myocarditis, carvedilol reduced only the collagen type I expression, which might be due to the early time point after infection. Therefore, long-term treatment with carvedilol in mice infected with coxsackievirus B3 may have further effects on the extracellular matrix architecture by attenuating the changes in the collagen gene expression. This may have a beneficial effect on disease progression and the evolution to dilated cardiomyopathy following the viral infection.
5. Conclusions
In a murine coxsackievirus-induced acute myocarditis model, we found that carvedilol treatment reduced myocardial IL-1β and MMP-8 mRNA abundance. IL-15 mRNA abundance was brought to the baseline levels. IL-10 mRNA abundance levels were induced, combined with a relevant decrease in CD4+ and an increased infiltration of the CD8+ T-lymphocytes. In addition, the carvedilol-treated group showed a significant improvement in left ventricular function. These effects were not produced by β1-selective blockade with metoprolol.
Despite several reports that support the involvement of carvedilol in the modulation of myocardial gene expression, the molecular mechanisms that underlie the multiple actions of carvedilol are yet to be uncovered. The spectrum of activity of carvedilol, a third-generation non-selective β-blocker with α1-blocking properties, can be extended to include anti-oxidant, immunomodulatory, cardioreparatory and antifibrotic characteristics.
Acknowledgement
This research was supported by the Deutsche Forschungsgemeinschaft (DFG Pa 369/2-1, Pa 369/2-3, Pa 369/3-1).




