NT-proBNP in severe chronic heart failure: rationale, design and preliminary results of the COPERNICUS NT-proBNP substudy
Abstract
Background:
Neither profiles nor prognostic value of cardiac N-terminal proBNP (NT-proBNP) have been prospectively evaluated in a sufficient number of patients with severe chronic heart failure (CHF) treated with carvedilol or placebo.
Methods:
Baseline and follow-up plasma concentrations of NT-proBNP were measured in the European part of the COPERNICUS Trial. This study enrolled patients with an ejection fraction <25% and symptoms of CHF at rest or on minimal exertion, equally randomized to placebo or carvedilol.
Results:
NT-proBNP concentrations were increased at baseline (mean±S.D.=579±822 pmol/l, median=322.5 pmol/l) with a marked decrease during follow-up in the carvedilol, but not in the placebo group. One-year mortality rates were 3.9, 12 and 27.9% in the lower, middle and upper tertiles of NT-proBNP, respectively. When mortality was calculated separately in the placebo and carvedilol group, rates were 0.8, 6.3 and 19.1% in the carvedilol treated but 6.7, 17.9 and 36.9% in the placebo treated patients.
Conclusions:
NT-proBNP was a powerful predictor of subsequent all-cause mortality in patients with severe CHF. This marker should therefore be further evaluated for risk stratification and monitoring of therapy in CHF.
1. Introduction
Chronic heart failure (CHF) is the only major cardiovascular disorder that is increasing in incidence and prevalence 1. The recognition of the importance of neurohormonal activation in CHF has resulted in a major change in our approach to its management, from the use of symptomatic treatment with diuretics and digitalis alone, to the utilization of specific means to counteract the neurohormonal derangement 2. Thus, angiotensin-converting enzyme inhibitors and, more recently, beta-blockers, have become crucial in the current management of CHF. The use of beta-blockers has been shown to enhance systolic function and to improve prognosis 3.
Association of neurohormonal activation with the severity of heart failure and cardiac events in CHF is well-established 4–6. In the 1980s, a family of peptides with potent natriuretic, diuretic, and vasorelaxant activity was detected in human heart disease 7. These natriuretic peptides, in particular brain natriuretic peptide (BNP) and amino-terminal pro-brain natriuretic peptide (NT-proBNP), can serve as biochemical indicators of impaired left ventricular function 8,9.
The predictive value of NT-proBNP has been documented after myocardial infarction 10,11 and in patients with established ischemic heart failure NYHA II–III 12, but few data exist in patients with severe symptoms of heart failure. In addition, natriuretic peptides have been proposed to tailor heart failure therapy. However, various interactions of heart failure therapy with natriuretic peptide levels have been reported, some increasing, others decreasing plasma concentrations with therapy 13–18. It is not known whether these interactions might influence the predictive power of the markers.
The COPERNICUS trial provided a unique opportunity to prospectively test the prognostic power of the new cardiac marker NT-proBNP and, in addition, to evaluate whether marker levels might be influenced by concomitant heart failure therapy.
2. Methods
COPERNICUS was a randomized, placebo-controlled, double-blind, parallel-arm multicenter trial. Two thousand and two hundred and eigthy-nine patients with severe chronic heart failure as a result of ischemic or non-ischemic cardiomyopathy were enrolled at 334 centers in 21 countries. Severe chronic heart failure was defined by the occurrence of dyspnea or fatigue at rest or on minimal exertion for at least 2 months and a left ventricular ejection fraction of <25%, despite appropriate conventional therapy. All patients provided written informed consent before enrolment in the study. Study design, protocol and major results have been presented in detail previously 19. Patients had to be pre-treated with diuretics and ACE inhibitors for at least 2 months and those with substantial fluid retention, need for intensive care, or treatment with iv inotropic agents or iv vasodilators within 4 days of screening were not to be enrolled. Patients were randomly assigned to receive either oral carvedilol or matching placebo in addition to their usual medications for heart failure. Carvedilol/placebo doses were up-titrated at 2-week intervals to a target dose of 25 mg twice daily. Patients then continued on the highest tolerated dose until the end of the trial. The primary endpoint of the study was death from any cause, and the combined risk of death or hospitalization for any reason was one of four pre-specified secondary end points. Patients were followed for up to 29 months. The trial program was stopped early because of significant beneficial effect of carvedilol on survival.
The present study was conducted as a substudy of COPERNICUS, involving the European part of the study. The substudy protocol was approved by the COPERNICUS Steering committee prior to the start of the study and coordinated by the authors in cooperation with Roche Diagnostics GmbH, Mannheim. The institutional ethical committees of the participating study sites approved the substudy protocol. Written informed consent was obtained from all patients.
As some centers were not able to participate in the substudy, 1048 patients out of the 1387 European patients could be prospectively included. Together with the samples for the safety laboratory, plasma samples for baseline neurohormonal levels were obtained on the day of randomization (n=1035) or, if not available, at the screening visit (n=13), using 10 ml EDTA syringes for sampling. After up-titration co-investigators were encouraged to collect follow-up samples at the clinical visits during the maintenance phase of the study. Plasma was separated, shipped to a core lab and stored at −80 °C until assays were performed.
Plasma levels of NT-proBNP were determined in a core laboratory at Roche Diagnostics, Penzberg, Germany by a novel, highly sensitive and specific enzyme-linked imunosorbent assay based on a sandwich format (ELISA: microtiter plate assay). This was the prototype for the subsequent commercial assay. The analytical detection limit of the assay was 2.7 pmol/l (3 S.D.). The intra-assay coefficients of variation were 5.7% (at 50 pmol/l) and 6.1% (at 250 pmol/l), while the inter-assay coefficients of variation were 15.8% at 15 pmol/l and 8.2% at 250 pmol/l 20.
2.1. Statistical methods
Marker levels were presented descriptively. As marker concentrations were apparently not normally distributed, statistical analyses were also done on logarithmically transformed values. Differences of group means and of interval-to-interval comparisons were analysed by appropriate test methodology.
To examine treatment effects for different aspects of the study population, NT-proBNP measurements of all patients with baseline and at least one post baseline measurement (n=972) were analyzed in the following ways: (1) changes from baseline at study end point (n=815), which include all patients with data at both baseline and end point; and (2) changes from baseline at maintenance visit one (M1: end of uptitration), four (M4: 3 months after M1) and seven (M7: 6 months after M1), including all patients with data at a given time point and at baseline. Mean baseline values for these analyses may differ. The number of patients represented at each subsequent time point decreases from M1 to M7 (M1: n=903; M4: n=303; M7: n=107).
Survival distributions were compared using two-sided log rank test. The effect of baseline NT-proBNP was characterized by hazard ratios (and corresponding 95% CI) on a Cox proportional hazard model. Kaplan–Meier curves were generated and 1-year mortality as well as annual hazard rates were calculated by stratifying according to tertiles of baseline NT-proBNP levels.
Statistical significance was defined as P<0.05 (two-tailed).
3. Results
3.1. Clinical data
In this analysis of the NT-proBNP substudy, 1048 European patients were included. Patient characteristics are indicated in Table 1. Substudy patients were equally distributed in the placebo or carvedilol treatment groups. 81% of patients were male, 66% had an ischemic aetiology of heart failure. An average left ventricular ejection fraction of 20.4% and an annual hospitalisation rate of 30% indicated severe heart failure. Concomitant medical treatment consisted of digitalis in 58%, diuretic in 99%, ACE inhibitor in 93%, AT II receptor antagonist in 6%, spironolactone in 26% and amiodarone in 18% of cases.
| All patients | |
|---|---|
| (n=1048) | |
| Age (years) | 62.5±11.0 |
| Sex (% men) | 81 |
| Cause of heart failure (% ischemic) | 66 |
| Left ventricular ejection fraction (%) | 20.4±3.6 |
| Hospitalized for heart failure within year (%) | 30 |
| Systolic blood pressure (mmHg) | 127±19 |
| Diastolic blood pressure (mmHg) | 79±10 |
| Heart rate (beats per min) | 83±13 |
| Patients receiving digitalis (%) | 58 |
| Patients receiving diuretic (%) | 99 |
| Patients receiving ACE inhibitor (%) | 93 |
| Patients receiving AT II receptor antagonists (%) | 6 |
| Patients receiving spironolactone (%) | 26 |
| Patients receiving amiodarone (%) | 18 |
- a All continuous data are expressed as mean±S.D. ACE indicates angiotensin converting-enzyme; AT, angiotensin.
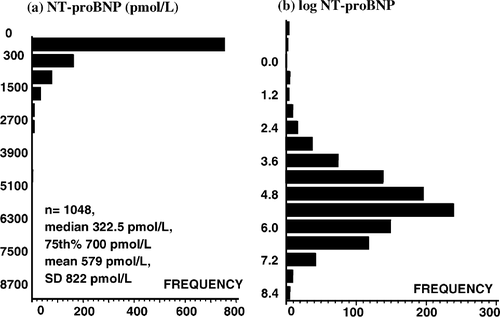
As known from other biochemical markers, baseline NT-proBNP concentrations were not normally distributed (Fig. 1a). The concentrations ranged from 0.49 (lower detection limit of the assay) to 9525 pmol/l with a median of 322.5 (25–75 percentiles, 151–700 pmol/l) and a mean±S.D. of 579±822 pmol/l. Logarithmic transformation of the values resulted in a bell-shaped distribution curve (Fig. 1b). Baseline NT-proBNP levels were not different in the placebo and the carvedilol treatment groups (331 vs. 319 pmol/l, P=n.s.). Concentrations in patients with ischemic aetiology of heart failure were slightly, however, significantly higher (341 vs. 302 pmol/l, P<0.0296).
3.1.1. Follow-up concentrations
Nine hundred and seventy-two patients with baseline NT-proBNP measurement and at least one post baseline measurement (476 patients in the Placebo group and 496 patients in the Carvedilol group) were available. Eight hundred and fifteen of the patients had a final NT-proBNP measurement at the individual end of study. An overview over all measurements per visit is provided in Table 2. Due to the short duration of the study, the number of samples decreased rapidly during the maintenance phase, making interpretation of the findings difficult.
| Baseline | M1 | M4 | M7 | Final | |
|---|---|---|---|---|---|
| n= | 972 | 903 | 303 | 107 | 815 |
| Placebo | 311.0 | 289.5 | 209.6 | 135.1 | 233.6 |
| Carvedilol | 308.5 | 329.8 | 212.9 | 210.8 | 261.5 |
| P= | n.s. | n.s. | n.s. | n.s. | n.s. |
- a M1, 4, 7 indicates maintenance visit 1, 4, 7.
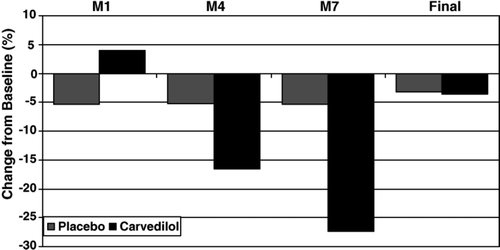
NT-proBNP plasma follow-up concentrations were compared between carvedilol and placebo treatment group. No significant differences were detected when the group medians of carvedilol and placebo treated patients at the different times were compared. However, when individual concentration profiles were analyzed, a different pattern was detected. NT-proBNP concentrations in the placebo group patients decreased slightly. In contrast, in the carvedilol treated patients, a slight increase at M1 was followed by a marked decrease at M4 and M7, indicating an influence of carvedilol treatment on marker concentrations. To illustrate these findings, the individual baseline values were regarded as 100%, the follow-up values were transformed relative to this baseline value and presented as differences in percentages to baseline (Fig. 2).
3.1.2. NT-proBNP and clinical events
In the substudy, a total of 83 patients died (53 placebo vs. 30 carvedilol) during follow up (median 157 days, range 1–488 days) (primary endpoint). One hundred and eighty seven patients died or were hospitalized for heart failure (107 placebo vs. 80 carvedilol), 212 patients died or were hospitalized for cardiovascular reasons (121 placebo vs. 91 carvedilol) and 302 patients died or were hospitalized for any reason (167 placebo vs. 135 carvedilol) (secondary endpoints). Table 3 indicates marker concentrations in patients with cardiac events compared to patients without events, irrespective of treatment group. Baseline NT-proBNP levels were significantly higher in patients with subsequent death or one of the secondary endpoints. Risk ratios and P-values confirm the prognostic significance of the marker for all endpoints.
| Event | No event | RR | P= | |||
|---|---|---|---|---|---|---|
| Median | 25–75 Perc | Median | 25–75 Perc | (95% CI) | ||
| All-cause mortality (n=83) | 585 | 292–1719 | 309 | 140–646 | 3.13 | 0.0001 |
| (1.94–5.07) | ||||||
| All-cause mortality or | 510 | 277–1266 | 290 | 130–593 | 3.11 | 0.0001 |
| hospitalization for heart | (2.27–4.27) | |||||
| failure (n=187) | ||||||
| All-cause mortality or | 481 | 251–1130 | 291 | 131–614 | 2.60 | 0.0001 |
| protocol specified CV | (1.95–3.46) | |||||
| hospitalization (n=212) | ||||||
| All-cause mortality or all- | 438 | 192–964 | 291 | 132–612 | 1.96 | 0.0001 |
| cause hospitalization | (1.56–2.48) | |||||
| (n=302) | ||||||
- a Perc indicates percentile; CV, cardiovascular; RR, risk ratio; CI, confidence interval.
In the next step, univariate Cox regression analysis was performed to investigate the impact of various baseline variables on survival. Clinically relevant classes were defined for continuous variables to characterize their impact on outcome. NT-proBNP (irrespective whether continuous or categorized), systolic blood pressure (continuous and categorized), age (continuous and categorized), creatinine (continuous), carvedilol treatment, and non-ischemic aetiology were shown to have an impact on mortality. Left ventricular ejection fraction (continuous and categorized) and creatinine (categorized) did not reach statistical significance. No influence was observed for treatment with amiodarone and AT II receptor antagonists. Risk ratios and P-values are indicated in Table 4.
| Variables | Risk ratio | 95% CI | P-value |
|---|---|---|---|
| NT-proBNP (10 pmol/l increase) | 1.005 | 1.003–1.006 | 0.0001 |
| NT-proBNP classes above/below | 3.13 | 1.94–5.07 | 0.0001 |
| median | |||
| Treatment group carvedilol/placebo | 0.56 | 0.36–0.88 | 0.01 |
| Age (1 year increase) | 1.03 | 1.01–1.05 | 0.006 |
| Age <65 years/≥65 years | 0.57 | 0.37–0.89 | 0.01 |
| LVEF (1% increase) | 0.95 | 0.90–1 01 | 0.08 |
| LVEF ≥20%/<20% | 0.68 | 0.43–1.06 | 0.09 |
| Creatinine (1 μmol/l increase) | 1.01 | 1.00–1.01 | 0.002 |
| Creatinine <125 μmol/l/≥125 μmol/l | 0.70 | 0.44–1.09 | 0.1 |
| SBP (1 mmHg increase) | 0.98 | 0.98–0.99 | 0.004 |
| SBP ≥100 mmHg/<100 mmHg | 0.28 | 0.15–0.51 | 0.0001 |
| Aetiology of heart failure non-ischemic/ | 0.56 | 0.34–0.91 | 0.0201 |
| ischemic |
- a CI indicates confidence interval; NT-proBNP, amino (N)-terminal pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure.
Kaplan–Meier analyses were performed and Kaplan–Meier survival curves were generated to illustrate the prognostic findings. Patients were subdivided in tertiles according to NT-proBNP concentrations. One-year mortality rates of 3.9%, 12% and 27.9% were calculated when NT-proBNP levels were <199 pmol/l, 199–504 pmol/l or >504 pmol/l, respectively. The differences between the three strata were significant (P=0.0001, log rank test) (Fig. 3).
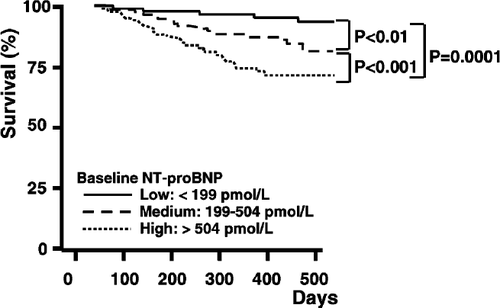
The combined 1-year risks for all-cause mortality or hospitalization for heart failure were 14%, 25.6% or 46.7% in patients with NT-proBNP levels <199 pmol/l, 199–504 pmol/l or >504 pmol/l, respectively. Again, the differences between the three strata were significant (P=0.0001, log rank test).
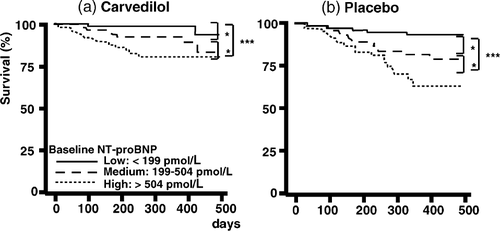
Mortality rates were calculated separately in the placebo and carvedilol group. One-year mortality rates were 0.8, 6.3 and 19.1% in the carvedilol treated but 6.7, 17.9 and 36.9% in the placebo treated patients when NT-proBNP levels were in the lower, middle and upper third. In both treatment groups, the differences between the three strata were significant (Fig. 4).
4. Discussion
The COPERNICUS NT-proBNP substudy investigated concentrations and the prognostic value of NT-proBNP in patients with severe heart failure due to ischemic and non-ischemic cardiomyopathy at baseline and during treatment with carvedilol or placebo. In these patients, NT-proBNP concentrations were markedly elevated at baseline with a wide range and higher values in the ischemic CHF patients. Although numbers of follow-up measurements were low, a marked decrease during follow-up was observed in the carvedilol, but not in the placebo treated patients.
NT-proBNP plasma levels on admission were strongly associated with mortality during follow-up. The substudy results indicate that plasma levels of NT-proBNP may add important information for risk stratification and therapy monitoring in patients with severe heart failure.
4.1. NT-proBNP concentrations in severe CHF
Several authors have observed elevated NT-proBNP concentrations in heart failure patients 21–25. However, the data were in most cases derived from small study groups of <100 individuals. Few data on NT-proBNP plasma concentrations in patients with severe CHF are available. The current study presents plasma NT-proBNP concentrations of more than 1000 severe heart failure patients. Although all patients were symptomatic at rest or on minimal exertion and had a left ventricular ejection fraction of <25%, some patients had very low marker concentrations.
4.2. Effects of treatment on NT-proBNP concentrations
Natriuretic peptides might be useful for the monitoring of heart failure therapy. This concept is supported by the findings of several studies. Tsutamoto et al. reported that 4 months of treatment with spironolactone improved left ventricular volume and mass, as well as decreased plasma levels of BNP in 37 non-ischemic patients with CHF 13. Latini et al. measured both BNP and noradrenaline at baseline and 4, 12 and 24 months after randomization to valsartan or placebo in 4284 patients of the Valsartan Heart Failure Trial (Val-HeFT). Valsartan caused sustained reduction in BNP and attenuated the increase in noradrenaline over the course of the study. These neurohormone effects of valsartan were consistent with the clinical benefits reported in the study 14.
Murdoch et al. sought to determine whether titration of vasodilator therapy according to plasma brain natriuretic peptide concentration might be of value in the individual optimization of vasodilator therapy in CHF. They found that plasma BNP concentration was chronically reduced by tailored vasodilator therapy in CHF. Furthermore, titration of vasodilator therapy according to plasma BNP was associated with more profound inhibition of the renin-angiotensin-aldosterone system and a significant fall in heart rate when compared with empiric therapy 26. Troughton et al. compared the usefulness of NT-proBNP in therapy monitoring with a clinical score in a pilot study in 69 DCM patients treated mainly with diuretics and vasodilators. In this randomized study, he reported a significant reduction in the cardiac event rates, when NT-proBNP was used to tailor heart failure therapy 27.
Conflicting findings have been reported regarding the influence of beta-blocker therapy on natriuretic peptide levels. In a study by Jansson et al., metoprolol suppressed the exercise-induced increase of ANP in patients with dilated cardiomyopathy, suggesting a favourable effect on ventricular performance 28. Kawai et al. reported that chronic beta-blocker therapy decreased circulating brain natriuretic peptide concentrations, interpreted as an indicator of response to treatment 29.
In other studies, the intake of beta-receptor antagonists resulted in an at least transient increase of plasma concentrations of the cardiac natriuretic peptides. In a retrospective analysis of data derived from a population-based study presented by Luchner et al., beta-receptor antagonists appeared to augment plasma ANP, BNP and cGMP concentrations. The authors speculated that this observation might be an indicator of a contribution of the cardiac natriuretic peptide system to the therapeutic mechanisms of beta-receptor antagonists 15. Similar effects on ANP concentrations have been reported earlier in hypertensive patients 16,17 and in healthy volunteers 18.
In the RESOLVD study, 24 weeks of therapy with metoprolol-CR induced a significant increase of NT-ANP and BNP. Especially BNP correlated with LV-function and volumes as well as with changes in cardiac volumes in response to metoprolol-CR 30.
In the CHRISTMAS study, 6 months carvedilol therapy resulted in significantly higher LV ejection fractions and in higher concentrations of natriuretic peptides. Interestingly, the increase in natriuretic peptides tended to be greater and the increase in LV ejection fractions smaller in patients without proof of hibernation, defined as mismatch of impaired echo wall motion but preserved myocardial perfusion 31.
These apparently opposite effects of beta-blockers on natriuretic peptide levels may in part be explained by different durations of treatment. Initiation of medication may result in a transient hemodynamic deterioration with increased marker levels. The subsequent recovery of cardiac function might then be followed by a decrease of neuropeptide concentrations.
In the COPERNICUS NT-proBNP substudy, interpretation of the follow-up concentrations during treatment with carvedilol or placebo is compromised by the short duration of the study. At M4 only 303 and at M7 only 107 samples were available. This may explain the lack of a significant difference when medians or mean values of NT-proBNP concentrations at the different times were compared. Alternatively, selective loss (due to death) of patients on placebo with high NT-proBNP levels may have occurred. However, when individual marker profiles were analyzed, it could be shown, that carvedilol reduced the marker levels at M4 and M7. In contrast, no significant difference was observed in the placebo patients. Why this difference was not present in the individual samples collected at the end of study is not clear, although it may partly be due to the wide variation in follow-up times and the short study duration in the majority of patients.
4.3. NT-proBNP as a predictor of prognosis in CHF
The prognostic value of commonly employed risk indicators including left ventricular ejection fraction, complex arrhythmias and heart failure symptoms is limited 32. Several groups proved the usefulness but also the limitations of different neurohormonal markers, in particular natriuretic peptides including ANP and BNP 5,33.
The Australia–New Zealand Heart Failure Study evaluated NT-proBNP for prediction of all-cause mortality and heart failure in 297 patients with established ischemic LV dysfunction (NYHA II–III, LVEF <45%) treated with carvedilol or placebo. NT-proBNP was superior to left ventricular ejection fraction in predicting mortality and heart failure 34.
The COPERNICUS NT-proBNP substudy extends this important finding to a large number of severe CHF patients with left ventricular ejection fractions <25% due to ischemic or non-ischemic cardiomyopathy. The COPERNICUS patients represent a high-risk population with an overall annual placebo mortality rate of 19.7%. In the NT-proBNP substudy, the risk of dying was more than five-fold higher in placebo patients with baseline NT-proBNP concentration in the upper third as compared to patients with NT-proBNP values in the lower third. When NT-proBNP was in the lowest third and carvedilol was applied, an extremely low 1-year mortality of approximately 1% was observed. The prognostic importance of NT-proBNP was highly significant irrespective of the thresholds or treatment groups and was comparable or superior to establish risk indicators such as treatment, cause of heart failure, left ventricular ejection fraction, age, and systolic blood pressure.
It is not known whether follow-up measurements of NT-proBNP might provide additional prognostic information. Normalization of previously elevated NT-proBNP levels indicates favorable treatment response and improved prognosis.
General application of the COPERNICUS NT-proBNP substudy results is restricted by the fact that the NT-proBNP blood levels and the discriminator values only relate to the specific assay used for the trial. In the present study, NT-proBNP was initially measured by a newly developed highly specific NT-proBNP ELISA that uses microtiter plate technology 20. During the evaluation of the study data, this technology was followed by a new assay based on electro chemi luminescence technique that will allow a standardized automated determination of NT-proBNP in CHF patients (Elecsys, proBNP assay). It is planned to confirm the presented data by this more sophisticated assay technology.
Acknowledgements
The substudy was supported by a grant from Roche Pharmaceuticals and NT-proBNP plasma level measurements were performed in a core laboratory at Roche Diagnostics. The authors Packer, Coats, Fowler, Katus, Krum, Mohacsi, Rouleau, Tendera and Castaigne have served as consultants to Roche Pharmaceuticals. The authors Amann-Zalan, Hörsch and Trawinski are employees of Roche Pharmaceuticals.




