Effects of an endothelial cell-conditioned medium on the hematopoietic and endothelial differentiation of embryonic stem cells
Abstract
We have examined the effect of mouse bone marrow endothelial cell-conditioned medium (mEC-CM) on hematopoietic and endothelial differentiation of mouse embryonic stem cells (mESCs). mEC-CM can efficiently promote the differentiation of mESCs into Flk+ cells and hematopoietic colony-forming cells. mEC-CM proved to be as potent as a cytokine cocktail comprised of VEGF, bFGF, IGF and EGF. After inducing mESCs with mEC-CM, cobblestone-like cells were mechanically selected and identified which had the ability to incorporate DiI-Ac-LDL. DiI-Ac-LDL-positive cells were endothelial-like cells due to their expression of CD31 and Flk1, ability to bind to UEA1 and capacity to form capillary-like tube structures on matrigel. In conclusion, mEC-CM can efficiently promote the differentiation of mESCs into endothelial cells and hematopoietic colony-forming cells. The differentiated endothelial-like cells can be isolated by using DiI-Ac-LDL labeling and mechanical selection.
1. Introduction
Endothelial cells (ECs) have a close relationship with hematopoiesis that occurs in the yolk sac, aorta-gonad-mesonephros, placenta, fetal liver, spleen, and adult BM (Lacaud et al., 2001; Li, 2005). ECs can produce a variety of hematopoietic stimulating factors and inhibitors. These cytokines play important roles in maintaining the properties of hematopoietic stem cells (HSCs), and in regulating proliferation and differentiation of HSCs (Kopp et al., 2005; Hu et al., 2006). Conditioned media generated from a murine bone marrow endothelial cell line can promote proliferation and differentiation of hematopoietic cells, and also stimulate the growth of bone marrow ECs (Wang et al., 1998; Zhao et al., 2003; Na et al., 2004; Zhou et al., 2005). These findings suggest that the cytokines in endothelial cell-conditioned medium (mEC-CM) may assist in the differentiation of mES cells into hematopoietic and endothelial cells.
We have used mEC-CM to induce the differentiation of mESCs into hematopoietic and endothelial progenitor cells and compared its differentiation efficiency with cytokine induced differentiation. Also in addition, cells were handpicked that incorporated DiI-Ac-LDL from differentiated mESCs, and these were analyzed in terms of their biological characteristics.
2. Materials and methods
2.1 Preparation of mEC-CM and PHACM
The mouse bone marrow endothelial cell line (a gift from Professor Qiru Wang, Central South University, China) was cultured in IMDM with 10% FBS, 100 μM non-essential amino acids, 2 mM l-glutamine, 10−4 M 2-mercaptoethanol (All from Invitrogen), 50 U/ml penicillin (Sigma) and 50 μg/ml streptomycin (Sigma). To collect the conditioned medium of mECs, cells were cultured until 90% confluent. The culture medium was replaced with 5 ml IMDM with 5% FBS in each 100 mm plate. After 24 h, the medium was collected and passed through a 0.22 μm filter before being stored at −20 °C before use. mEC-CM was used within a week after being thawed.
Bone marrow cells were harvested from the femurs of experimental mouse and cultured in IMDM, 20% FBS, 10−4 M 2-mercaptoethanol and 10 μg/ml PHA (Sigma) for 7 days. Conditioned media (PHACM) were harvested and passed through 0.22 μm filter and stored at −20 °C before use.
2.2 Differentiation of mouse ES cells
129/SVEV ES cells were maintained and allowed to spontaneously differentiate into embryoid bodies (mEBs) as previously described (Nishikawa et al., 1998). After 4 days, 50 mEBs (medium-sized, diameter 200–250 μm) were selected under a microscope and cultured on fibronectin-coated plates for 10 days in media A, B, C and D. Medium A consisted of IMDM with 20% FBS, 100 μM non-essential amino acids, 2 mM glutamine, 10−4 M 2-mercaptoethanol, 50 U/ml penicillin and 50 μg/ml streptomycin. Medium B consisted of medium A supplemented with 10% mEC-CM. Medium C consisted of medium A plus cytokines, including 20 ng/ml recombinant (r) human (h) VEGF(R&D), 10 ng/ml rhbFGF (Invitrogen), 10 ng/ml rhEGF(R&D) and 10 ng/ml rhIGF-1(R&D). Medium D consisted of medium C plus 10% mEC-CM.
2.3 Colony Forming Unit (CFU) assays
For the generation of BFU-E colonies, adherent EB cells (105 cells/ml) were plated in IMDM with 1% methylcellulose (Sigma, St. Louis, MO) supplemented with 30% horse serum (lot selected for growth of erythroid cells, Sijiqing Company, Hangzhou, China), 2 mM l-glutamine, 10−4 M 2-mercaptoethanol, 10 U/ml rhEPO (Kirin Brewery Co. Ltd., Japan) and 20% PHACM. For CFU-GM colony growth, cells (105 cells/ml) were plated in IMDM with 1% methylcellulose, 30% horse serum (lot selected for growth of granulocyte/macrophage, Sijiqing Company, Hangzhou, China) and 50 U/ml r-murine (m) GM-CSF (Biogen, Geneva, Switzerland). For the generation of CFU-GEMM colonies, cells were plated in IMDM with 1% methylcellulose, 30% horse serum (lot selected for growth of granulocyte/macrophage), 50 U/ml rmGM-CSF, 50 ng/ml rhSCF (R&D), 20 ng/ml rhIL-6(R&D), 20 ng/ml rhIL-3(R&D) and 10 U/ml rhEPO. Cells were incubated at 37 °C in air with 5% CO2 for 7 days (BFU-E, CFU-GM) or 9 days (CFU-GEMM).
2.4 Flow cytometry analysis
Single-cell suspensions were generated by dissociation of attached EBs. Cells were incubated with goat anti-mouse Flk1 antibody (R&D) and with FITC-conjugated bovine anti goat IgG (Santa Cruz Biotechnology) secondary antibody. Samples were analyzed with a FACS Calibur (Becton Dickinson) while being gated to exclude debris. Flow cytometric data were analyzed using the BD CELLQuest software.
2.5 Selection of DiI-Ac-LDL-positively-labeled cells
Four-day mEBs (4dEBs) were cultured on fibronectin-coated plates for 10–16 days in medium B before being incubated with 10 μg/ml DiI-Ac-LDL (Biomedical Technologies Inc.) for 4 h. Cell clusters that stained positively for DiI-Ac-LDL showed red fluorescence and were mechanically dissociated into small clumps, aspirated out and seeded onto a fibronectin-coated plate in EGM-2 (Cambrex). Following 7 days of expansion, cells that stained positively for DiI-Ac-LDL were mechanically selected again. Three or four repetitions were sufficient to get a pure cobblestone-like cell population with DiI-Ac-LDL staining.
2.6 Wright–Giemsa staining
Hematopoietic colonies were aspirated from methylcellulose, washed in PBS, and smeared onto glass slides. After fixing in an 80:20 methanol and acetone solution, cells were stained with Wright–Giemsa (Sigma).
2.7 Immunophenotype and functional analysis for DiI-Ac-LDL-positive cells
Cells to be tested by ICAM1 and VCAM1 antibodies were first incubated in EGM-2 medium with 5 ng/ml TNFα and 20 ng/ml IL-1 overnight. Then all the cells for immunocytochemistry were fixed in situ by 4% paraformaldehyde. Endothelial markers were detected using the HRP-AEC kit (Lab Vision Corp., USA), according to the manufacturer's protocol. Primary antibodies included goat anti-mouse Flk1 (R&D), rat anti-mouse CD31, ICAM1, ICAM2 and VCAM1 (Biolegend) monoclonal antibodies. Positive staining was indicated by red staining. HEK 293 cells were used as negative controls.
For endothelial specific lectin binding studies, DiI-Ac-LDL-positive cells were fixed in situ and incubated with UEA1-FITC (Sigma) for 1 h. The cells were then observed under a fluorescence microscope.
To test the ability of purified DiI-Ac-LDL-positive cells to undergo angiogenesis in vitro, cell suspension, containing 1–2 × 104 DiI-Ac-LDL-positive cells supplemented with 50 ng/ml VEGF, were added onto the matrigel (Becton Dickinson, pharmingen) in the 96-well plate. The cultures were incubated at 37 °C, in 5% CO2 in air, and observed after 4–6 h.
2.8 Statistical analysis
The experiments were repeated 3 times. Results from the proportion of Flk1+ cells or the counting of CFU colonies were expressed as means ± SD. The data were submitted to a one-way ANOVA followed by the Newman–Keuls's test. Difference were considered statistically significant at P < 0.05.
3. Results
3.1 mEC-CM promotes mESC differentiation into Flk1+ cells
Flk1 (VEGFR2) is considered to be an important marker of hematopoietic and endothelial progenitor cells (Chung et al., 2002). Differentiation of 4dEBs was driven with 4 different culture conditions: A (control), B (mEC-CM), C (cytokines) and D (mEC-CM combined with cytokines). The percentage of Flk1 positive cells in each culture condition was determined. The proportion of Flk1+ cells in medium B (14.9 ± 3.0%), C (13.1 ± 2.5%) and D (17.0 ± 2.9%) was significantly higher than that for those cultured in medium A (7.53 ± 2.4%; P < 0.05), but there were no significant differences among groups B, C and D, indicating that mEC-CM and/or cytokine cocktail could promote differentiation of mESCs to mesodermal precursor cells, combination of mEC-CM with cytokine cocktail did not show significant synergism (Fig. 1A).
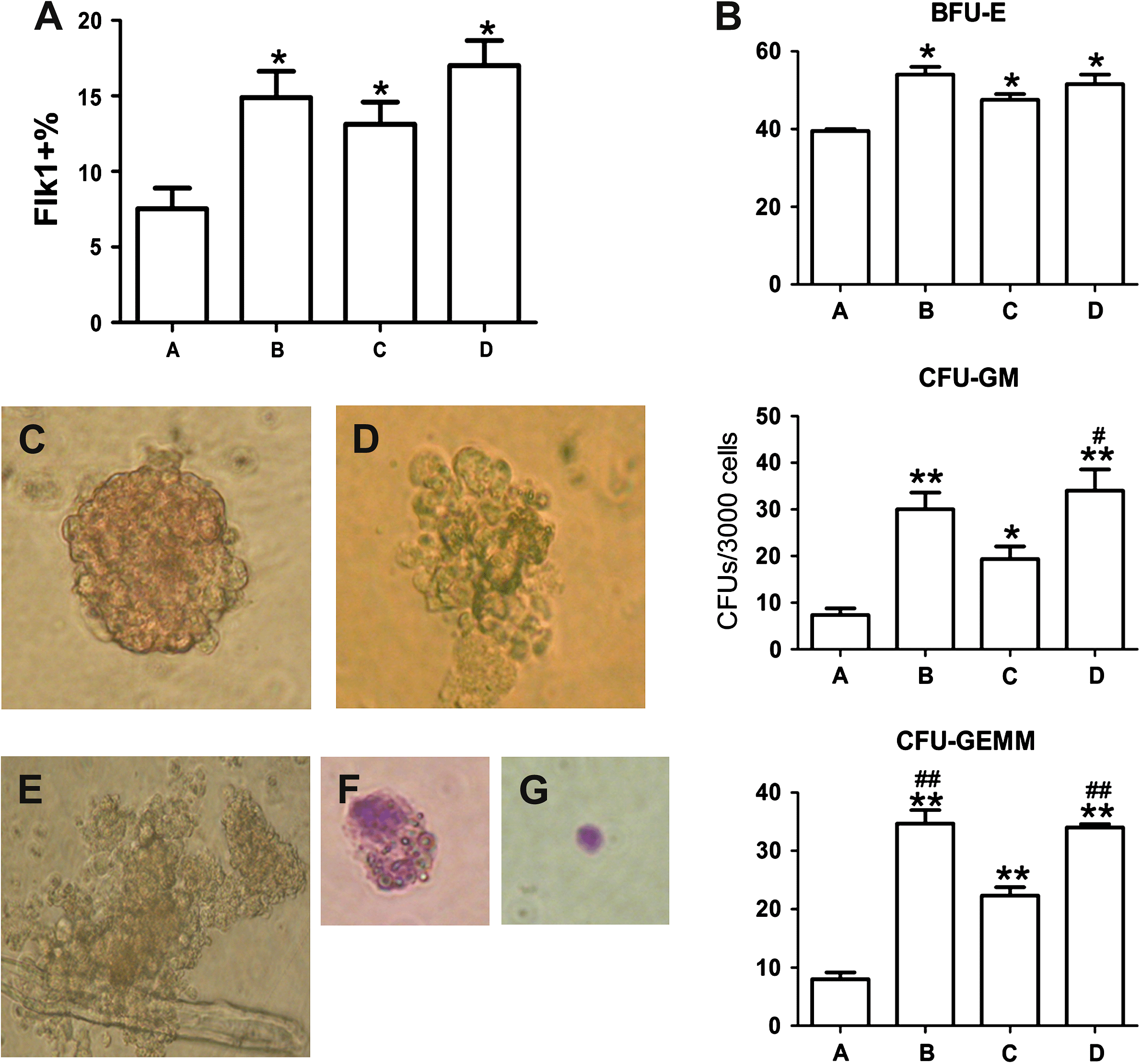
Proportion of Flk1+ cells in differentiated mESCs under different inducing conditions and their ability to form CFUs. (A) FACS results for Flk1 expression under 4 culture conditions. Group B, C, D all have significant differences from group A. *P < 0.05 (n = 3, by ANOVA). (B) The number of colonies that developed from 3 × 104 differentiated cells. Group B, C and D contained more hematopoietic progenitors (BFU-E, CFU-GM and CFU-GEMM) than group A (*, P < 0.05; **, P < 0.01; n = 3, by ANOVA). Group B and/or D showed stronger effects than group C in inducing the formation of CFU-GM and/or CFU-GEMM (?, P < 0.05; ##, P < 0.01; n = 3, by ANOVA). The morphology of BFU-E(C), 200×; CFU-GM(D), 200×; CFU-GEMM(E), 200×; monocytes(F), 400×; erythroid cells(G), 400× derived from differentiated mESCs.
3.2 mEC-CM significantly promotes differentiation of mESCs into hematopoietic progenitors
To determine whether mEC-CM was efficient in promoting differentiation of 4dEBs into hematopoietic progenitor cells, hematopoietic colony-forming assays were performed. After 7 days (CFU-GM and BFU-E) or 9 days (CFU-GEMM) of culture, hematopoietic colonies developed in methylcellulose semi-solid medium (Fig. 1C–E) and the cell components of the colonies were identified by Wright–Giemsa staining. Monocytes were found in CFU-GM and CFU-GEMM, and erythroid cells in BFU-E and CFU-GEMM (Fig. 1F and G). Fig. 1B shows the number of corresponding colonies. As expected, the addition of mEC-CM, cytokine cocktail or both showed significant effects on the formation of various types of hematopoietic colonies when compared with control (P < 0.05). There were no significant differences among the B, C, D groups in inducing the formation of BFU-E. However, groups given both mEC-CM and cytokine cocktail (group D) responded more strongly than the group given the cytokine cocktail (group C) in inducing the formation of CFU-GM (P < 0.05) and CFU-GEMM (P < 0.01); group B, which simply contained mEC-CM, also showed a stronger effect than group C in inducing the formation of CFU-GEMM (P < 0.01). These results suggest that mEC-CM has a significant impact on inducing various hematopoietic progenitors.
3.3 DiI-Ac-LDL-positive cells can be mechanically selected from differentiated mESCs
After adherent culture of 4dEBs in medium B (containing mEC-CM) for 10–16 days, the cells were stained by DiI-Ac-LDL. Cells that incorporated DiI-Ac-LDL usually appeared in the center region of the attached EBs (Fig. 2B). Most cells were linearly aligned and appeared as a clump of red cells under a fluorescent microscope. We collected the cellular regions with red fluorescence, and cultured them in EGM-2. After 4–7 days of culture, cobblestone-like cell colonies emerged on the plate (Fig. 2C). Almost all the cells in the colonies incorporated DiI-Ac-LDL. These cells were once more mechanically selected and seeded on a new plate. After repeating this procedure 3–4 times, we obtained highly purified DiI-Ac-LDL-labeled cobblestone-like cells (Fig. 2D and K).
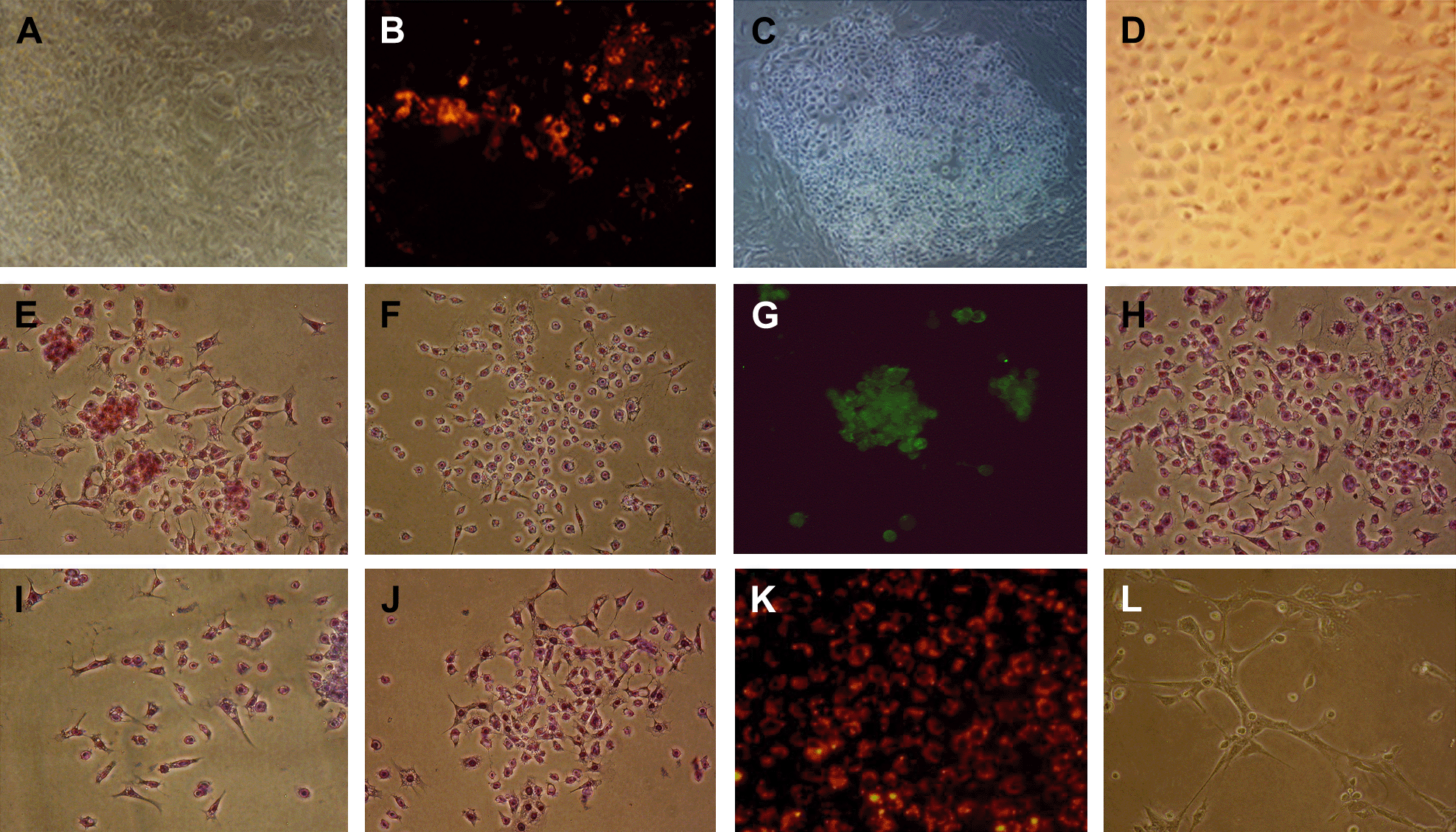
Derivation of DiI-Ac-LDL-positive cells from mESCs and phenotypic and functional analysis of these DiI-Ac-LDL-positive cells. (A) 4dEBs attached on fibronectin pretreated plates for 10 days, 200×; (B) Some regions of the attached EBs incorporated DiI-Ac-LDL and showed red fluorescence, 200×; (C) Cobblestone-like cell colonies emerged, 100×; (D) DiI-Ac-LDL-positive cells proliferated into a pure cobblestone-like cell population, 200×. This pure cell population positively stained for CD31 (E), Flk1 (F), ICAM1 (H), ICAM2 (I), VCAM1 (J); bound UEA1 (G), incorporated DiI-Ac-LDL (K), and could form vascular-like tubes on matrigel (L) (magnifications of E–L: 200×).
3.4 Phenotypic and functional analyses of DiI-Ac-LDL-positive cells derived from mESCs show that they are endothelial-like cells
Purified DiI-Ac-LDL-positive cells were stained for endothelial cell specific markers. The cells expressed CD31 (Fig. 2E), Flk1 (Fig. 2F) and ICAM2 (Fig. 2I), and also expressed ICAM1 (Fig. 2H) and VCAM1 (Fig. 2J) once they had been stimulated with TNFα and IL-1. In contrast, HEK 293 cells, a negative control, did not express these markers. The purified DiI-Ac-LDL-positive cells (referring to which? Ed.) could bind UEA1 lectin (Fig. 2G) and form vascular-like structures on matrigel (Fig. 2L). The results suggest that the purified cell population consisted of endothelial-like cells.
4. Discussion
VEGF, bFGF, IGF-1 and EGF are cytokines that play important roles in the development of endothelial cells and/or hematopoietic differentiation (McCloskey et al., 2003; Kennedy et al., 2007; Kilroy et al., 2007). In our study (which experiment(s)? Those just mentioned?: Ed), mEC-CM promoted the generation of Flk1+ mesodermal precusor cells and various kinds of hematopoietic progenitors from 4dEBs. Its effect was as potent as a cytokine cocktail (combination of VEGF, bFGF, IGF and EGF), which suggests that the growth factors in mEC-CM could also induce differentiation of ESCs into endothelial and hematopoietic cells. Previous study has proved that mEC-CM could support the derivation of HSCs from mESCs before 4dEBs (Zhao et al., 2003). From the hematopoietic colony-forming assay data, the addition of mEC-CM to the medium showed a significant effect on derivation or maintenance of the hematopoietic progenitor cells after 4dEBs were formed. The effect of mEC-CM is as potent as the cytokine cocktail in inducing the formation of BFU-E and GM-CFU, and even stronger than cytokine cocktail in inducing GEMM-CFU. mEC-CM, which has been verified to contain many endothelial and hematopoietic growth factors such as SCF, GM-CSF, IL-1, IL-6, TGF β, and IL-11(Li et al., 2000; Zhou et al., 2005), is helpful in endothelial and hematopoietic differentiation from ESCs; and mEC-CM also contains inhibitors such as thymosin β4 and AcSDKP, which can exert inhibitory effects on the proliferation of hematopoietic progenitors (Huang and Wang, 2001).
DiI-Ac-LDL can label both vascular endothelial cells and macrophages, without affecting cell viability, allowing labeled cells to be continuously cultured (Voyta et al., 1984). We have successfully isolated endothelial cells from a complex cell population derived from mESCs by the mechanical selection of DiI-Ac-LDL-positive cobblestone-like cells. Three or four repetitions of this procedure can result in a highly purified cobblestone-like population and becomes an option to isolate endothelial-like cells.
5. Conclusions
mEC-CM can efficiently promote the differentiation of mESCs into Flk1 positive cells and hematopoietic colony-forming cells. The induction of differentiation of mESCs by mEC-CM, followed by mechanical selection of DiI-Ac-LDL-positive cobblestone-like cells provides an efficient and economic method for purifying endothelial-like cells from mESCs.
Acknowledgements
We are grateful to Professor Qiru Wang (Central South University, Changsha, China) for the mouse bone marrow endothelial cell line. This study was supported in part by grants from the National Basic Research Program of China (973 program No. 2007CB947901), the Hi-Tech Research and Development of China program (863 program No. 2006AA02A102) and Hunan Natural Science Foundation (No. 08JJ3075).




