Increase in cytosolic calcium maintains plasma membrane integrity through the formation of microtubule ring structure in apoptotic cervical cancer cells induced by trichosanthin
Abstract
This study investigates the role of dysregulated cytosolic free calcium ([Ca2+]c) homeostasis on microtubule (MT) ring structure in apoptotic cervical cancer (HeLa) cells induced by trichosanthin (TCS), a type I ribosome inactivating protein (RIP). The TCS-induced decrease in cell viability was significantly enhanced in combination with the specific calcium chelator, EGTA-AM. Sequestration of [Ca2+]c markedly disrupted the special MT ring structure. Furthermore, TCS tended to increase LDH release, whereas no significant differences were observed until 48 h of the treatment. In contrast, combined addition of EGTA-AM or colchicine (an inhibitor of tubulin polymerization) significantly reinforced LDH release. The data suggest that TCS-elevated [Ca2+]c maintains plasma membrane integrity via the formation of the MT ring structure in apoptotic HeLa cells.
1. Introduction
Trichosanthin (TCS) is a type I ribosome inactivating protein (RIP) isolated from the root tubers of the Chinese medicinal herb, Trichosanthes kirilowii. It has been used for termination of pregnancy for >1000 years in China, inducing abortion due to its high toxicity to trophoblasts (Maraganore et al., 1987). Increasing evidence suggests that TCS has a broad spectrum of biological and pharmacological activities, including anti-tumor activities (Tsao et al., 1986; Chan et al., 2000; Ru et al., 2000). Our previous reports have shown that TCS inhibits HeLa cell proliferation by activating the apoptotic pathway (Wang and Li, 2007). Furthermore, a novel mechanism of intracellular cAMP signal was mediated by TCS in apoptosis (Wang et al., 2007). In particular, PKA probably contributes in a limited way to cAMP-dependent activation of TCS-induced apoptosis (Wang et al., 2008). Therefore, the possibility is raised that TCS has a specific toxicity on HeLa cells.
Apoptotic cells undergo a series of molecular and morphologic changes. To permit the dramatic morphological changes that occur concomitant with the execution phase, an apoptotic cell undergoes a series of profound cytoskeletal breakdown and reformation (Veselska et al., 2003). How the cytoskeleton is regulated during the apoptotic execution phase has been the topic of much research, which has resulted in an emphasis on the roles of microfilament (MF), whereas all other cytoskeleton elements were dismantled (Cervinka et al., 2004). But recent studies showed that an important and hitherto overlooked contribution is made by a novel apoptotic microtubule (MT) array (Moss et al., 2006; Moss and Lane, 2006; Sanchez-Alcazar et al., 2007). In particular, our recent reports showed that a typical MT ring structure was formed in apoptotic HeLa cells induced by TCS (Wang and Li, 2007). However, little is known about mechanisms underlying the process of apoptotic cytoskeletal rearrangements. In particular, no information is available about the effects of special MT rearrangements during HeLa cell apoptosis.
One of the main biochemical features of apoptosis is the disruption of cytosolic free Ca2+ ([Ca2+]c) homeostasis (Nicotera et al., 1992). Increase in [Ca2+]c preceding death in cells undergoing apoptosis has been reported (Takadera and Ohyashiki, 1997; Nakamura et al., 2000), and Ca2+ chelators have been observed to block apoptosis in different cell types (Lin et al., 2006). In addition, sustained [Ca2+]c elevation was seen in apoptotic cells (Wang et al., 2008). A growing body of evidence indicates a requirement for Ca2+-dependent signaling in the regulation of MT dynamics (Melander Gradin et al., 1997; Facanha et al., 2002). However, the role of [Ca2+]c on MT ring structure formation in TCS-treated HeLa cells remains unknown.
We have therefore investigated whether TCS-dysregulated [Ca2+]c homeostasis was involved in the regulation of MT ring structure formation and the maintenance of apoptotic cell morphology.
2. Materials and methods
2.1 Cell culture
Hela cells (ATCC, USA) were cultured as previously reported (Wang and Li, 2007; Wang et al., 2007, 2008). For cell viability and [Ca2+]c assays that involved the use of a microplate reader, cells were seeded in 96-well black-clear bottom plates (Greiner Bio-One, Longwood, FL, USA) at 6 × 104/ml.
2.2 Treatment with drug in vitro
Cells were treated with TCS (100 μg/ml, Jinshan pharmacy company, Shanghai, China) for 24 h (except when otherwise indicated). Inhibitors of tubulin polymerization, colchicine (COL, 2 μM, Sigma, St. Louis, MO, USA) and ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetoxymethyl ester (EGTA-AM EGTA-AM, 50 μM, Calbiochem, San Diego, CA) were added 12 h after TCS treatment.
2.3 Cell viability assay
To assess viability, cell counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) was used, as previously described (Wang and Li, 2007; Wang et al., 2008).
2.4 Fluorescent staining of MF, MT and nucleus
MFs, MTs and nucleus were stained as previously described (Wang and Li, 2007). Coverslips were washed twice with PBS and examined under a confocal scanning microscopy (Bio-Rad, Hercules, CA, USA).
2.5 Analysis of apoptotic nuclei
Apoptotic features were assessed by observing PARP expression and nuclear fragment. In each case 10 random fields and >500 cells were counted.
2.6 Determination of [Ca2+]c
Fluo-4-acetoxymethyl ester (AM) (Molecular Probes, Eugene, OR, USA) is a cell-permeant fluorophore used to measure temporal changes in [Ca2+]c (Lim et al., 2006). Briefly, an aliquot of the AM stock solution in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) was mixed with an equal amount of pluronic (Molecular Probes, Leiden, Netherlands) and added to the assay buffer consisting of HBS, 20 mM HEPES (pH 7.4), and 2.5 mM probenecid (Molecular Probes, Leiden, Netherlands), to give a final concentration of 10 μM. After cells had been treated with TCS and/or inhibitors for 48 h, the supernatant from each well was removed and cells were incubated with Fluo-4-AM for 1 h at 37 °C. After removing Fluo-4 from the well, the change in fluorescence (excitation 485 nm, emission 520 nm) was monitored in a fluorescence microplate reader. Ionomycin calcium salt (20 μM, Molecular Probes) was used as a positive control.
2.7 Western blotting analysis
Total cell lysate was prepared by lysis with radioimmunoprecipitation assay buffer and protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA). For Western blotting analysis, 50 μg of each sample were processed as previously described (Wang et al., 2007). The following antibodies were used: monoclonal anti-actin and cleaved PARP (Promega, Southampton, UK), according to the recommendations of manufacturer. The secondary antibodies were coupled to horseradish peroxidase and detected by chemiluminescence (Bio-Rad, Hercules, CA).
2.8 LDH determination
Lactate dehydrogenase (LDH) activity was determined spectrofluoremetrically with a commercial kit (Promega, Madison, WI, USA). In the presence of LDH, resazurin is converted to resorufin, which was quantified in a microplate reader (excitation 560 nm, emission 590 nm).
2.9 Statistical analysis
All experiments were repeated for 3 times. The data were expressed as means ± SD. ANOVA was used to evaluate the statistically significant differences among various treatments. Statistical analysis of different treatments was performed by Dunnett's pairwise multiple comparison t test. Significance was attributed when p < 0.05.
3. Results
3.1 TCS-decreased cell viability was enhanced by the combination of EGTA-AM
Exposure HeLa cells to TCS resulted in a time- and concentration-dependent cytostatic effect on cell proliferation (Fig. 1A). To ascertain whether cell death induced by TCS was mediated by intracellular calcium level, viability was determined in the presence of EGTA-AM. EGTA-AM alone had no distinct effect on cell viabilities. Combined addition of EGTA and TCS, however, enhanced the decrease of cell viability which was induced by TCS alone (Fig. 1B).
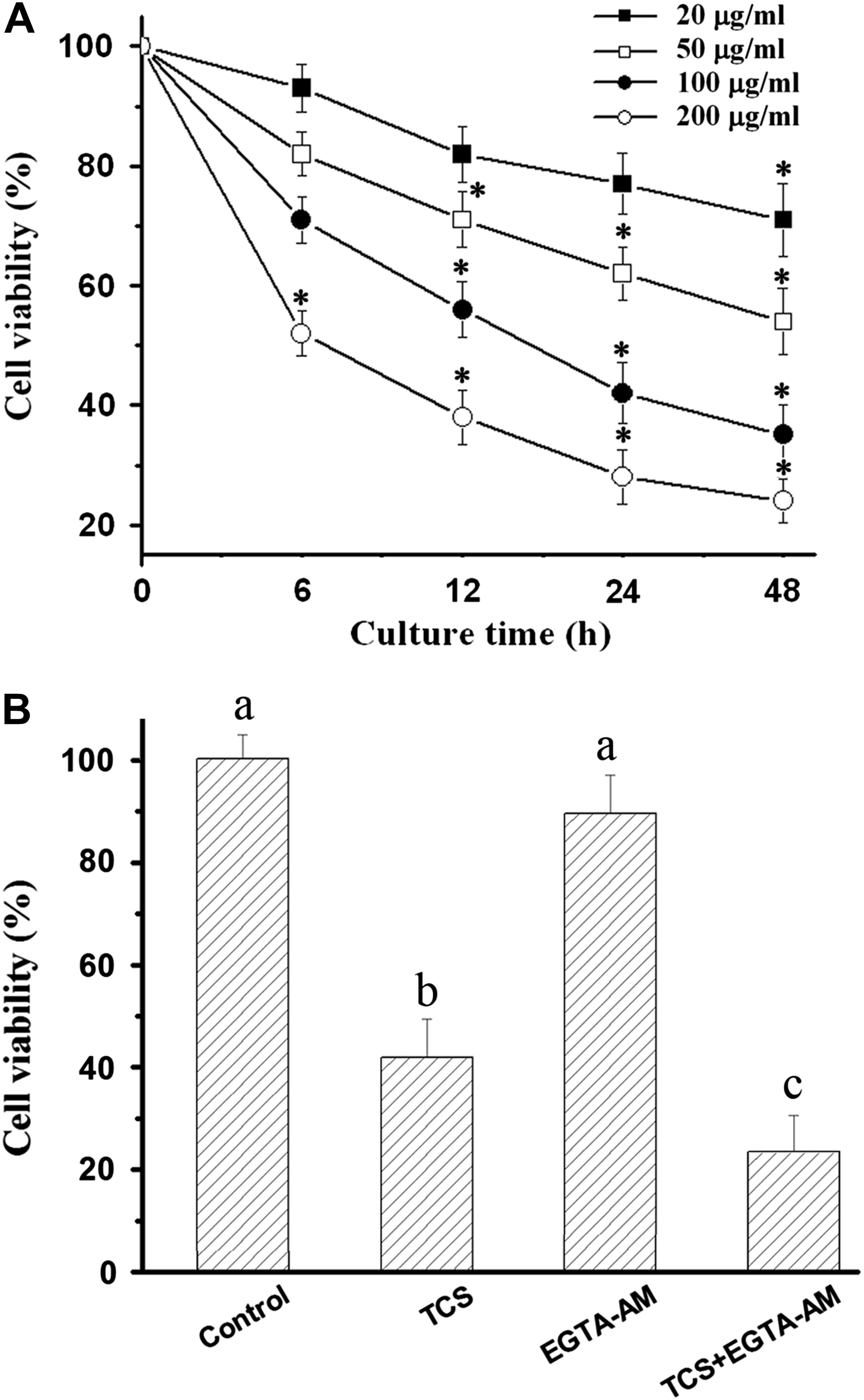
TCS-decreased cell viability was enhanced by the combination of EGTA-AM. Cytostatic effect induced by TCS was shown to be a time- and concentration-dependent manner (A). TCS-decreased cell viability was enhanced in cells treated with TCS + EGTA-AM for 24 h (B). *p < 0.05 compared with 0 h, significant differences among different stimulators are indicated with different letters (p < 0.05). Data presented as means ± SD of 8 replicates from 8 independent experiments.
3.2 A typical MT ring structure was induced in apoptotic HeLa cells
We examined whether MT array was reorganized in vitro during the execution phase of HeLa cell apoptosis, as reported in other cell lines (Pittman et al., 1994; Moss and Lane, 2006; Sanchez-Alcazar et al., 2007). HeLa cells were treated with TCS, and immunostained for β-tubulin, rhodamine–phalloidin and DAPI. Similar results were observed to those previously described (Wang and Li, 2007). In brief, depolymerized MFs were intensely stained in apoptotic HeLa cells, a typical MT ring structure was formed to enclose most of the cell interior, including the apoptotic nuclei and bodies (Fig. 2).
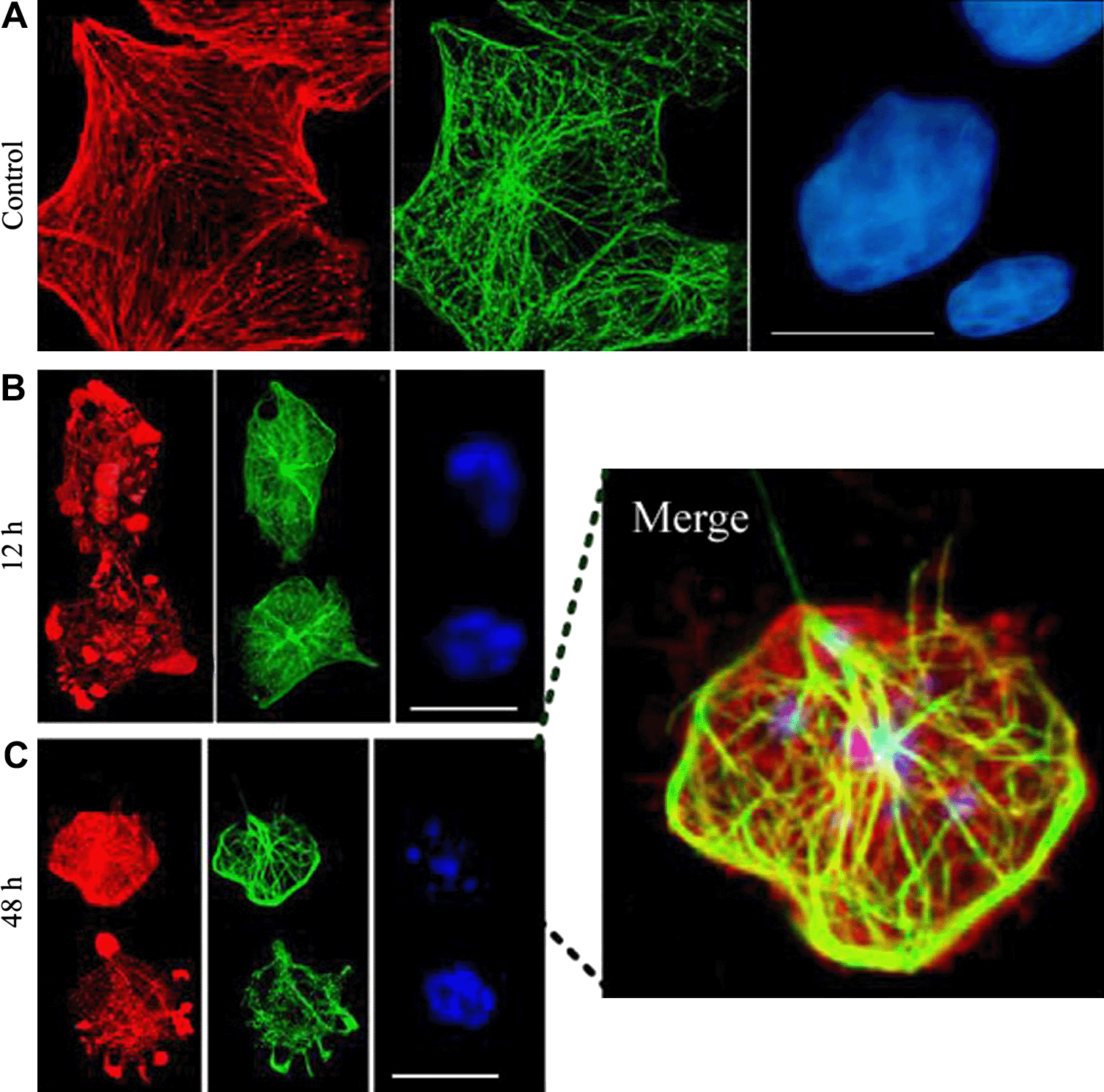
TCS-induced a typical MT ring structure in apoptotic HeLa cells. Confocal images of cells labeled with rhodamine–phalloidin (red), β-tubulin antibody (green) and DAPI (blue). Control cells (A), cells were treated with TCS for 12 h (B) and 48 h (C). A special ring structure of MT was formed in cells characterized with the fragmented chromatin (merged image). Scale bars, 20 μm.
3.3 Apoptotic MT ring structure was mediated by TCS-increased [Ca2+]c
To analyze the possible effects of temporal changes in cytosolic free calcium concentrations, [Ca2+]c was measured with the highly specific calcium probe Fluo-4. TCS caused transiently increase of the fluorescence signal. The peak occurred early (3 h) in cells treated with higher concentration of TCS (100 and 200 μg/ml). Additionally, the increase in cells with the treatment at the lower concentration (50 μg/ml) peaked at 24 h. The quantitative increase of [Ca2+]c was comparable to those induced by the positive control, ionomycin (Fig. 3A).

Apoptotic MT network mediated by intracellular calcium. Time- and concentration-dependent effects of TCS on [Ca2+]c (A). The presence of EGTA-AM disrupted the apoptotic ring MT structure (B). A significant increase of apoptotic nuclear fragmentation occurred in cells treated with calcium sequestration compared with control (C). *p < 0.05 compared with 0 h, significant differences among different stimulators are indicated with different letters (p < 0.05). Data presented as means ± SD of 3 independent experiments. Scale bars, 20 μm.
To assay whether the increased [Ca2+]c was causally involved in triggering the MF and MT arrangements, cells were concomitantly treated with TCS and/or EGTA-AM. Combined addition of TCS and EGTA-AM distinctly disrupted the typical MT ring structure, whereas no obvious effect was observed on the depolymerized MFs (Fig. 3B). Furthermore, a significant increase in apoptotic nuclear fragmentation and PARP cleavage expression continued to be found in cells with calcium sequestration compared with controls (Fig. 3C).
3.4 TCS-increased LDH release was enhanced by the combined treatment of EGTA-AM
To determine the functional significance of MT networks on plasma membrane integrity, the LDH release in cells treated with EGTA-AM was measured. TCS tended to increase LDH release, whereas no significant differences were observed until 48 h of treatment. In addition, TCS-increased LDH release was significantly reinforced by combined treatment of EGTA-AM, EGTA-AM alone having no effect on LDH release. Interestingly, TCS-caused LDH release was significantly enhanced by COL (Fig. 4).
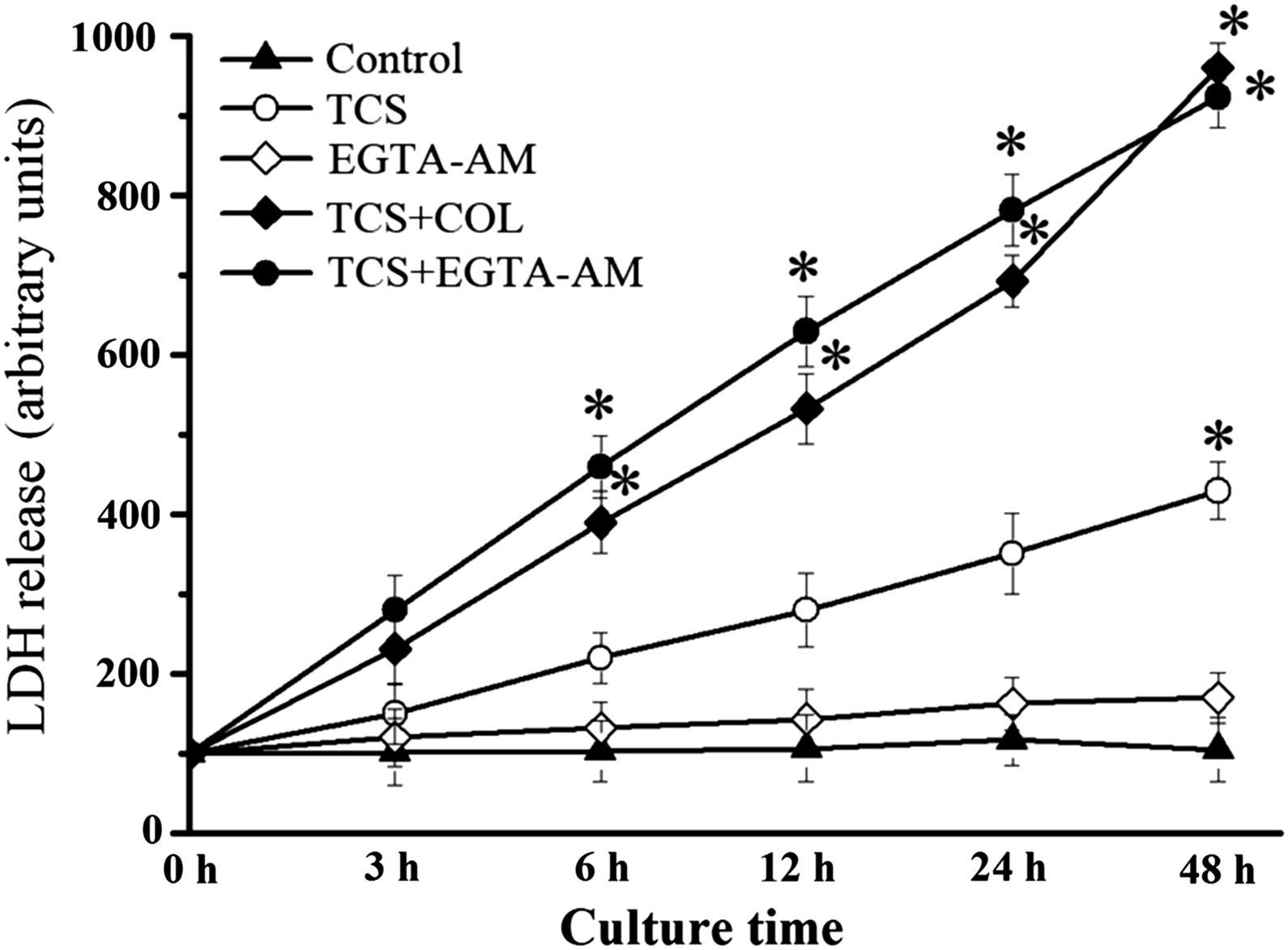
TCS-increased LDH release was enhanced by the combined treatment of EGTA-AM. *p < 0.05 compared with 0 h. Data presented as means ± SD of 3 independent experiments.
4. Discussion
The aim of this study was to explore the special effects of MT ring structure in TCS-induced HeLa cell apoptosis, and in particular the role of [Ca2+]c on the formation of the MT ring structure. An important question was whether the more proximal interactive characters existed among these potential changes in cell injury. HeLa cell injury caused by TCS was significantly enhanced in the presence of EGTA-AM, and sequestration of [Ca2+]c markedly disrupted the TCS-induced special MT ring structure. TCS tended to increase the LDH release, whereas addition of EGTA-AM/COL significantly reinforced the LDH release. These results confirm and extend our earlier reports that TCS induces cell apoptosis (Wang et al., 2007), cytoskeletal rearrangements (Wang and Li, 2007), and elevation of [Ca2+]c (Wang et al., 2008). However, these reports focused on their respective character of molecular events in cell apoptosis, the more proximal interactive features with MT rearrangement leading to cell death being unknown. We have now shown that TCS-elevated [Ca2+]c plays a pivotal role in maintaining plasma membrane integrity through MT ring structure formation in apoptotic HeLa cells.
These conclusions were derived from a number of observations seen in Figs. 1 and 2. These data are in accord with the earlier studies showing that MF depolymerization occurring at the onset of apoptosis, and that the MT network is required to maintain the later apoptotic morphology (Mills et al., 1999; Moss and Lane, 2006; Sanchez-Alcazar et al., 2007). Secondly, typical TCS-induced MT structure disruption occurs in combination with EGTA-AM (Fig. 3). Furthermore and importantly, TCS-increased LDH release is significantly reinforced by combined EGTA-AM or COL treatment (Fig. 4). Therefore, sequestration of the increased [Ca2+]c causing less protection of viability is related to plasma membrane permeability altered by the absence of the MT structure.
Normally, Ca2+ plays a key role in signal transduction in eukaryotic cells. A transient elevation in [Ca2+]c stimulates several Ca2+ binding proteins that elicit downstream signaling pathways. Increasing evidence has demonstrated that intracellular Ca2+ signal plays a pivotal role in the regulation of MT dynamics (Melander Gradin et al., 1997; Facanha et al., 2002). Higher levels of [Ca2+]c have been implicated in destabilizing MTs since the original study of Weisenberg (1972). Since that time, [Ca2+]c has been shown by bulk biochemical techniques to inhibit the assembly or induce the disassembly of MTs, and this has been demonstrated in dynein and purified tubulin systems (O'Brien et al., 1997; Sakato and King, 2003). Although Ca2+/calmodulin-dependent protein kinases (CaMKs) were thought to regulate the activity of actin-based myosin V (Karcher et al., 2001), recent reports have confirmed that an increase in calcium signaling has no effect on F-actin and myosin V distribution as well as the MF network during apoptosis (Adamikova et al., 2004; Sanchez-Alcazar et al., 2007). Based on this information, we propose that TCS-increased [Ca2+]c plays a critical effect on the formation of MT ring structure in apoptosis of HeLa cells.
Under physiological conditions, the cytoskeleton helps maintain plasma membrane integrity. Changes in the cytoskeletal network beneath the plasma membrane could therefore contribute to increase membrane permeability. TCS treatment combined with EGTA-AM disrupts the ring structure of MT, and enhances membrane permeability as well as the release of LDH. These results are in line with the increased permeability induced by colchicine in apoptotic cells (Sanchez-Alcazar et al., 2007). Hence, the weaker protection of cell viability due to TCS and EGTA-AM is related to the increase of plasma membrane permeability.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (No. 30800577), the Qianjiang Talent Project of Zhejiang Province of China (No. 2009R30057) and KC Wong Magna Fund in Ningbo University.




