Epidemiologic evidence linking oxidative stress and pulmonary function in healthy populations
Abstract
Respiratory health in the general population declines regardless of the presence of pulmonary diseases. Oxidative stress has been implicated as one of the mechanisms involved in respiratory dysfunction. This review was to evaluate studies that relate oxidative stress factors with pulmonary function among the general population without prior respiratory illnesses. The search yielded 54 citations. Twenty-one studies qualified for incorporation in this review. Owing to the heterogeneity of the review, studies were discussed based on identified oxidative stress factors responsible for pulmonary dysfunction. Oxidative stress biomarkers, including gene polymorphisms of nuclear factor erythroid 2-related factor 2, heme oxygenase 1, glutathione S transferase, superoxide dismutase, and lipid peroxidation products were involved in lung function decline. In addition, the antioxidant status of individuals in reference to dietary antioxidant intake and exposure to environmental pollutants affected oxidative stress and pulmonary function, as indicated by forced expired volume in one second, forced vital capacity, and forced expiratory flow at 25%–75%. This review indicated that oxidative stress is implicated in the gradual decline of lung function among the general population, and gene polymorphism along the antioxidant defense line and/or their interaction with air pollutants reduce lung function. Different polymorphic forms among individuals explain why the rate of lung function decline differs among people. Dietary antioxidants have respiratory health benefits in antioxidant gene polymorphic forms. Therefore, the genetic composition of an individual may be considered for monitoring and identifying people at risk of respiratory illnesses.
Introduction
Accumulating evidence from previous studies suggests that oxidative stress is a significant contributor to many respiratory diseases.1-3 However, oxidative stress with reduced pulmonary function among a healthy population has not been well appraised. The healthy people in this context include people who have not previously reported any respiratory diseases. Such people experience a gradual respiratory decline, which can be attributed to some oxidative stress-related factors, including exposure to air pollution, genetic polymorphisms, dietary factors, and lifestyle.
Oxidative stress results from an elevated amount of reactive free radicals or a depressed antioxidant defense system in the body. The lungs are regularly exposed to oxidants from ambient air and are liable to oxidative damage.4 The ability of an individual to ward off oxidative lung damage depends partly on endogenous antioxidant systems and exogenous antioxidant intake. Research has suggested that oxidative stress is implicated in the pathobiology of most respiratory disorders such as chronic obstructive pulmonary disease (COPD), bronchitis, asthma, emphysema, and cystic fibrosis.5-8
Owing to anatomical positioning, the respiratory tract is exposed to many environmental assaults, mainly air pollutants such as cigarette smoke and particulate matter, which have pro-oxidant effects.9 Air pollutants can activate other reactive oxygen species (ROS)-producing reactions in the lungs or directly elicit their oxidizing effect in the body. These pro-oxidants present in the ambient air stimulate oxidases and myeloperoxidases (MPOs) in neutrophils, macrophages, epithelial cells, and endothelial cells. ROS produced from these internal sources could cause mitochondrial DNA damage and disruption of the electron transport chain, leading to an elevated amount of oxidants in the internal milieu.10 The chemical constituents of the air pollutants also engage in direct oxidizing reactions. For instance, quinone/hydroxyquinone present in cigarette smoke tar reduces oxygen to superoxide anions (O2•−) with consequent formation of hydrogen peroxide with hydroxyl radicals,11,12 and also hypochlorites in the presence of hypochlorous acid.13 Gaseous puffs from cigarette smoke also contain nitric oxide radicals (NO•), nitrogen–II–oxide, and peroxynitrite (ONOO−).14 NO• spontaneously reacts with superoxide anion, and natural lipid peroxyl radicals (ROO•) to produce peroxynitrite and alkyl peroxynitrites (ROONO), respectively, both of which are highly cytotoxic radicals and could affect the healthy population close to smokers.15 Particulate matter containing iron-rich compounds engage in the Fenton reaction to generate hydroxyl radicals (•OH) in the lungs.16 Volatile organic compounds, including aldehydes, toluene and xylene, polychlorinated dibenzo-dioxins, and poly-chlorinated dibenzo-furans, have also been previously reported to impair pulmonary function through oxidative stress-related mechanisms.17,18
Despite the presence of endogenous antioxidants in the lungs, continual exposure to oxidants could overwhelm the antioxidant state of the respiratory tract. Previous reports have shown that antioxidant status plays a valuable role in maintaining respiratory health.19 Holistically, the antioxidant defense against free radicals can either come from endogenous or exogenous sources. Exogenous sources of antioxidants include thiol-containing molecules (e.g., N-acetyl-l-cysteine),20 vitamins (e.g., C, D, and E),21-23 polyphenols, and flavonoids. Endogenous sources of antioxidant enzymes include catalase (CAT), glutathione peroxidases, superoxide dismutase (SOD), and peroxiredoxins.24-27 In addition, the activation of some antioxidant genes contributes to the defense of the body against free radicals. Examples of antioxidant genes that influence respiratory health include those for heme oxygenase 1(HMOX1), glutathione, CAT, glutathione S transferase (GST), nuclear factor erythroid 2-related factor 2 (Nrf2), and SOD.28-30 A summary of oxidative stress factors involved in reduced pulmonary function through an interplay of oxidants and antioxidants is given in Fig. 1.
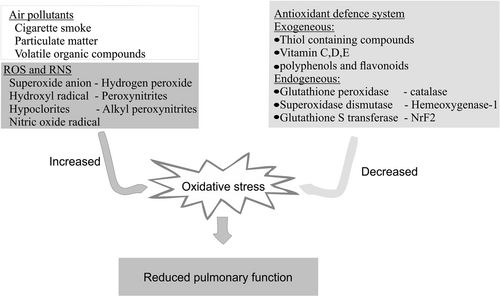
Mechanism of the interplay of pollutant, oxidant, and antioxidant in reduced pulmonary function. NrF2: Nuclear factor erythroid 2-related factor 2; ROS: Reactive oxygen species; RNS: Reactive nitrogen species.
The interplay between oxidants and antioxidants contributes to the changes in respiratory health status among diseased and non-diseased general populations. Previously, a review of epidemiologic studies associated with antioxidant status and obstructive airway events reported a potential protective role of dietary antioxidants dotted with some inconsistencies.31 However, a similar epidemiologic review considering other oxidative stress factors on lung function decline in the general population has not been studied.
Forced expired volume for the first second (FEV1) has generally been utilized in expressing lung physiology in epidemiological studies.31 Reduced FEV1 has been shown to correlate with increased mortality among people with or without respiratory diseases regardless of their smoking status.32 In this review, FEV1 has been selected to depict pulmonary dysfunction because it is reproducible and appropriate for pulmonary function analysis over time.33 Hence, studies correlating respiratory dysfunction (represented by FEV1 alteration) and oxidative stress in the general population were evaluated. Studies showing a relationship between impaired lung function test and oxidative stress in a population presumed to be healthy were discussed.
Search strategy
A literature search for studies that connect oxidative stress with FEV1 among general populations with no prior airway obstructive diseases was conducted. Peer-reviewed articles from PubMed and Google Scholar up to 2020 were used as primary references. Secondary references from the articles obtained from the preliminary search were also used. The following keywords were used for the literature search: “FEV1”, “oxidative stress”, “general population”, “antioxidant gene polymorphism”, and “lung function”. Population-based epidemiological studies correlating lung function decline with oxidative stress factors were included. Most of these were retrospective or prospective studies that lasted for at least one year. This search was conducted to examine the pattern of altered lung parameters among subjects following their daily lifestyle and environmental exposure. The search strategy ensured that studies involving only human subjects were included. In addition, studies that involved diseased states such as COPD and asthma, were excluded.
The search procedure yielded a total of 54 citations. After excluding non-human studies (n = 4),4 those involving subjects with prior respiratory illnesses (n = 13),13 those not relevant to the purpose of the review (oxidative stress parameters not evaluated or not population studies, n = 12),12 and duplicate studies (same articles drawn out from different search portals, n = 4),4,21 the remaining 21 studies met the inclusion criteria and were considered for the evaluation. A review of oxidative stress and lung function decline among healthy populations exposed to ambient air could result in different results because of the diverse categories of exposure evaluated.
Translating the outcomes into quantifiable outlines would have been simpler if it were a single result and exposure. The literature search results that met the search criteria and the aim of the review focused on the role of antioxidant gene polymorphisms, antioxidant dietary intake, and exposure to environmental pollutants as determinants of oxidative stress. In addition, the outcomes for measuring lung function decline were FEV1, forced vital capacity (FVC), and forced expiratory flow at 25%–75% (FEF25-75). The instruments used for assessing the independent variables were serum antioxidant levels, genotypes of antioxidant genes, questionnaires on food frequency, smoking, interviews, air and food samples for pollutants, and urinary and blood metabolites of oxidative stress. The outcome measured was primarily through spirometry and was for FEV1, FVC, or FEF25-75. The differences in the study exposure variables led to substantial heterogeneity among studies. Bearing this in mind, we evaluated the relationship between oxidative stress factors and lung function decline. We present studies related to the biomarkers of oxidative stress in lung function decline, followed by studies on the factors that influence oxidative stress in altered lung function in the general population.
Biomarkers of oxidative stress in altered lung function
Antioxidant gene polymorphism as a biomarker
Polymorphisms in antioxidant genes influence enzyme activities and result in varying functions that affect the overall antioxidant activity. This section presents some antioxidant gene polymorphisms and their effect on lung function in general population studies.
The transcriptional component Nrf2 regulates antioxidant activity.34,35 Nrf2 has also been reported to improve inflammation induced by elastase in some animal models of emphysema.36 The genetic variation seen in the promoter region of the Nrf2 gene has been associated with lung injury.37 A study among the general population of Japan (n = 915) was conducted to examine the association between the Nrf2 gene and FEV1 reduction stimulated by cigarette smoking.38 The study reported that the rs6726395 genotype of Nrf2 interacts with exogenous substances (cigarette smoke) and causes a gradual decline in FEV1. This suggests a gene–environment interaction in the pathophysiology of respiratory health decline among the general population.
Similarly, an Nrf2 controlled antioxidant enzyme, HMOX1, was studied for its possible interaction with environmental pollutants to elicit lung-function decline. Antioxidant enzymes such as HMOX1, act against oxidants and confer some protection on individuals exposed to air pollutants such as smoke. However, polymorphism in antioxidant genes for this enzyme may be responsible for the increased susceptibility of people exposed to environmental oxidants. Guenegou et al39 reported that the presence of a long (L) heme oxygenase gene promoter among heavy smokers could be responsible for their susceptibility. This study was conducted among 749 subjects from the European Community Respiratory Health Survey (ECRHS) and suggests that individuals, although exposed to the same type of pollutant, may develop a different degree of reduced lung function due to some genetic underpinnings. Conversely, polymorphisms in the HMOX1 (GT)n alleles were reported to not be associated with pulmonary function deterioration among smokers in the National Heart and Lung study conducted in Canada.40
Furthermore, studies showing the modulating effect of oxidative stress-related genes on air pollution were investigated to check for the interplay of more than one gene. About 669 adults were evaluated based on their exposure to tobacco smoke and respirable particulate matter (PM10) over 10 years. Single nucleotide polymorphism (SNP) genotyping was performed to identify oxidative stress-linked genes and mechanisms involved in lung physiology reduction. Of the 152 genes studied, alpha-synuclein (SNCA), Parkin RBR E3 ubiquitin-protein ligase (PARK2), and cysteine-rich secretory protein 2 (CRISP2) were identified with a potential modulatory role in lung function decline. While SNCA was reported to accelerate lung function decline, PARK2 attenuated FEV1 decrease, and CRISP2 was found to interact with PM10-induced lung function decline.41 From these long-term retrospective studies, it is evident that crosstalk exists between air pollutants and some oxidative stress genes, which predicts lung function decline.
To further delineate the role of environmental and genetic risk factors in the general population, a study investigated the role of antioxidant enzyme gene deficiency. The lack of the GST gene was studied to elucidate its effect on lung physiology in older adults. It has been reported that deletion of the GSTT1 genotype of GST correlates strongly with accelerated lung decline irrespective of the smoking habit of the subject, and this correlation was more significant in men than in women.42 This suggests that genetic mutations in the form of antioxidant gene deletion can cause accelerated lung function decline. Similarly, polymorphisms in the GST gene were linked with rapid lung function decline among smokers in a 5-year cohort study conducted in Canada.40 Polymorphisms in three submembers of the GST super-family (GSTM1, GSTT1, and GSTP1) were reported to correlate with reduced lung function as depicted by FEV1.40 This indicates a possible important role of GSTs in air-pollutant-induced lung function decline.
Previously, researchers have studied the effect of GST polymorphism on lung function among children taking acetaminophen for treatment since birth. The presence of some genotypes may expose children to dangerous respiratory outcomes of acetaminophen intake later in life. It was noted from this study that children who had taken twice as much acetaminophen and with the GSTP1 Ile/Ile allele had reduced lung function capacity (FEV1/FVC) compared to the others. Consequently, these children might be at risk of developing obstructive diseases such as asthma, later in life.43
Furthermore, the role of genetic polymorphism in HMOX1 and glutathione S-transferase pi (GSTP1) have been investigated in an ozone-induced reduction in pulmonary function among the adult population in Boston. Two single nucleotide polymorphisms (SNPs) from GSTP1 (Ile105Val and Ala114Val) and HMOX1 (Long and short GT repeat polymorphisms) were genotyped in a 10-year cohort study. The long (GT)n repeat in HMOX1 and Val105 in GSTP1 SNPs were noted to be associated with reduced lung function. Although exposure to ozone was associated with lung-function decline, these SNPs worsened it. Individuals who possess these two SNPs showed a more significant reduction in lung function.44
Genetic variation in the antioxidant pathway could increase susceptibility to air pollution and cause reduced lung function. A change in the glutathione synthesis pathway was correlated with the vulnerability of children to lung function deficits when exposed to air pollutants such as ozone, NO2, PM10, and carbon. The glutathione gene haplotype 0100000 was found to play a protective role against lung function decline induced by air pollutants.45
Dahl et al46 studied the effect of the SOD3 polymorphism on lung function decline. It has been shown that SOD3 inhibits oxidative fragmentation of the lung matrix, thereby preventing lung function decline.46,47 Subsequently, genotyping for the SOD3 was performed to evaluate its different polymorphic forms and the one responsible for pulmonary function. Two polymorphism types were identified: E1/I1 homozygote and E1/I1 heterozygotes. After evaluation of two sizeable general population studies in Denmark, Dahl, in 2008, noted that the E1/I1 homozygous form showed a correlation with lung function decline and a higher probability of developing COPD. This study revealed that antioxidant genes play a protective role in preventing lung function decline. However, different polymorphic forms of these genes determine individual susceptibility to altered lung function.46
A similar study of SOD was conducted in the Netherlands in the Vlagtwedde-Vlaardingen cohort study consisting of 1390 subjects.48 Two single nucleotide polymorphisms of SOD2 and SOD3 were analyzed for their association with bronchial hyperresponsiveness and lung function. The SOD2 polymorphism types were considerably associated with COPD menace in the general population. In contrast, SOD3 polymorphisms were correlated with whether or not there was a quick or slow decline in the vital lung capacity. The results of this study indicate the role of SOD3 in lung function decline in the general population.46
Genetic polymorphic forms of CAT, MPO, and SOD were examined for their role in phthalate exposure among some Korean elderly population. The urinary phthalate level was used as a marker to indicate the status of phthalate exposure. Genetic modifications in CAT, SOD and MPO were determined, and lung functions were evaluated using spirometry (FEV1/FVC, FEF25-75). Some polymorphic forms of CAT, SOD, and MPO genes were suggested to play a role in the decreased pulmonary function induced by phthalate, as indicated by high urinary phthalate levels.49
Wei et al50 investigated the tendency of genetic variants in the antioxidant defense system to modify the effect of lead and cadmium on the reduction in pulmonary function among 1243 coke-oven employees. Among the 2666 SNPs of 345 oxidative stress-linked genes studied, NQO1 rs2917670 was noted to influence a lead-induced reduction in lung function.50 There was no significant association reported for cadmium. Thus, it highlights the outcome of lead on pulmonary function decline and the involvement of a reduced impression of the antioxidant gene NQO1 produced by the rs2917670-C allele. A summary of findings related to antioxidant gene variants as a biomarker of oxidative stress in lung function decline is shown in Table 1.
| Origin and reference | Sample size, n | Instrument used in assessing exposure | Exposure studied | Adjusted outcome measure | Comments |
|---|---|---|---|---|---|
| Japan38 | 915 | Questionnaires, interviews, blood tests, pulmonary function test, and SNP of the Nrf2 gene. | Cigarette smoke, allergens, air pollution, Nrf2 genotype. | FEV1 | Nrf2 response to exogenous stimuli [smoking] influences lung function decline. Interaction between smoking and rs6726395 genotype of Nrf2 correlates significantly with lung function decline. |
| France39 | 749 | Questionnaires, lung function test, and HMOX1 promoter microsatellite polymorphisms. | Length inconsistency of (GT)n repeats in the HO-1 promoter. | A steep decline in FEV1, low FEV1, and FEV1/FVC ratio in heavy smokers. | Long [L] HO-1 gene promoter in heavy smokers is connected with susceptibility to generate airway obstruction. |
| Switzerland41 | 669 | Questionnaires on smoking information and exposure to PM10. | Smoking and PM10. | FEV1, FEV1/FVC, and FEF25-75 SNP genotyping. |
SNCA expedites FEV1/FVC, PARK2 attenuated FEV1 decline, and CRISP2 shows a significant interplay with PM10. The oxidative stress gene mediates PM10 and tobacco smoke-induced lung physiology decline. |
| Switzerland42 | 4686 | Interview on respiratory health, occupational and lifestyle exposures, and spirometry. | Smoking, gender, and GST genotype deletion. | FEV1, FVC, and FEF25-75 GST genotypes. |
GSTT1 deficiency correlates strongly with accelerated lung function decline. |
| Denmark46 | 35,635 9093 |
Genotyping for SOD3 polymorphism. Spirometry |
SOD3 polymorphism | FEV1 and FVC | For the two population studies, homozygous E1/I1 for SOD3 polymorphism exhibited more significant lung function decline than subjects with heterozygous E1/I1 polymorphism SOD3. |
| Netherlands48 | 1390 | Genotyping of SNP in SOD2 and SOD3. | SOD2, SOD3, and SNPs | Bronchial hyper-responsiveness FEV1 |
SOD2 is associated with BHR and COPD. SOD3 polymorphisms were associated with altered vital capacity. |
| Canada40 | 621 | Smoke, antioxidant gene, PCR for GSTM1, GSTT1, GSTP1, and HMOX1 genotyping. | Smoking, antioxidant genes GSTM1, GSTT1, GSTP1, and HMOX1. | FEV1 predicted | There was a relationship between the rapid decline of lung function and the presence of all the three GST polymorphisms. There is no relationship between HMOX1 (GT)n alleles and the rate at which pulmonary functions decline. |
| Australia43 | 620 | Interviews/questionnaires on acetaminophen use, spirometry, and GST genotyping. | Acetaminophen, and glutathione S transferase polymorphism. | FEV1/FVC | Increased acetaminophen use reduced FEV1/FVC. GSTM null and GSTT1 present was linked to reduced FEV1. Increased use of acetaminophen was connected to asthma at 18 years in children with GSTP1 Ile/Ile. |
| The United States45 | 2106 | Questionnaire, spirometry, integrated filter samples, and genotyping of glutathione synthesis genes. | Ozone, Nitrogen dioxide, particulate matter, and carbon. | FEV1, FVC, and FEF25-75 | Variation in GSS was connected with differences in susceptibility to adverse effects of pollutants on the functional growth in the lung. |
| Korea49 | 418 | Questionnaire, spirometry, urine phthalate metabolites, and genotyping. | Phthalate, CAT, SOD2, and MPO polymorphism. | FEV1/FVC and FEF25–75 | Urinary phthalate metabolic product levels are associated with declining pulmonary function, and CAT, SOD2, and MPO polymorphisms are suggested to modify it. |
| Boston44 | 1100 | Questionnaires, Ambient Ozone level, Antioxidant genotyping, and spirometry. | Ozone, HMOX1, and GSTP1 genotyping. | FEV1 and FVC | Increased ozone exposure was associated with FEV1 declined. Long (GT)n repeat in HMOX1 and Val105 in GSTP1 were connected with worsened lung physiology individually and collectively. |
| China50 | 1243 | Questionnaires, lung function test, and genotyping. | Lead, Cadmium from coke-oven, SNPs in oxidative stress-related genes. | FEV1 | A gene–environment interaction exists between NQO1 rs2917670 and Pb exposure on the decrease of FEV1. |
| The United States51 | 132 | Interviews, blood tests, and spirometry. | TBARS, serum bilirubin, glutathione, glutathione peroxidase, and trolox identical antioxidant capacity. | FEV1 | The lowest quartile of FEV1% showed higher levels of p-TBARS and low bilirubin compared to those in the more upper quartile of FEV1. |
| The United States52 | 2346 | Blood test | TBARS, glutathione, glutathione peroxidase, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid [Trolox]-identical antioxidant capacity. | FEV1, FVC | TBARS is inversely connected with FEV1 and FVC. Glutathione peroxidase is positively linked with FEV1 and FVC. Significance was seen in males but not females. |
- SNP: Single nucleotide polymorphism; Nrf2: Nuclear factor erythroid 2-related factor 2; FEV1: Forced expired volume in 1 second; PM10: Respirable particulate matter less than 10 mm; FEV1/FVC: Forced expired volume to forced vital capacity ratio; FVC: Forced vital capacity; CAT: Catalase; SOD: Superoxide dismutase; MPO: Myeloperoxidase; GSTP1: Glutathione S transferase pi; HMOX1: Heme oxygenase 1; SNCA: Synuclein Alpha; PARK2: Parkin RBR E3 ubiquitin-protein ligase; CRISP2: Cysteine-rich secretory protein 2; TBARS: Thiobarbituric acid reactive substances; GST: Glutathione S transferase; COPD: Chronic obstructive pulmonary disease.
Lipid peroxidation product as a biomarker
Different studies have suggested that exposure to oxidants could decrease lung function, while the intake of antioxidant vitamins could improve lung function. A survey evaluated the oxidant and antioxidant markers in blood samples from a general population and correlated this with their lung function. The subjects in this study were non-smokers. This study reported that subjects with lower FEV1 had a higher amount of thiobarbituric acid reactive substances (TBARS), an indicator of lipid peroxidation. In addition, serum bilirubin (an antioxidant marker) was lower among subjects with low FEV1. This indicates that elevated lipid peroxidation resulting from oxidative stress is linked with a decline in lung physiology.51
Similarly, a study in the general population (n = 2346) of Western New York reported an inverse correlation between lipid peroxidation product (TBARS) and FEV1. This supports the fact that an increased oxidant state that precipitates lipid peroxidation causes a reduction in lung function parameters. This study also showed a positive correlation between FEV1 and glutathione peroxidase, suggesting a beneficial role of the high antioxidant state on lung function.52 The summary of these findings can be seen in Table 1 on lipid peroxidation as a biomarker of oxidative stress in altered lung function.
Factors that affect oxidative stress and pulmonary function
Antioxidant status concerning dietary antioxidants intake
One of the ways to evaluate oxidative stress involves the measurement of antioxidant biomarkers in the serum. It has also been suggested that the antioxidant state of an individual can predict susceptibility to respiratory diseases. Antioxidant status depends on the consumption of antioxidant dietary sources, absorption, and bioavailability of antioxidant nutrients, alcohol intake, exposure to ultraviolet radiation, exercise, medication, environmental pollutants, and physiological state, including age.52 Some dietary antioxidant sources have been previously studied in population-wide cohort studies among healthy populations.
Flavonoids are known to regulate oxidative stress in vitro, therefore recommending a potential protective effect on the respiratory tract. A study of the administration of the six subclasses of flavonoids was evaluated using the GA2LEN Food Frequency Questionnaire in a general population of European adults. This study reported that an increased FEV1/FVC was associated with higher intakes of total flavonoids and pro-anthocyanidins, which depict a positive connection between flavonoid intake and proanthocyanidins in pulmonary physiology, and a negative association with spirometry limitation among European adults.53 This suggests that individuals who have consumed more flavonoids over time are likely to have better pulmonary function parameters than those who have not.
Polyphenol, a dietary compound with known antioxidant properties, was also studied to assess the effect of its consumption on lung physiology among the general population of the Molise region of Italy. The dietary intake of about 4551 women and 5108 men were evaluated using the European Prospective Investigation into Cancer and Nutrition-Food Frequency Questionnaire (EPIC-FFQ) and the Eurofir-eBASIS were used to calculate polyphenol intake. This study showed a positive correlation between polyphenol intake and lung function parameters, suggesting that overall polyphenol content in diet could predict better respiratory health among the general population.54 However whether similar correlations will be observed among younger people aged <35 years remains to be unraveled because the reported study focused on subjects older than 35 years.
Furthermore, a study conducted by the European Community Respiratory Health Survey [ECRHS] investigated the effect of different antioxidants on lung function decline. It was hypothesized that a low level of antioxidants could result in a higher risk of FEV1 reduction, as there would be reduced protection from oxidative stress. Serum beta-carotene (β-carotene), alpha-carotene, vitamin A, and vitamin E were evaluated by Guénégou and his colleagues in this 8-year study. β-carotene was observed to be associated with a slower reduction in FEV1 compared with other antioxidants studied. Conversely, the most severe lung function decline was observed among heavy smokers, combined with a low level of beta-carotene and vitamin E.55 A summary of these results is presented in Table 2.
| Origin and reference | Sample size, n | Instrument used in assessing exposure | Exposure studied | Adjusted outcome measure | Comments |
|---|---|---|---|---|---|
| 18 countries in Europe53 | 55,000 | GA2LEN food frequency questionnaire [FFQ]. | Flavonoids | FEV1/FVC | Total flavonoid intake correlates positively with ventilatory function and negatively with spirometric restriction. |
| Italy54 | 4551 women and 5108 men | EPIC-FFQ for dietary assessment and Eurofir-eBASIS for polyphenol intake. | Polyphenol intake | FEV1, FVC, FEV1% predicted, and FVC% predicted. | Polyphenol intake is positively associated with lung function parameters. |
| France55 | 1194 | Blood test for antioxidants using high-performance liquid chromatography and spirometry. | Serum beta-carotene, alpha-carotene, and vitamins A and E. | The mean annual decrease in FEV1 is 29.8 mL/year. | An increase in beta-carotene between the two surveys was associated with a slower decline in FEV1. No relationship was seen between alpha-carotene, vitamin A, or vitamin E, and FEV (1) reduction. |
| France56 | 523 | Serum samples and smoking status. | Smoking, beta-carotene level, and heme oxygenase genotype. | FEV1 | A high level of beta-carotene might disrupt the equilibrium effects on FEV1 decline. |
| Netherlands57 | 2542 subjects | Semi-quantitative food frequency questionnaire (Vitamin C intake), and SNP for glutamate-cysteine ligase. | GCL polymorphism, smoking, and vitamin C. | FEV1 and FVC | GCLC polymorphisms were considerably connected with lowered pulmonary function levels in interplay with pack-years smoked. The effects are more among subjects with low vitamin C intake. |
| South Korea17 | 154 | Urine and blood samples. | Metabolites of VOC, MDA, and 8-oxo-29-deoxyguanosine. | The decline in FEV1, FEV1/FVC, and FEF25-75. | Metabolites of toluene and xylene are associated with lung physiology decline and oxidative stress markers. |
| China18 | 201 foundry workers and 222 controls. | Air, food, and serum samples. | PCDD/Fs, and serum content of PCDD/Fs. | FEV1 decrease, and FVC decrease. | PCDD/Fs exposure was connected to a 0.47 L decline in FVC and a 0.25 L reduction in FEV1. |
- FFQ: Food frequency questionnaire; PCDD/Fs: Polychlorinated dibenzo-dioxins and polychlorinated dibenzo-furans; VOC: volatile organic compounds; MDA: Malondialdehyde; FEV1/FVC: Forced expired volume to forced vital capacity ratio; FVC: forced vital capacity; FEV1: forced expired volume in 1 second; GCL: Glutamate-cysteine ligase.
Antioxidant diets modulate the effect of antioxidant gene polymorphism
Previously, it has been reported that the long heme oxygenase promoter genes correlate with susceptibility to lung function decline.39 A subsequent study revealed a beneficial effect of the consumption of antioxidant nutrients regardless of the presence of L-allele heme oxygenase polymorphism. Dietary use of a high level of β-carotene has been reported to counteract L-allele polymorphism in heme oxygenase genes. Subjects who reported a high level of β-carotene consumption showed better lung function parameters regardless of the presence of the heme oxygenase L-allele. This same effect was not observed in subjects with low β-carotene concentrations.56 Thus, the significance of antioxidant state derived from dietary consumption in modulating lung function decline as a result of gene polymorphism in the general population was demonstrated.
Similarly, the role of antioxidant enzyme genes such as glutamate-cysteine ligase (GCL), or antioxidants such as vitamin C, was investigated by Siedlinski et al57 in 2008 in smoking-induced oxidative stress in pulmonary dysfunction. This study consisted of two cohorts with exclusively different subjects in the same country. Polymorphism in the GCLC subunit of GCL correlated significantly with diminished lung function in connection with pack-years smoked. However, this effect was notably worse among subjects with low vitamin C intake than among those with higher vitamin C levels.57
Two studies were identified on the role of antioxidants in modulating the effect of antioxidant gene polymorphism on pulmonary function, and a summary of the findings is presented in Table 2.
Environmental air pollutant and pulmonary function
Many studies have reported the adverse effects of air pollutants from different sources on pulmonary function.58-60 One of these studies investigated evidence linking volatile organic compounds with impaired lung function and oxidative stress in the elderly. The study was conducted in three communities in South Korea. Indoor amounts of toluene, benzene, ethylbenzene, and xylene were estimated, and urinary concentrations of the metabolites of different volatile organic compounds (VOCs) were examined. Furthermore, oxidative stress markers (MDA and 8-oxo-dG) and pulmonary function variables (FEV1, FEV1/FVC, and FEF25-75) were evaluated. This study reported an association between the metabolites of VOCs [Toluene and Xylene] and reduced lung function (FEV1, FEV1/FVC, and FEF25-75). The urinary concentration of hippuric acid and methyl hippuric acid (metabolites of toluene and xylene) correlates with oxidative stress parameters, which suggests that environmental exposure to VOCs, especially toluene and xylene, could be harmful to the respiratory health of the elderly population.17
A case–control investigation was conducted in China among foundry workers and the general residents of central China to evaluate the effect of polychlorinated dibenzo-dioxins and polychlorinated dibenzo-furans (PCDD/Fs) on lung pulmonary decline. The serum levels of these chemicals were studied to assess the body load and then compared with FEV1 and FVC for lung function decline. It was found that an increase in PCDD/F hazard and serum PCDD/F content correlated with FEV1 and FVC decline. In addition, urinary 8-hydroxy-2-deoxyguanosine (8-OHdG) is an indicator of oxidative DNA damage associated with lung function decline.18 This study suggests that exposure to PCDD/Fs (common among foundry workers) was associated with decreased lung function parameters, and oxidative mediated DNA damage could be involved in this event. A summary of the studies correlating environmental pollutants with lung function decline is presented in Table 2 under the factors influencing oxidative stress in lung function decline.
Conclusions
Oxidative stress-related factors play a role in the gradual decline in lung function over time among the general population. Different polymorphic forms of antioxidant genes among individuals contribute to environmental oxidant/pollutant susceptibility, highlighting gene–environment interactions in lung function decline. However, some studies suggest that adequate antioxidant intake could modulate the effect of these gene polymorphisms. In this review, the effect of antioxidant gene polymorphism and the beneficial role of antioxidants in reducing lung function decline among the general population is prominent. Notably, β-carotene, polyphenols, flavonoids, and vitamin C are suggested to be good sources of antioxidants with respiratory health benefits.
Therefore, clinicians need to consider antioxidant gene polymorphic forms in designing appropriate therapy for obstructive lung diseases. Another translational benefit of this review is that the knowledge of different antioxidant gene polymorphisms among the general population could help clinicians understand the susceptibility of an individual to obstructive respiratory diseases and therefore assist clinicians in making decisions about the management of some respiratory diseases. Specific risk alleles in Nrf2, GST, and HMOX1 should be considered when evaluating patients at risk of pulmonary diseases. This review is also of public health importance as it creates awareness of the benefits of including antioxidants in diet and consuming antioxidant supplements to prevent pulmonary function loss. Additional studies on antioxidant genes are required to elucidate the genetic determinants of lung functions among those exposed to environmental oxidants and the general population. More prospective, long-term cohort studies are needed in other regions of the world to form a robust body of knowledge, which will provide a better understanding of a holistic approach to preventing the growing number of pulmonary diseases.
Conflict of interest
None.




