Chronic lung disease, lung regeneration and future therapeutic strategies
Abstract
Chronic lung diseases have been recognized as one of the world's leading causes of death in recent decades. Lacking effective treatments brings the patients not only bad quality of life but also higher risk for lung cancer development. By increasing the understanding of deeper mechanism of how lung develops and regenerates, researchers now focus on studying lung regenerative medicine, aiming to apply different and more efficient therapies to treat chronic lung diseases. This review will provide a wide picture of both basic lung developmental, regeneration mechanism and different designed strategies for treating chronic lung diseases in the future decades.
Introduction
Chronic degenerative lung diseases are recognized as untreatable pathological diseases, significantly affecting both life span and life quality of human-beings. Chronic obstructive pulmonary disease (COPD) presents a group of lung diseases which block the airflow and lead patients difficult to breathe, is predicted to be the third leading cause of death by the end of 2020s according to the World Health Organization. It is also widely established that patients with COPD are at increased risk for the development of primary lung cancer. Based on current medicine and technologies, the only treatment for COPD is lung transplantation, while there is always acute shortage of donor lungs. It has been reported that adult lung has the capacity for both regeneration and regrowth.1, 2 However, loss of alveolar units and the emerging of pulmonary fibrosis often trigger repeated episodes of injury, thus resulting in unceasing consumptions of endogenous lung repair capacity.3 This makes the authentic therapeutic lung regeneration still a long-term goal. Recent emerging regenerative medicine has shed a light on the path for finding a new way to treat the COPD instead of seeking lung transplantations. Approaches include not only generating new lungs in vitro and replenishing exogenous stem cells to the damaged parts,4 but also using pharmacologically selection for promoting the healthy cells survival other than damaged ones. All these methods are based on numerous basic researches focusing on lung developmental and regeneration pathways. Therefore, this review will mainly discuss the current and newest lung regrowth and developmental mechanism discovered, with the possibilities of serving future applications for the therapeutic lung regeneration.
Developmental mechanism of lung
The discussion of the lung regeneration mechanisms requires a thorough understanding of how lung generates during normal developmental process. The majority researches focusing on mammalian lung development are conducted by using mice models. With the effort of numerous studies, now we know that the mammalian lung development can be divided into three phases: (1) Specification of lung progenitors; (2) Branching morphogenesis; (3) Sacculation and alveolarization.5
The initiation of lung development is relied on the specification of lung endoderm which derives from the ventral side of anterior foregut endoderm (Fig. 1). The biomarker for this phase is the highly expressed NK2 homeobox 1 (Nkx2.1),6 who is one of the developmental pioneer transcription factors family. Embryonic day 9 (E9.0) in mice is the approximate time for this event happening. Around embryonic day 9.5 (E9.5), a proximal-distal patterning of endoderm begins with the differential expression of sex determining region Y-Box 2 (Sox2). Then the Sox9 and inhibitor of differentiation 2 (Id2), which determine the most distal tips of branching epithelium, are proximally demarcated by the expression of Sox2. This is the signal of entering the step 2 phases. The cells that highly express the Sox9 and Id2 are multipotent before embryonic day 13.5 (E13.5), and will be restricted in the fate for generating alveolar type 1 and alveolar type 2 cells (AT1 and 2 cells) after E13.5.7 This point is one of the important dates for various lung regeneration models, while the differentiation of the Sox9+, Id2+ cells contribute essentially for generating functional alveolar gas exchanging units. However, recent researches indicate that a bipotent progenitor may be responsible for the generation of lung alveolus, the detailed mechanism still is little understood.
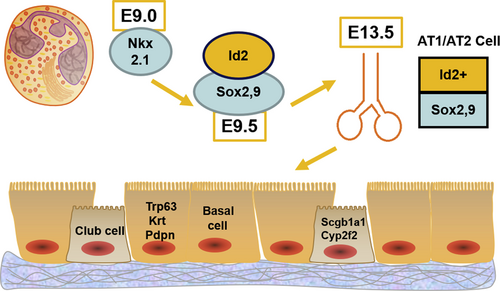
E9.0- E13.5 mice embryonic specification of lung progenitors. Scheme for mice embryonic specification of lung progenitors, from E9.0- E13.5, a continuous process of different pioneer transcription factors that differentially expressed, leading to the formation of adult lungs. E9.0: embryonic day 9; Nkx2.1: NK2 homeobox 1; Id2: inhibitor of differentiation 2; E13.5: embryonic day 13.5; AT1/AT2 cell: alveolar type 1 and alveolar type 2 cell; Sox2,9: sex determining region Y-Box 2,9; E9.5: embryonic day 9.5; Trp63: tumor protein P63; Krt: keratin; Pdpn: podoplanin; Scgb1a1: secretoglobin family 1A member 1; Cyp2f2: cytochrome P450 family 2 subfamily F member 2.
Airway epithelial lineages are determined by the Sox2 progenitors, whereas other lineages are also produced during the Sox2 early endoderm differentiation, this majorly reflects the developmental process reaches phase 3. Tumor protein P63 (Trp63), keratin (Krt) and podoplanin (Pdpn) are the markers of basal cells, who generate multi-ciliated cells after airway injury from adult trachea.8 Club cells in which secretoglobin family 1A member 1 (Scgb1a1) and cytochrome P450 family 2 subfamily F member 2 (Cyp2f2) are highly expressed, with the basal cells will generate trachea and bronchi. In addition to the basal cells, Sox2 cells also generate pulmonary cells secreting neuro-endocrines, who play an essential role in regulation of postnatal immune response in the lung,9 in which calcitonin related polypeptide alpha (Cgrp) is expressed as a marker. Those cells will be enriched in the bronchioles later. Meanwhile, development of airway epithelium is regulated by Notch signaling, which also plays a major role in the total embryo development. Notch act with both ciliated and non-ciliated cell fate occurs in proximal progenitors. It can silence the ciliated program in the cells that are going to continue expanding and differentiating into secretory cells.10 Last but not least, subsets of secretory cells are also reported to be having self-renew and differentiating ability after injury.11, 12
Developmental pathways in lung regeneration
Multiple progenitor lineages and stem cells are involved in the lung regeneration after injuries.13-15 The activation of Trp63 + basal cells in the large airway and differentiation into secretory and multi-ciliated epithelial cells are mainly determined and regulated by Notch signaling pathway.16 Different Notch protein regulates the regenerative signaling differently, as Notch-2 is necessary for alveologenesis,10 where Notch-3 suppresses basal cell over-expansion.17 Notably a rare population of progenitors termed lineage-negative epithelial progenitors (LNEP), are reported to migrate from the airways to the inflamed interstitial space.18, 19 It remains unknown how many subpopulations of LNEPs there are. However, recent researches show that they can either regenerate alveolar epithelium, or form pod-like structures in which the alveoli are not repaired. Those fate decisions can also be regulated by Notch pathway, as well as hypoxia signal and wingless-int (Wnt) signaling.20
In the more distal lung airways, the expansion of bronchioalveolar stem cells (BASC), which were recently identified and thought to be the tissue stem cells that lead to the repopulation of the terminal bronchiolar and the alveolar epithelia after injury,21 is reported to be regulated by Wnt signaling pathway, with the process involved with GATA binding protein 6 (Gata6), a key transcription factor in lung development.22 BASC differentiation was reported to be regulated by bone morphogenetic protein (BMP) pathway, which is initiated by expression of BMP4, promoting thrombospondin 1 (TSP-1) expression in the lung endothelium.23 Those findings demonstrated that all these developmentally conserved pathways also work efficiently in regenerative process, towards both epithelium and supporting mesenchymal lineages. For instance, it was widely established that sonic hedgehog signaling (Shh) plays a key role in the adult lung mesenchymal maintenance and quiescence. Peng et al27 showed that inhibition of Shh signaling can promote epithelial proliferation at homeostasis or after injury.
Epigenetic pathway in lung development and diseases
Besides of the conserved developmental pathways, epigenetic regulation is also involved in and associated with adult lung diseases, such as COPD. Histone codes include DNA methylation, histone methylation and histone acylation. During lung regeneration and development, histone acetylation, which is regulated by histone acetyltransferase (HAT) and histone deacetylase (HDAC) protein families, presents certain patterns among different developmental stages. A recent report showed that asthma patients have a higher HAT activity and a lower HDAC activity in airway epithelium.24 A correlation is also built in COPD patients, showing that decreased HDAC2 expression is related to disease severity. This implies that genetic manipulation of HDAC proteins could be used for addressing the lung regenerative medicine. Other than affecting COPD patients, HDAC2 also plays an important role in regulating the expression of Interleukin 6 (IL-6), one of the essential cytokine members for inhibiting the alveolar response from long-time injury.25 In addition to histone acetylation, protein arginine methylation, which is recognized as a relative novel posttranslational modification, also contributes to the lung disease development. Asymmetric dimethylarginine (ADMA), whose producing is regulated by protein arginine methyltransferases (PRMT), influences pulmonary cell function thus determining the development of chronic lung diseases, such as COPD and pulmonary arterial hypertension (PAH). This suggests a good future in the investigation for evaluating the functional contribution of arginine methylation in lung homeostasis and disease by genetic modifying PRMT protein families.26
Animal models for studying lung regeneration
Though the cell-based experiments showed that multiple dysregulated developmental pathways could have great impacts on lung regrowth and regeneration, the in vivo animal models are still characterized as golden standards for evaluating their physiological significance. The mouse model has been proven to be an excellent model for studying both the lung regenerative mechanisms and drug efficacy, pharmacodynamic and toxicological profiles. A mouse model of influenza-instigated acute lung injury mimics many aspects of human influenza infection including the keratin-5-expressing epithelium and robust immune responses.27 However, there are still insurmountable obstacles for studying lung disease and regeneration by using mice due to its incapability of reflecting all aspects of human conditions. Bissig et al have developed several types of human liver chimeric mice by using the genome engineering and editing system clustered regularly interspaced short palindromic repeats (CRISPR/Cas9), which implied a good research direction for creating and manipulating human lung chimeric mice for future studies.28, 29 All these mice models provide critical information for lung regeneration after chronic and acute injuries. Knowledge gained from these in vivo models is also useful implication in pre-clinical lung regenerative study trials.
Directions to fulfilling therapeutic lung regeneration
Our knowledge is still far from sufficient for understanding the total mechanisms of how lung develops and regenerates, though recent surprising findings continue to characterize those endogenous mechanisms very well. To translate those research results into therapies against chronic lung diseases such as COPD, both researchers and doctors have developed three main therapeutic lung regeneration strategies, including (1) Engineering new tissues/organs in vitro30; (2) Transplanting healthy stem/progenitor cells into a damaged lung; (3) Low dose of bleomycin treatment31(Fig. 2). Though all those three methods remain in the process of early development, several tools have showed their capacities for providing certain possibilities to success. The advent of induced pluripotent stem cell (iPSC)32 brought amazing opportunities for the researchers to evaluate the ability of human lung progenitor cells for self-renewal, differentiation and proliferation. iPSCs have been already derived at increased efficiencies from several easily accessible human cell types, including blood cells and fibroblasts etc.33-36 To avoid using lentivirus transfection, small-molecules based on iPSCs are also available after several studies.37-40 Recent findings showed that iPSCs can be differentiated into lung epithelial cell lineages, including both the surfactant protein C (Sftpc)+AT2s and secretoglobin family 1A member 1 (Scgb1a1)+ secretory cell lineages.41, 42 More recently, a research group also found a direct way to differentiate iPSCs into functional lung alveolar epithelial cells by regulating Nkx2.1 + primordial progenitors with three-dimensional cell culture methods.43 All those results hint the strong possibility that extracting fibroblast cells from patients and then culturing them in the medium, with finally inducing them differentiating to lung cell lineages, representing a probability of low level immune responses. Another major advantage of patient-derived iPSCs is their capacity to harbor genetic mutations affecting respiratory function, like cystic fibrosis or alpha-anti-trypsin deficiency.44
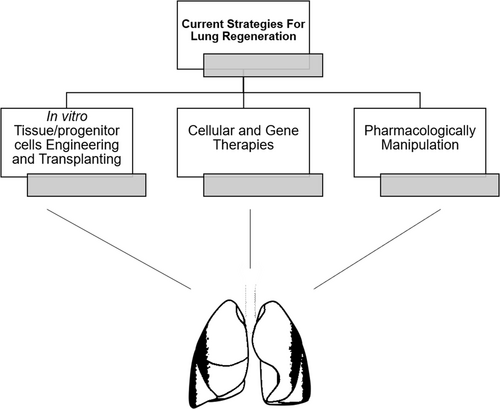
Strategies designed for treating chronic lung diseases in future, though all three are still in early developing stages.
The second strategy relied on genome engineering technology and gene/cell therapy. iPSCs are attractive and useful. However, it is also widely acknowledged that iPSCs have safety problems such as high tumorigenesis rate. Although mesenchymal stromal/stem cells have not been reported to be beyond a small anti-inflammatory paracrine function, they still have the possibility for lung regeneration which needs future researches. Recently, CRISPR/Cas9 has been developed as an efficient genomic editing tool. A Chinese research group reported a modified type of T-cell by CRISPR/Cas9 used for treating metastatic non-small cell lung cancer in 2016.45 This clearly implied that the gene therapy combined with immunotherapy may increase the chance for beating chronic lung diseases based on precision medicine methods. The third therapeutic strategy showed in Fig. 2 is the pharmacological manipulation of the lung endogenous developmental and repair pathway. This could be very attractive, if drugs, such as small molecules with certain nano-particles, can be delivered directly to the lungs to activate naturally-occurring processes that could function efficiently for regeneration and repair. However, the concern is that it is still hard for lung homeostatic maintenance by taking drugs, which can result in certain possibilities of unbalanced proliferating status. Together, despite the facts that all three strategies are in early development stages, tremendous progress has been made already in treating chronic lung diseases based on lung regeneration methods.
Summary and conclusion
Basic lung developmental and regenerative medicine studies reveal the mysteries of human lung developmental process as well as shed light on the path for treating the uncontrollable diseases such as COPD or even lung cancers. Other than traditional lung transplantation, three different strategies designed for future remedies towards COPD remain a long-term goal to fulfill. Future researches should focus on not only discovering new potential target for lung regeneration, but also a better disease model, which can further accelerate researches into novel therapeutic approaches for chronic lung diseases.
Conflicts of interest
The authors declare no competing financial interests.




