An immunohistochemical study of metroplastic surgical specimens from patients with a septate uterus
Abstract
Purpose
To elucidate the etiology of recurrent pregnancy loss in patients with congenital uterine anomalies, an immunohistochemical technique was used to quantitatively evaluate the vascular arrangement of septate uteri with respect to vascular density and morphology.
Methods
Nine specimens obtained from patients who had undergone metroplastic surgery for the treatment of a septate uterus and 10 control specimens from patients who had undergone a hysterectomy because of cervical carcinoma were used in this study. Formalin-fixed paraffin-embedded uterine specimens were then immunostained for CD34, which is specifically expressed in vascular endothelial cells.
Results
The mean blood vessel count (mean ± SD) for the myometrium was 149.7 ± 22.7/field in the septate uteri and 162.2 ± 36.4/field in the control uteri; these values were not significantly different. However, the total vessel cross-sectional areas, as evaluated quantitatively using the KS400 image analysis system, were 10350.4 ± 1024.3 μm2/field for the septate uteri and 12002.9 ± 2232.3 μm2/field for the control uteri; these values were significantly different (p < 0.05). The vessel morphology expressed by vessel irregularity and deformity showed a characteristic change in the septate uterus.
Conclusions
A significant difference in the distribution of the blood vessels existed between the septate and control uteri, presumably impairing blood flow in the myometrium and the adverse pregnancy outcome.
Introduction
In women, uterine development starts during the 4th to 5th week of gestation with the emergence of the metanephric ducts and their connection with the cloacae, followed by the union of the two Müllerian ducts during the 10th week of gestation. Fusion starts in the middle part of the conjugated area and extends caudally. By the 20th week of human gestation, the typical human uterine shape has been completed as a result of cellular proliferation in the upper part of the original uterus and the dissolution of the cells in the lower part [1]. Thus, the fusion of the Müllerian ducts, which develop into a single reproductive tract in humans, is induced at three different embryonal levels over three different periods of time. Therefore, uterine malformations can easily be induced during these complicated developmental processes.
Congenital uterine anomalies can be clinically diagnosed utilizing hysterosalpingography, ultrasonography and hysteroscopy as well as magnetic resonance imaging. The reported incidence of congenital uterine malformations varies between 10–15%, presumably because of differences in diagnostic procedures [2, 3]. A few studies have examined how congenital uterine anomalies may induce recurrent pregnancy loss, e.g., by limiting the space available for fetal growth in utero [4], abnormal vascularity [5], suppression of enzymatic activity [6], and hormonal response of the endometrium [7]. Nevertheless, the indications and efficacy of surgical treatment, such as metroplastic surgery, for uterine cavity deformity after recurrent pregnancy wastage remain controversial.
In this study, the vascular arrangement of the septate uterus was examined using an immunohistochemical technique to elucidate how congenital uterine malformation induces reproductive wastage.
Materials and methods
Patients
An immunohistochemical study was performed using formalin-fixed paraffin-embedded uterine fundal longitudinal sections. A total of nine patients (Table 1) with septate uterus, who had consecutively undergone transabdominal metroplasty between March 1999 and March 2002 at Tokai University Hospital, were examined in this study. These patients had visited our Department of Obstetrics and Gynecology because of recurrent reproductive losses or infertility. The mean age of the patients at the time of the metroplasty procedure was 33.5 ± 3.8 years, ranging from 27 to 40 years age. The preoperative reproductive history of the patients included 2 infertilities, 1 premature delivery, and 6 repeated spontaneous abortions (2–5 times). The number of previous spontaneous miscarriages was 2.0 ± 1.6. Other causes of infertility and abortion were screened out using routine examinations for infertility, such as sperm analysis, postcoital test, antiphospholipid antibody analysis, endocrine evaluation, chromosomal analysis and ultrasonographic examination. The diagnosis of uterine malformation was based on a combination of hysterosalpingography, hysteroscopy and ultrasonography, and was classified in accordance with the American Fertility Society classification for uterine anomalies [8] and by our proposed X/M ratio [9]. As controls, 10 tissue specimens of the uterine fundal portion were obtained from women (mean age 35.9 ± 3.9 years, range 28–40 years) who had undergone a hysterectomy because of cervical carcinoma (Table 1). The number of previous pregnancies was 2.3 ± 2.2, and the number of previous abortions was 0.1 ± 0.3. The detailed clinical stages of the controls were as follows: 3 patients had carcinoma in situ, 5 patients presented with stage I, 1 patient presented with stage II, and 1 patient presented with stage IIIa. The clinical staging was based on the clinical staging of the International Federation of Gynecologists and Obstetricians (FIGO) for invasive cervical cancer. All the patients provided their written consent.
| Septate uterus (n = 9) | Controls (n = 10) | p value | |
|---|---|---|---|
| Agea | 33.5 ± 3.9 | 35.9 ± 3.8 | NS |
| Age range (years) | 27–40 | 28–40 | – |
| No. of previous pregnanciesa | 2.0 ± 1.6 | 2.3 ± 2.2 | NS |
| No. of previous abortionsa | 2.0 ± 1.6 | 0.1 ± 0.3 | p < 0.01 |
- NS not significant
- aMean ± SD
Immunohistochemical technique
All the tissue specimens were fixed in 10% buffered formalin and embedded in paraffin wax. Tissue blocks were sectioned (4 μm) and the sections were mounted on glass slides. Immunostaining was performed as follows. After dewaxing in xylene and dehydration in graded ethanol, the sections were exposed to 3% hydrogen peroxide for 15 min. After washing with phosphate buffered saline, they were then incubated in 10% normal bovine serum for 5 min, followed by incubation in a humidity chamber at room temperature with a primary antibody against CD34 (mouse monoclonal antibody QBEnd/10; Novocastra Laboratory, Newcastle, UK) for one night. Biotinylated anti-mouse immunoglobulin and anti-rabbit immunoglobulin (Dako LSAB kit; Dako Japan, Kyoto, Japan) were used as the second antibody and peroxidase-labeled avidin (Dako Japan) was used. Finally, for the chromogen, diaminobenzidine (Dako Japan) was used as the substrate. The sections were counterstained with hematoxylin.
Analysis of microvessel density and morphology
The immunostained sections were viewed using Carl Zeiss Axiophot microscopy (Carl Zeiss, Oberkochen, Germany) under low power (×8 objective), and a computer-assisted image analysis was performed using the KS400 image analysis system (Carl Zeiss) with the following image analysis parameters: vessel count, area, maximum diameter, minimum diameter, area percentage. For the analysis of microvessel morphology, the parameters of the F-circle (4 × area/π × max diameter2) and the F-shape (min diameter/max diameter) were determined [10]. The detailed process was as follows: the full immunostained sections were examined under low power (×8 objective) to identify the largest vascular area. For each section, the five most vascular-rich areas around the largest vessel were randomly selected. A ×20 objective field (approximately 0.19 mm2) (Fig. 1) in each of these five areas was examined using the KS400 image analysis system (Carl Zeiss). Each stained lumen was regarded as a countable microvessel. Even if only a single CD34-positive cell or CD34-positive cell cluster was visible with no lumen, the cell or cell cluster was interpreted as a microvessel. Stained spots with a maximum diameter <20 μm were excluded from the count.
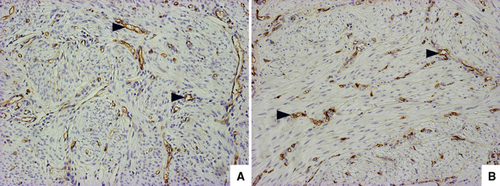
An example of the region around the largest vessel for measuring the vascular morphometric variables in myometrium immunostained with CD34 (×20 objective). a Blood vessels assessed by immunostaining for CD34 in normal myometrium of a patient with cervical carcinoma. b Blood vessels assessed by immunostaining for CD34 in myometrium of a patient with septate uterus. Intense brown CD34-positive cells are vascular endothelial cells (arrowheads)
Statistical analysis
A database file was set up using Microsoft Excel for Windows (Microsoft USA) to facilitate data entry and retrieval. Statistical analysis was performed using Statview 5.0 (SAS Institute Inc, Cary, NC, USA) for Windows. Data are presented as the mean ± SD. A one-way analysis of variance was used with the Scheffe test as a post-hoc comparison, when necessary. An unpaired t test or Mann–Whitney U test was used for comparisons between groups. A p value of less than 0.05 was considered statistically significant.
Results
Analysis of microvessel density in the myometrium
Intense brown CD34 staining was found in variable blood vessels (Fig. 1). No significant stain intensity was observed between the septate uteri and the control uteri. The vessel counts are shown in Table 2. A total of 45 fields of the microvessel area in the septate uteri and 50 fields in the controls were observed (5 fields/patient). The septate uteri had a vessel count/field (0.19 mm2) of 149.7 ± 22.7, while the control uteri had a value of 162.2 ± 36.4. The microvessel count was visibly lower for the septate uteri than for the controls, but no significant difference was found. Interestingly we observed that the total vessel area/field and the vessel area percentage were significantly different between the septate and the control uteri (p < 0.05). The total vessel area/field in the septate uteri was 10350.4 ± 1024.3 μm2 while that in the controls was 12002.9 ± 2232,3 μm2, corresponding to a vessel area percentage of 5.47 ± 0.54 and 6.35 ± 1.18%, respectively.
| Blood vessels | Septate uterus (n = 45)a | Controls (n = 50)a | p value |
|---|---|---|---|
| Vessels count | 149.7 ± 22.7 | 162.2 ± 36.4 | NS |
| Area (μm2) | 10350.4 ± 1024.3 | 12002.9 ± 2232.3 | p < 0.05 |
| Area percentage (%) | 5.47 ± 0.54 | 6.35 ± 1.18 | p < 0.05 |
- NS not significant
- a5fields/patient; values are expressed as mean ± SD
Analysis of microvessel morphology
The microvessel morphology was quantified using the parameter F-circle (4 × area/π × max diameter2) and F-shape (min diameter/max diameter). A total of 6735 microvessels in the septate uteri and 8108 microvessels in the controls were counted and examined in this study (Table 3). The area per vessel in the septate uteri was smaller than that in the controls, but no significant difference was found between the two groups. The F-circle value (irregularity of microvessels) of the septate uteri group was 0.627 ± 0.003 compared with 0.634 ± 0.002 for the control group; these values were significantly different (p < 0.05). Furthermore, a comparison of the F-shape value (deformity of microvessels) between the septate uterus group and the control group also revealed a significant difference (p < 0.0001). The F-shape value for the septate group was 0.534 ± 0.002 compared with 0.566 ± 0.002 for the control group.
| Septate uterus (n = 6735)a | Controls (n = 8108)a | p value | |
|---|---|---|---|
| Area per vessel (μm2) | 69.2 ± 3.4 | 74.0 ± 2.6 | NS |
| F-circle | 0.627 ± 0.003 | 0.636 ± 0.003 | p < 0.05 |
| F-shape | 0.534 ± 0.002 | 0.566 ± 0.002 | p < 0.001 |
- NS not significant
- aTotal blood vessel count in myometrium (5 fields/patient × no. of patients); values are expressed as mean ± SD
Discussion
In humans, the formation of the uterine cavity starts with the union of the two Müllerian ducts during approximately the 10th week of development. After fusion starts in the middle, it extends caudally and cephalically, and the typical uterine shape is formed by cellular proliferation in the upper portion and the dissolution of cells at the lower pole. During the 20th week of gestation, the uterine septum separates from an upper thick wedge of tissue and the characteristic uterine cavity develops. The failure of this process results in congenital uterine anomalies, which are associated with poor reproductive performance [11, 12]. The American Fertility Society classifies these congenital anomalies into seven categories [13]. Among these seven categories, septate uterus is a popular clinical target for obstetricians because of its association with variable reproductive failure, including early recurrent spontaneous abortions, preterm delivery, abnormal fetal presentations, intrauterine growth retardation, and infertility [11, 14, 15]. Burchell et al. [16] put forward a hypothesis to explain the poor reproductive outcome for women with septate uteri with regard to uterine artery anomalies. They suggested that a compromised blood flow into the endometrial tissue may result in dyssynchronous decidualization, inadequate hormone responsiveness, and abnormal placentation. However, a quantitative analysis of the blood vessel distribution in septate uteri has not been previously reported.
The present study is the first quantitative immunohistochemical evaluation of the blood vessel distribution in the myometrium of septate uteri. CD34 is a heavily glycosylated transmembrane protein that is expressed in vascular endothelial cells [17]. As an endothelial cell marker, anti-CD34 antibody was both more sensitive and more specific than other endothelial cell-specific antibodies, such as anti-vWF antibody [18]. In the present study, we used immunostaining for CD34 to observe the blood vessels in septate uteri and noted changes in their relative area, size and shape compared with blood vessels of the myometrium in normal uteri. Our data quantitatively demonstrated that the blood vessel area of a uterine septum was significantly smaller than that of a normal uterus. This result may indicate that the blood flow to the endometrium was compromised. We have also shown for the first time that a septate uterus exhibits abnormal blood vessel morphology. Since the blood vessel count was not significantly decreased, this observation strongly suggests that the significant decrease in the blood vessel area contributed to the deformity in the vessel shape; however, the mechanism responsible for vessel deformity remains to be elucidated.
Our data were consistent with the widely accepted theory that the uterine septum consists of fibroelastic tissue with inadequate vascularization. This feature of septate uteri results in compromised fetal placentation [19–21]. Dabirashrafi et al. [22] examined 64 biopsy specimens obtained from four different regions of 16 septate uteri and showed that the histologic feature of the septum showed a lower connective tissue and a higher muscle content. They also observed a reduction in the number of the vessels, although the reduction was not statistically significant. Anomalies in anatomical structure are another cause of adverse effects on reproductive performance. The reduced volume of the uterine cavity as well as cervical incompetence have been suggested as possible etiologic factors [23, 24]. Premature delivery may be caused by increased intrauterine pressure with relative cervical incompetence. Deficiencies in estrogen and progesterone receptor in malformed uteri are also thought to cause uncoordinated contractibility, leading to reproductive loss [25].
Our previous report demonstrated that a septate uterus results in an insufficient blood supply to the endometrium [26]. In the present study, we quantitatively evaluated the blood vessel arrangement in the septum and determined that an abnormal blood supply arising from blood vessel deformity might be the major cause of poor reproductive outcome. Surgical intervention for septate uterus has been traditionally performed using a transabdominal approach [27, 28]. Ayhan et al. [29] evaluated the reproductive performance of 102 patients who underwent conventional metroplasty for the treatment of septate uterus and reported that the miscarriage rate was reduced from 90% before surgery to 17% after abdominal metroplasty. In our recent statistical data [30], we performed modified metroplastic surgery in 173 women: 101 with septate uterus, 59 with arcuate uterus, 10 with a T-shape uterus and 3 with a double uterus. As demonstrated in this study, the subsequent reproductive outcome was distinctly improved, with a reduction in the miscarriage rate from 93.2% to 12.5%, and an increase in the term delivery rate from 1.3% to 63.4%. In this kind of study, the efficacy of treatment cannot be evaluated without an appropriate control study. Therefore we performed a control study in which the patients had uterine anomalies but did not receive metroplasty. The control group consisted of 47 randomly selected women with congenital uterine anomalies not treated by metroplasty. This study revealed that 152 out of 161 subsequent pregnancies (94.4%) terminated in spontaneous abortion [30]. Thus, metroplasty for the treatment of septate uterus appears to be an appropriate surgical intervention with the ability to improve the subsequent reproductive outcome.
The exact reason why surgical intervention is capable of improving the outcome of pregnancy is not yet clear. However, this surgical maneuver can be used to rebuild the uterine anatomical structure and may improve the blood supply to the endometrium and allow the establishment of reproductive function. Therefore, appropriate surgical intervention seems to be a relevant choice in the treatment of women with anomalous uterine anatomies and a history of recurrent reproductive wastage.
In conclusion, our results show that a quantitative difference in the blood vessel area exists between septate uteri and normal uteri, and appropriate surgical manipulation may change the intrauterine environment enabling a favorable pregnancy outcome. Further studies should focus on implantation and placentation before and after metroplasty.




