Diagnosis of Menopause in a Captive Sumatran Orangutan (Pongo abelii)
ABSTRACT
Humans were long thought to be the only mammal to experience menopause, the permanent cessation of reproduction followed by a long post-reproductive lifespan. More recently, evidence has been found for the existence of menopause in other long-lived mammals, including chimpanzees and gorillas. However, orangutans, which have the longest interbirth interval of any primate, have rarely been studied in this period of their lives. In this paper, we describe clinical, ultrasound, endocrine, and histological evidence consistent with a natural menopause in a captive, previously fertile, Sumatran orangutan (Pongo abelii), aged approximately 50. Consecutive serum samples showed low levels of estradiol and high levels of follicle-stimulating hormone. Transvaginal ultrasound revealed an atrophic uterus with an antero-posterior diameter of 2.36 cm, an endometrial thickness of 2 mm, and inactive ovaries. Following this female's death from a subdural hematoma, histological examination of the ovaries showed a dense stroma with corpora albicantia, in comparison to the numerous primordial follicles seen in the ovaries of a stillborn infant female orangutan. These multiple lines of evidence suggest that Sumatran orangutans can now be added to the list of mammals which undergo a true menopause, which may ensure that females' final offspring can be reared to independence.
Summary
-
Low estradiol and high FSH levels, few primordial follicles, and an atrophic endometrium indicated menopause in a female Sumatran orangutan.
-
Zoos could establish a database of reproductive hormone levels to facilitate research and diagnosis.
1 Introduction
As female mammals age, the ovarian oocyte pool becomes depleted, and folliculogenesis fails in response to increasing levels of follicle-stimulating hormone (FSH), and luteinizing hormone (LH) (Soules et al. 2001). Reproductive senescence occurs in several species, with signs including irregular estrous cycles, reduced fertility, longer interbirth intervals, increased incidence of aneuploidy, and poorer offspring survival (e.g., Atsalis and Margulis 2008; Tardif et al. 2008; Campos et al. 2022; Charalambous et al. 2023).
Menopause, the point at which menstruation permanently ceases (Utian 1999), occurs at an average of 51 years in humans. Complete cessation of reproduction followed by a long postreproductive lifespan was for some time thought unique to humans (Peccei 2001; Alberts et al. 2013). More recently, evidence for menopause has emerged in other long-lived mammals such as Asian elephants (Lahdenperä, Mar, and Lummaa 2014) and cetaceans (Ward et al. 2009; Pavelka et al. 2018; Ellis et al. 2024), and a growing number of studies in captivity and the wild now suggests that both gorillas and chimpanzees may also experience a true menopause (Atsalis and Margulis 2006; Walker and Herndon 2008; Videan et al. 2006; Wood et al. 2023).
However, while captive female orangutans decline in fertility with age (Caro et al. 1995), Wich et al. (2004) found no evidence of menopause in an analysis of wild orangutan life history data. As the orangutan has the longest interbirth interval of all primates (7–9 years; Wich et al. 2004; Knott, Emery Thompson, and Wich 2009; van Noordwijk et al. 2018), it has been more difficult to decide whether cessation of menses is true ovarian failure, or secondary to ongoing lactation or illness. However, the latest age at reproduction in captivity (41 years) is considerably less than likely lifespan in the wild, conservatively estimated at 53 years for females (Shumaker, Wich, and Perkins 2008).
To help with captive management and conservation efforts, there is therefore a need for more information on reproduction in orangutans as they age. Here we report evidence that a captive, previously fertile, Sumatran orangutan (Pongo abelii), approximately 50 years old, underwent a natural menopause.
2 Materials and Methods
Gina (Jersey Zoo ID M229), estimated to have been born between August 1963 and August 1965, arrived at Jersey Zoo in 1968 and died on August 15, 2014. Postmortem examination revealed a major subdural bleed in the right hemisphere without external trauma. Histopathology showed severe nephritis and mild arteriosclerosis.
Sections of Gina's ovaries were compared with those taken from a fully developed female orangutan infant (Jersey Zoo ID M3062), stillborn at Jersey Zoo on May 23, 2009, and frozen for later histological analysis. Selected tissue samples were placed in 10% neutral buffered formalin. Sections 5-µm thick were prepared and examined using routine histological methods (Bancroft 2008).
We collated data on Gina's health and reproduction from Jersey Zoo's animal records (ZIMS; Species360 2023). We also compared serum parameters in blood samples taken from Gina and her daughter, Mawar (M1462, born March 16, 1989), aged 22 at the time, to assess the possibility that the values we obtained might be normal for adult female orangutans. All blood samples were taken under anesthesia during health assessments.
2.1 Ethics Statement
This research complied with protocols approved by Durrell Wildlife Conservation Trust's Ethics Committee, and adhered to the Animal Welfare (Jersey) Law 2004.
2.2 History and Results
Gina had her first pregnancy in 1972, followed by five more births with an average interbirth interval of 6.4 years (range 3.5–8.8 years). During her final pregnancy, Gina developed labial edema and was diagnosed with dystocia. An ultrasound showing placenta previa prompted an emergency cesarean section on May 13, 2004. The male infant was successfully reared by Gina. Before this pregnancy, she had menstruated most months and copulated around estrus.
After her last offspring was weaned, she was given the combined oral contraceptive pill (Mercilon, Organon Pharma UK) starting on August 4, 2010. In early 2014, staff decided to assess if she was medically fit to conceive again. Her contraception was stopped on February 28, 2014, 2 months before the examination, and during that time no menstruation was seen.
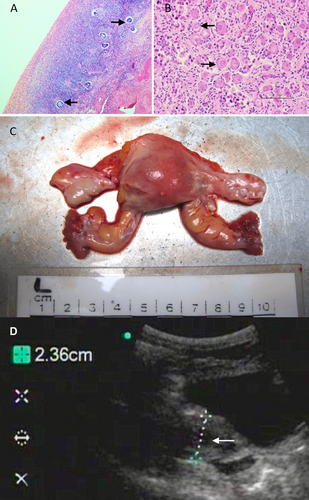
A full medical check was performed under anesthesia on April 28, 2014. Blood analysis (Table 1) revealed an elevated FSH level and a low serum estradiol level. Gina's prolactin level was higher than her daughter's, but lower than typical values for gorillas. Though her potassium level was normal, her creatinine and urea levels were raised, suggesting renal disease. The N-terminal pro B-type natriuretic peptide (NT-proBNP) level was also high in 2014, consistent with renal and/or cardiac disease. Transvaginal ultrasound (Figure 1D) revealed a uterus with an antero-posterior diameter of 2.36 cm, an atrophic endometrium 2 mm thick, and ovaries with no active follicles.
| Mawar | Gina | ||||
|---|---|---|---|---|---|
| Test (units) | August 23, 2011 | May 13, 2004 | April 28, 2014 | May 22, 2014 | Reference values* |
| FSH (IU/L) | 4.3 | — | > 200 | > 200 | Maximum c. 30 in premenopausal chimpanzees (Videan et al. 2006) |
| Estradiol (pmol/L) | 301 | — | 128 | 146 | 180–918 in cycling females (Bentley 1999); minimum of 206 in luteal phase (Nadler, Collins, and Blank 1984) |
| Prolactin (mU/L) | 480 | — | 921 | 924 | 1062–1994 (gorillas; Laughlin, Meehan, and Zinaman 2008) |
| NT-proBNP (pg/mL) | — | 2650 | 1750 | 23.51 ± 21.63 SD (gorillas; Murray et al. 2019) | |
| Urea (mmol/L) | 2.1 | 2.7 | 12.1 | 16.3 | 1.4–8.1 (Species360 2023); 10.8 ± 13.4 SD (Kilbourn et al. 2003) |
| Creatinine (μmol/L) | 49 | 105 | 389 | 374 | 35–133 (Species360 2023); 123.8 ± 194.5 SD (Kilbourn et al. 2003); 118.0 ± 11.7 SEM (females; Schmidt et al. 2006) |
| Potassium (mmol/L) | 5.5 | — | 4.7 | 4.8 | 3.2–5.6 (Seng, Meng, and Abdullah 2007; Species360 2023) |
- Abbreviations: FSH, follicle-stimulating hormone; NT-proBNP, N-terminal pro-brain natriuretic peptide.
- * Units used in the original sources have been converted to SI standards if necessary. Reference values are from orangutans unless otherwise stated.
Three weeks later, on May 22, 2014, she was reassessed under anesthetic and serum levels confirmed the menopausal picture, along with cardiac and renal disease of unknown etiology (Table 1). These results contrast with those from her daughter Mawar, which showed normal levels.
Following Gina's death, postmortem analysis of her reproductive organs (Figure 1C) revealed small ovaries (approximately 10 × 15 mm), both of which contained corpora albicantia along with thick vessels and a dense stroma depleted of primordial follicles (Figure 1A). Examination of the stillborn infant's ovaries showed numerous primordial follicles (Figure 1B).
3 Discussion
While the menstrual cycle in orangutans has been described (Durgavich, Harwell, and Knott 2023), no previous studies have indicated the occurrence of menopause. In this case study, however, we found several lines of evidence that suggest that a female Sumatran orangutan had undergone menopause.
Although Gina had been taken off contraception 2 months before her reproductive status was assessed, she had shown no signs of menstruation; furthermore, contraception leads to lower, not higher, FSH levels (Kallio et al. 2013). Amenorrhea can also be secondary to a hypothalamic–pituitary axis disorder resulting from stress or illness (Klein, Paradise, and Reeder 2019; Meczekalski et al. 2022). However, Gina's environment had not changed, she had a proven record of successful breeding in Jersey, and amenorrhea resulting from stress would have been associated with low or normal FSH levels, and the presence of antral follicles.
Postmortem analysis, renal function serology, and markedly raised BNP levels did indicate that Gina had both heart and renal disease, which are often linked in orangutans (Lowenstine, McManamon, and Terio 2016; Murray et al. 2019). These conditions could be associated with a downregulated pituitary resulting in secondary functional hypothalamic amenorrhea, and the atrophy of Gina's genital tract could be a result of this. However, other evidence suggests that this was not the case. Consecutive serum samples showed altered estradiol and FSH levels consistent with menopause. Gina's estradiol levels were lower than those found in cycling female orangutans in previous studies (Bentley 1999; Nadler, Collins, and Blank 1984; see Table 1) and in a sample taken from her 22-year-old daughter. Nadler et al. (1985) also found much higher levels of estradiol in cycling female chimpanzees (minimum 232 pmol/L, mean 631 pmol/L). If amenorrhea was the result of an HPA disorder, we would have expected to see raised estradiol levels (Vaikkakara et al. 2017), and more antral follicles.
Gina also had markedly elevated levels of FSH in comparison to her daughter, and to premenopausal chimpanzees (maximum approx. 30 IU/L; Videan et al. 2006). Her prolactin levels, however, were slightly lower than those reported for cycling gorillas (which are typically higher than normal human levels; Laughlin, Meehan, and Zinaman 2008), but higher than those in a serum sample from her cycling daughter.
Ultrasound examinations of Gina's reproductive anatomy also suggested that she had entered a post-reproductive phase. Her endometrium was measured at 2 mm thick, much thinner than values found by Kinoshita et al. (2020), who reported that endometrial thickness varied from 4 to 9.2 mm in three female orangutans aged between 24 and 50 years, and that it was correlated with urinary estrone (E1G) levels. Finally, postmortem comparison of the reproductive system and ovarian tissue with that of an infant female clearly showed a difference in density of the primordial follicles, with Gina's ovaries depleted.
One of the main theories to explain menopause suggests that an older mother would do better to invest resources in their existing offspring, rather than face the increased risks of stillbirth, birth defects, and her own death in childbirth (O'Connell, Hawkes, and Blurton Jones 1999). The orangutan has the longest birth interval of all great apes, with the young dependent on maternal care for learning life skills for some 9 years (van Noordwijk et al. 2018). An extended period of post-reproductive life would allow juveniles born to older mothers to successfully reach independence, and our data suggest that Sumatran orangutans experience true menopause.
Although so few studies have been done on aging orangutans, zoos need to be aware that the reproductive life of female orangutans may be shorter than previously believed. Any opportunity to obtain serum FSH, LH, and estradiol levels should be taken; monitoring any behavioral indicators of menopause would also be very helpful. Ultimately, a global database would provide information vital for understanding both captive and wild population dynamics in this critically endangered species.
Acknowledgments
We are very grateful to the many people who have cared for the orangutans at Jersey Zoo.
Open Research
Data Availability Statement
All relevant data are presented in the text.




