Abstract
The reaction of CuCl with WOCl3 at 400 °C leads to a mixture of Cu1−x[W2O2Cl6] (1) and Cu1−x[W4O4Cl10] (2) in form of black lustrous needles. Both compounds crystallize in space group C2/m with a = 12.7832(5) Å, b = 3.7656(2) Å, c = 10.7362(3) Å, β = 119.169(2)° for 1 and a = 12.8367(19) Å, b = 3.7715(7) Å, c = 15.955(3) Å, β = 102.736(5)° for 2. The structures are made up of WO2Cl4 octahedra. In the case of 1 two octahedra are edge-sharing via chlorine atoms to form pairs which are linked via the trans-positioned oxygen atoms to form infinite double strands 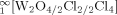 . In the structure of 2 two of these double strands are condensed via terminal chlorine atoms to form quadruple strands
. In the structure of 2 two of these double strands are condensed via terminal chlorine atoms to form quadruple strands 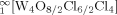 . Like for all members of the Mx[W2O2X6] structure family (X = Cl, Br) nonstochiometry with respect to the cations M was observed. The copper content of 1 and 2 was derived from the site occupation factors of the respective structure refinements. For several crystals examined the copper content varied between x = 0.27 and 0.17 for 1 and x = 0.04 for 2. In both structures the oxochlorotungstate strands are negatively charged and connected to layers by the monovalent copper ions, which are tetrahedrally coordinated by the non-bridging chlorine atoms of the strands. The structure models imply disorder of the Cu+ ions over closely neighboured sites.
. Like for all members of the Mx[W2O2X6] structure family (X = Cl, Br) nonstochiometry with respect to the cations M was observed. The copper content of 1 and 2 was derived from the site occupation factors of the respective structure refinements. For several crystals examined the copper content varied between x = 0.27 and 0.17 for 1 and x = 0.04 for 2. In both structures the oxochlorotungstate strands are negatively charged and connected to layers by the monovalent copper ions, which are tetrahedrally coordinated by the non-bridging chlorine atoms of the strands. The structure models imply disorder of the Cu+ ions over closely neighboured sites.