The effects of COVID-19 on IBD prescribing and service provision in a UK tertiary centre
Abstract
Background
To quantify the effects of COVID-19 on our inflammatory bowel disease (IBD) unit, including service provision, prescribing practices and use of therapeutic drug monitoring (TDM).
Methods
We performed a single centre retrospective observational cohort study. Data was extracted from our IBD database, electronic patient records and radiology/endoscopy reporting systems between 16/3/20-17/4/20 and the corresponding period in 2019.
Results
A similar number of patients commenced biologic therapy before COVID-19 (n = 37) and during the pandemic (n = 36). Patients in the pre-COVID-19 cohort were older (median 36 vs 29 years, P = 0.009) with a longer median disease duration (9.3 vs 5.2 years, P = 0.02). During COVID-19 there was a nonsignificant increase in prescribing of vedolizumab (8/37, 22% vs 14/36, 39%, P = 0.13) and a higher proportion of patients were anti-TNF-naïve (3/17, 18% vs 18/24, 74%, P = 0.0004). There was a reduction in use of concomitant immunomodulators (22/29, 76% vs 4/34, 12%, P < 0.0001) and increased biologic use in thiopurine-naïve patients (3/37, 8% vs 15/36, 42%, P = 0.001). Use of TDM fell by 75% (240 vs 59 tests). Outpatient appointments fell by 68% and were conducted via telemedicine. MRI scanning, endoscopy, luminal surgery and inpatient numbers fell by 87%, 85%, 100% and 82% respectively. IBD Clinical Nurse Specialist and Pharmacist helpline contacts increased by 76% and 228% respectively.
Conclusions
We observed prescribing differences during COVID-19, bypassing the initiation of immunomodulators and/or anti-TNF therapy in favour of vedolizumab with a reduction in immunomodulator prescribing. We also observed a rapid reorganisation of service provision, including a shift towards telemedicine and online solutions.
1 INTRODUCTION
The World Health Organisation declared the coronavirus outbreak a pandemic on 11 March 2020. National Health Service (NHS) England subsequently issued a collective plan on 17 March 2020 to all NHS trusts on how to respond to the crisis including cancellation of non-urgent procedures and elective work and staff re-deployment by mid-April. This has had wide-ranging impact across the NHS including on Inflammatory Bowel Disease (IBD) services.
The ever-changing nature of the pandemic, difficulties of remotely delivered care and an overburdened helpline have negatively impacted access to care for IBD patients. This was further exacerbated by a reduction in clinic capacity and delay or cancellation of procedures and investigations. We aimed to quantify the effect of the pandemic on service provision at a tertiary centre.
The risk of COVID-19 is of particular relevance to patients with IBD given a high proportion take therapies that affect the immune system.
The impact of immunosuppression on the risk and severity of COVID-19 infection is unclear. Consideration of this unknown risk balanced with the risk of active disease and subsequent hospitalisation is required by those prescribing immunomodulators and advanced therapies for the treatment of IBD during the pandemic. Prescribing decisions are made in a multidisciplinary meeting (MDM) with consideration of many factors including disease phenotype, patient preferences, cost and potential adverse effects. This study aimed to evaluate how the additional threat of COVID-19 infection affected prescribing practices.
2 METHODS
We compared our IBD service provision and prescribing practices between 16 March-17 April 2020 and the corresponding period in 2019. Assessment of service provision and methods for respective data collection are described below.
2.1 Prescribing practices
All patients who were referred to our virtual multidisciplinary biologic and immunomodulator meeting for the treatment of active IBD (identified by abnormal faecal calprotectin, endoscopy or cross-sectional imaging) during the two time periods were included. Demographic data, previous treatments and treatment decisions were recorded. All patients newly initiated on biologic treatment were discussed at our biologic and immunomodulator meeting. The pharmacy database was reviewed to identify referrals for initiation of thiopurines and methotrexate.
2.2 Comparison of investigation frequency
Computerised Radiology Information System (CRIS®) [Healthcare Software Solutions Limited, UK] and Endosoft® [Endosoft LLC, New York, USA] were searched for all MRI scans and endoscopies performed respectively. Data generated were crosschecked with the electronic patient record to exclude patients without known or suspected IBD.
To quantify the number of patients undergoing therapeutic drug monitoring (TDM), patients with IBD were identified from the contemporaneously recorded digital records between 1 April 2016 and 29 April 2020. These patients were cross referenced with electronically stored laboratory results for adalimumab, infliximab and thiopurine metabolite levels performed during the two study periods using a custom-built package (EndoMineR®) in R 3.6.1 [R Foundation for Statistical Computing, Vienna, Austria].
2.3 Service evaluation
Clinic codes were used to search Patient Identity Management Service [Custodix NV, Belgium] to calculate the number of patients seen in each clinic.
The numbers of patients starting exclusive enteral nutrition (EEN), taking part in clinical trials, attending the IBD infusion unit, being discussed at multidisciplinary meetings (MDM), or undergoing abdominal surgery and the numbers of inpatients and IBD helpline contacts were extracted from the respective local databases.
2.4 Analysis
To compare the cohorts we used Fisher's exact test for categorical data and Mann-Whitney test for continuous variables. We used descriptive statistics for service provision. Continuous data are presented as medians with ranges in brackets. Analyses were performed using GraphPad Prism v8.4.2.
2.5 Ethics
This work was carried out as part of a service evaluation exercise and therefore, ethical approval was not required.
3 RESULTS
3.1 Prescribing practices
Table 1 details the characteristics of patients commencing, or changing class of biological therapy or tofacitinib. Patients in the pre-COVID-19 cohort were older (36 (22-76) vs 29 (17-91) years, P = 0.009) and had a longer disease duration (9.3 (0.4-36.2) vs 5.2 (0.2-21.2) years respectively, P = 0.02). Fewer patients were also thiopurine-naïve (3/37, 8% vs 15/36, 42%, P = 0.001) and anti-TNF-naïve (10/37, 27% vs 26/36, 72% P = 0.0002) compared with the COVID-19 cohort. Otherwise the cohorts were comparable.
| Pre-COVID-19 period | COVID-19 period | P-value | |
|---|---|---|---|
| Total | 37 | 36 | |
| Median age, years (range) | 36 (22-76) | 29 (17-91) | 0.02 |
| Age at diagnosis | |||
| 16 years or younger (A1) | 10 | 9 | >0.99 |
| 17 to 40 years (A2) | 23 | 23 | >0.99 |
| Older than 40 years (A3) | 4 | 4 | >0.99 |
| Median disease duration, years | 9.3 (0.4-36.2) | 5.2 (0.2-21.2) | 0.009 * |
| Female, n (%) | 17(46) | 13 (36) | 0.82 |
| Phenotype, n (%) | |||
| Ulcerative colitis | 12 (32) | 16 (44) | 0.46 |
| Proctitis (1) | 1 (8) | 3 (19) | 0.61 |
| Left sided (2) | 5 (42) | 6 (38) | >0.99 |
| Extensive (3) | 6 (50) | 7(44) | >0.99 |
|
Crohn's disease Location |
25 (68) | 20 (56) | 0.33 |
| Ileal (L1) | 8 (32) | 8 (40) | >0.99 |
| Colonic (L2) | 4 (16) | 3 (15) | >0.99 |
| Ileocolonic (L3) | 13 (52) | 9 (45) | 0.44 |
| Upper GI (L4) | 4 (16) | 5 (25) | 0.74 |
| Perianal | 10 (40) | 5 (25) | 0.24 |
| Behaviour | |||
| Inflammatory (B1) | 12 (48) | 9(45) | 0.61 |
| Stricturing (B2) | 6 (24) | 9(45) | 0.40 |
| Penetrating (B3) | 7 (28) | 2(10) | 0.15 |
| Total thiopurine naïve | 3 (8) | 15 (42) | 0.001 * |
| Total anti-TNF naïve | 10 (27) | 26 (72) | 0.0002 * |
- * Denotes a significant P-value ≤ 0.05.
Table 2 displays the treatment decisions made for patients with active IBD before and during the pandemic. During COVID-19, more patients started de novo biologic therapy or tofacitinib (29/45 (64%) compared to pre-pandemic (19/50 (38%)) (P = 0.01). Change of biologic class, or to tofacitinib, occurred in 7/45 (16%) during COVID-19 compared with 18/50 (36%) pre-pandemic (P = 0.01). The remaining patients were dose escalated on their current therapy or commenced azathioprine alone. Two patients in the pre-COVID-19 cohort were recruited to a clinical trial of an investigational product.
| Treatment Decision, n (%) | |||
|---|---|---|---|
| Treatment decision | Pre-COVID cohort (n = 50) | COVID cohort (n = 45) | P value |
| Dose escalation | 8 (16) | 8 (18) | >0.99 |
| Start biologic/tofacitinib | 19 (38) | 29 (64) | 0.01 * |
| Switch biologic/tofacitinib |
20 (40) 2 referred for clinical trial and not included in the below data. |
7 (16) | 0.01 * |
| Start immunomodulator | 3 (6) | 1 (2) | 0.61 |
| Disease characteristics and prior treatment | ||||
|---|---|---|---|---|
| Biologic initiation or class change |
Pre-COVID cohort, (n = 37) |
COVID cohort (n = 36) | P value | |
| Adalimumab |
Total, n (%)
|
7(19) 1 (14) 6 (86) |
9 (25) 1 (11) 8 (89) |
0.58 |
|
Anti-TNF naïve, n (%) -UC -CD |
5 (71) 1 (20) 4 (80) |
7 (78) 1 (14) 6 (86) |
0.54 | |
| Thiopurine naïve, n (%) | 1 (14) | 5 (56) | 0.14 | |
| Monotherapy, n (%) | 1 (14) | 9 (100) | 0.0004 * | |
| Infliximab | Total, n (%) | 5 (14) | 1(3) | 0.21 |
|
5 (100) |
1(100) |
||
| Anti-TNF naïve, n (%) | 2 (40) | 0 (0) | >0.99 | |
|
2 (100) |
|||
| Thiopurine naïve, n (%) | 1 (20) | 0 (0) | >0.99 | |
| Monotherapy, n (%) | 0 (0) | 1 (100) | 0.29 | |
| Vedolizumab |
Total n, (%)
|
8 (22) 3 (38) 5 (62) |
14 (39) 12 (86) 2 (14) |
0.13 |
|
Anti-TNF naïve, n (%)
|
2 (25) 2 (100) |
9 (64) 8 (89) 1 (11) |
0.18 | |
| Thiopurine naïve, n (%) | 1 (13) | 5 (36) | 0.35 | |
| Monotherapy, n (%) | 3 (38) | 11 (79) | 0.08 | |
|
Ustekinumab† |
Total, n (%)
|
9 (24) 9 (100) |
10 (28) 1(10) 9 (90) |
0.61 |
| Anti-TNF naïve, n (%) | 1 (11) | 9 (90) | 0.01 * | |
|
0 (0) | 1(11) | ||
|
1 (11) | 8 (89) | ||
| Thiopurine naïve, n (%) | 0 (0) | 4 (40) | 0.09 | |
| Monotherapy, n (%) | 3 (33) | 10 (100) | 0.003 | |
| Tofacitinib |
Total, n (%)
Anti-TNF naïve, n (%) Thiopurine naïve, n (%) |
8 (22) 8 (100) 0 (0) 0(0) |
2 (5) 2 (100) 1 (50) 1 (50) |
0.10 0.2 0.2 |
| Total prescribed as monotherapy | 7/29 (24) | 31/34 (91) | <0.0001 * | |
- * Denotes a significant P-value ≤ 0.05. †Unlicensed for UC in 2019.
The data for patients commencing biologic therapy or tofacitinib are also shown in Table 2 (n = 37 pre-COVID-19, n = 36 during COVID-19). During the pandemic there was a nonsignificant increase in the use of vedolizumab (14/36, 39%), compared to the pre-COVID-19 period (8/37, 22%, P = 0.13). Although treatment escalation to an advanced therapy increased overall, de novo prescriptions for infliximab and tofacitinib fell (14% vs 3%, P = 0.21 and 22% vs 5%, P = 0.10, respectively). Ustekinumab and adalimumab prescribing remained similar. In those patients commenced on vedolizumab or ustekinumab, 18/24 (74%) were anti-TNF naïve compared with 3/17 (18%) before the pandemic (P = 0.004) (Figure 1). Across all biologic classes there was a reduction in concomitant immunomodulator use; biologics were prescribed as monotherapy in 7/29 (24%) in the pre-COVID-19 cohort compared with 31/34 (91%, P < 0.0001) in the COVID-19 cohort. A significant change in practice was demonstrated in relation to adalimumab specifically as it was prescribed as monotherapy in 1/7 (14%) in the pre-COVID 19 cohort compared with 9/9 (100%, P < 0.0004) in the COVID-19 cohort. Prescribing vedolizumab and ustekinumab as monotherapy was not unusual prior to COVID-19 (prescribed in 33% and 38% of patients respectively) however, there was a shift towards commencing biologics in immunomodulator-naïve patients (3/37, 8% pre-pandemic vs 15/36, 42% during COVID-19, P = 0.001)(Figure 1).
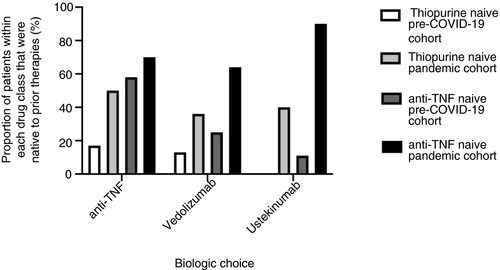
Use of immunosuppressants fell during the COVID-19 period. There were 28 referrals (including referrals not discussed at MDM) to commence immunomodulators in the pre-COVID-19 cohort but only one during COVID-19. Similarly, eight patients were initiated on tofacitinib in the pre-pandemic group compared to two patients during COVID-19.
Differences in the indication for EEN as a treatment for active luminal Crohn's disease were also noted. EEN prescriptions in the pre-COVID-19 cohort were for bridging to vedolizumab (n = 1) and as pre-surgical optimisation (n = 1). In the pandemic cohort EEN was prescribed to enable surgery to be delayed (n = 1), to enable steroid weaning (n = 1) and as an adjunct to medical therapy for symptom management (n = 2).
3.2 Investigations
Two hundred and twenty-four MRI scans of the small bowel or pelvis were performed/requested for IBD patients in the pre-COVID-19 cohort compared with 18 during the pandemic (−87%). Similarly, endoscopy for IBD decreased dramatically; 115 procedures were performed in the pre-pandemic cohort compared with 17 procedures during COVID-19. Six flexible sigmoidoscopies were performed during the COVID-19 period all of which were for assessment of clinically active colitis. Eleven colonoscopies performed during the pandemic were all undertaken within 48 hours of receipt of NHS England guidance regarding discontinuation of elective activity, in patients who had already started bowel preparation.
The frequency of TDM was reduced during the pandemic across all drugs for which it is available (despite the increase in the use of adalimumab monotherapy). Thiopurine metabolite measurements decreased from 143 to 44 (−69%), infliximab levels from 46 to 3 (−93%) and adalimumab levels from 51 to 12 (−76%).
3.3 Service provision
The effects of the pandemic on service provision are shown in Table 3. Outpatient services were significantly affected during the pandemic with a 68% reduction in appointments, and 100% were conducted via telephone. Prior to the pandemic, only clinical nurse specialist (CNS) and pharmacist appointments were performed telephonically. Conversely, helpline contacts dramatically increased; there was a 76% increase in contacts to the IBD CNS advice line and a 228% increase in gastroenterology pharmacy helpline contacts (Figure 2). A particular surge was noted after the government issued advice on shielding high-risk groups on 16/3/2020.
| Cohorts | Pre-COVID-19 cohort | COVID-19 cohort | Percentage change (%) |
|---|---|---|---|
| Outpatient clinical contacts | |||
| Total | 1036 | 334 | −68 |
| Physician | |||
| Face-to-face | 835 | 0 | N/A |
| Telephone | 0 | 235 | N/A |
| IBD Clinical Nurse Specialist | 21 | 21 | 0 |
| Pharmacy | 85 | 43 | −49 |
| Psychology | 56 | 25 | −55 |
| Helpline contacts - total | 1521 | 2881 | +89 |
| IBD Clinical Nurse Specialist | 1391 | 2455 | +76 |
| Pharmacy | 130 | 426 | +228 |
| IBD in-patients | 17 | 3 | −82 |
| IBD infusion unit total | 274 | 298 | +9 |
| Infliximab | 166 | 165 | −0.6 |
| Vedolizumab | 105 | 127 | +21 |
| Ustekinumab | 3 | 6 | +100 |
| Multidisciplinary meetings | |||
| IBD MDM | 59 | 57 | −3 |
| Perianal virtual clinic | 21 | 2 | −90 |
| Virtual | 13 | 2 | −85 |
| Face-to-face | 8 | 0 | −100 |
| Trial participation | |||
| Recruitment | 48 | 0 | −100 |
| Follow-up | 28 | 24 | −14 |
| Radiology | |||
| MRI pelvis | |||
| Requested | 30 | 1 | −97 |
| Performed | 22 | 3 | −86 |
| MRI small bowel | |||
| Requested | 79 | 2 | −97 |
| Performed | 93 | 12 | −87 |
| Endoscopy | |||
| Lower gastrointestinal endoscopy | 114 | 17 | −85 |
| Colonoscopy | 66 | 11 | −83 |
| Indications: | |||
| Assessment of activity | 59 | 8 | −86 |
| Surveillance | 7 | 3 | −57 |
| Faecal microbiota transplant | 1 | 0 | −100 |
| Flexible sigmoidoscopy | 41 | 6 | −85 |
| Indication: Assessment of activity | 41 | 6 | −85 |
| Pouchoscopy | 7 | 0 | −100 |
| Indications: | |||
| Assessment of activity | 2 | 0 | −100 |
| Surveillance | 5 | 0 | −100 |
| Luminal surgery | |||
| Total number performed | 6 | 0 | −100 |
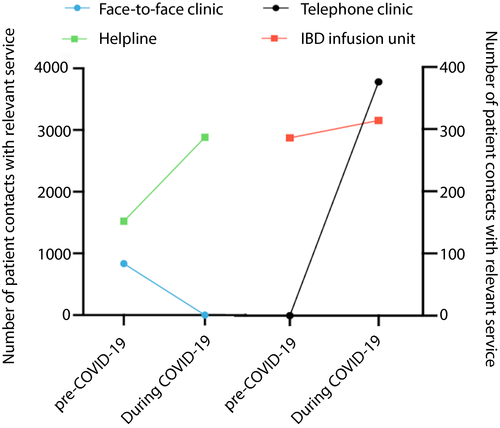
IBD infusion unit attendances increased, largely due to an increase in vedolizumab initiation. Inpatient numbers were reduced. Although there was a reduction in the frequency of cross-sectional imaging there was no change in the numbers of patients discussed in the IBD radiology MDM although video conferencing was used to enable social distancing. This is likely due a backlog of MDM referrals and because imaging during COVID-19 was limited to clinically urgent cases where MDM discussion would more often be required. All new recruitment to non-COVID-19 trials was halted on 17 March 2020 whilst follow up for administration of investigational medicinal products continued. Finally, no operations for luminal IBD were performed; all elective surgery was suspended and there were no emergency IBD cases during the COVID-19 period.
4 DISCUSSION
COVID-19 is having unprecedented effects on healthcare provision. Services across all specialties adapted rapidly to enable staff and resources to be diverted to managing the pandemic. Despite a reduced specialty workforce, a degree of ongoing service provision was required to manage urgent cases and, for patients with chronic diseases, to maintain remission. In line with other organisations, The British Society of Gastroenterology (BSG) has advised that the risks and benefits of immune-modifying drugs be carefully considered, investigations be performed only where absolutely necessary, telemedicine be the new status quo and endoscopy be limited to emergencies or reviewed on a case-by-case basis.1, 2 The changes in practice described in this paper largely reflect this advice.
We describe a clear change in prescribing practices with regards to treatment positioning and drug preference. The number of new biologic prescriptions in the COVID-19 cohort fits with the general annual growth at our centre however the number of patients started on de novo biologic therapy was greater in the COVID-19 period and stands out as an outlier when looking at prescribing trends over the last year. Our pre-COVID-19 practice was broadly in line with guidance from The National Institute for Health and Care Excellence (NICE) which generally recommends escalation to a biologic agent only in patients who have failed to respond to immunomodulators, or who are intolerant to, or have contraindications to such therapies.3 However, with the onset of COVID-19, there was a shift to prescribing biologics in thiopurine-naïve patients. The COVID-19 cohort were younger with a shorter disease duration perhaps highlighting our change in practice with escalation to biological therapy earlier in their disease course. In addition, NICE also advises that where a choice exists, the cheapest drug should be used which, currently, is often a biosimilar anti-TNF. However, during COVID-19, vedolizumab was the predominant agent used in UC (71%) and ustekinumab was the predominant agent used in CD (47%) with frequent use in anti-TNF-naïve patients (89% in both cases). Finally, we saw a marked decrease in the use of concomitant immunomodulation.
These changes in prescribing practices are due to several reasons. First, the COVID-19 pandemic has raised concerns with regards to the use of immunosuppressing medications. Biologic agents, particularly anti-TNF therapy, are associated with an increase in infection, albeit mainly bacterial.4, 5 In addition, immunomodulator therapies and tofacitinib are associated with an increased risk of viral infection6-8 including severe influenza.9 The concern with regards to increased influenza risk relates to pooled data from the H1N1 epidemic which demonstrated that immunocompromise predicted a more severe disease course. However, these data may not be generalisable to the IBD population nor do they relate specifically to SARS-CoV-2 infection.10 Data from the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle-Eastern respiratory syndrome (MERS) epidemics of 2002 and 2014 suggest immunocompromise did not seem to increase the risk of infection.11 Since the prescribing decisions were made (that our data relates to), much data has emerged. The Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)-IBD registry has published a preliminary analysis of 525 IBD patients infected with SARS-CoV-2 associating corticosteroids and combination therapy with worse outcomes (odds ratios 6.9 (2.3-20.5) and 5 (2-12.3) respectively) whereas, anti-TNF monotherapy did not influence outcomes.12 These data are open to a number of biases, particularly underlying active disease. There has been no documented worsening of SARS-CoV-2 pneumonia in patients on anti-TNF therapy and it may be that anti-TNF therapy could be protective; especially in relation the ‘cytokine storm’ associated with life-threatening SARS-CoV-2 pneumonia.13 Concerns over the potential risks of corticosteroid use may have caused an increase in the prescribing of steroid-sparing biologic treatment. However, the results of the RECOVERY trial suggest that corticosteroid use, namely dexamethasone, may be beneficial.14 Further research is required to clarify the true impact of corticosteroids on outcomes from COVID-19 infection.
Both vedolizumab and ustekinumab have a favourable safety profile with regards to infection4, 15-17 and in the recent International Organisation for the Study of Inflammatory Bowel Disease (IOIBD) RAND panel, it was agreed they were unlikely to be associated with an increased risk of COVID-19 infection or worsening disease course.18 Moreover, these drug classes have lower rates of immunogenicity compared to anti-TNF therapy so there is less, or no need for concomitant immunomodulation.19, 20 In addition, adalimumab is delivered subcutaneously thus avoiding the need for hospital attendance and is also less immunogenic than infliximab.19 These reasons are likely to have led to the increases we observed in use of these agents during the pandemic.
In addition, side effects of therapy may have more significant sequelae during the pandemic and should be considered along with the practicalities of initiating treatments. There are a number of factors that make thiopurine treatment less attractive in the current climate. Particularly, the intensive blood monitoring required on initiation and risk of adverse effects which may lead to hospitalisation. Both of these factors may increase the risk of nosocomial transmission. Furthermore, adverse effects such as flu-like syndrome and lymphopaenia may mimic and confuse symptoms and signs associated with SARS-CoV-2 infection. In a recent RAND panel the appropriateness of commencing thiopurine therapy in SARS-CoV-2 negative patients was deemed uncertain for the aforementioned reasons.21 Our practice reflects these concerns with a substantial decrease in our rate of thiopurine initiation alone or as combination therapy.
Likewise, tofacitinib is associated with a pro-thrombotic risk.22 Though this complication has not been observed in UC patients and identified in an older cohort of patients with rheumatoid arthritis with at least one cardiovascular risk factor there may have been concerns regarding use of this agent when considered alongside the recognised thrombotic complications associated with COVID-19.23 Favourable features of tofacitinib, such as its oral administration (thus avoiding hospital visits), rapid onset of action and its short half-life explained our occasional use of this drug. It is also relevant that tofacitinib is being investigated in clinical trials to treat COVID-19 pneumonia, the outcome of which may alter practice.24
Conversely, concerns about the need for hospital attendance for infusions did not decrease our use of intravenous treatments. There have been variable approaches to this in different units. Our infusion unit is located away from acute areas of the hospital theoretically reducing the risk of nosocomial acquisition of SARS-CoV-2 for attendees. In addition, we were able to continue running our infusion unit at almost full capacity; changes in staffing levels due to redeployment and/or staff illness means that this was not feasible at other centres. Indeed some trusts have proactively switched patients from infused to subcutaneous therapy for this reason.25
Modifications to usual care, including deferring non-essential blood tests, have been recommended, and both clinical and laboratory resources have been diverted to managing COVID-19. Thus the use of TDM was reduced.26 This observation has been made elsewhere and if prolonged may have consequences, with the potential for inappropriate dosing, drug toxicity or loss of response.27 The development of point of care assays28 and methods for remote TDM (for example, using dried blood spots)29 may allow us to overcome these new barriers to TDM.
The COVID-19 pandemic has necessitated a significant shift in our working pattern, most notably the use of telemedicine, enabling us to maintain acute services. There are many obstacles to developing telemedicine services, especially over such an abridged timeframe, but a randomised controlled trial has shown that they reduce hospital visits and IBD admissions compared to standard of care.30, 31 Whilst remote monitoring may be preferable to some patients, others may prefer, or have symptoms that require, attendance for review or investigation. The pandemic has resulted in a step-change in the use of telemedicine in the UK and as the pandemic subsides, it is likely to remain a significant aspect of future outpatient services.
Finally, the cancellation of large numbers of clinic appointments, investigations, elective imaging and procedures enabled acute services to cope with the influx of patients with COVID-19. However, the huge backlog of work created, and the potential for “collateral damage” to patients who avoided or were unable to access healthcare for non-COVID-19-related issues will undoubtedly affect service provision for months or even years to come. Endoscopy in particular, since it is an aerosol generating procedure, remains hugely problematic with most units only just beginning to resume services, initially, with a significant reduction in capacity in most cases.1
This study builds upon our existing understanding of the impact that COVID-19 has had on IBD services in the UK. That knowledge, largely based upon a survey of IBD clinician opinions towards the beginning of the pandemic,25 has been corroborated and added to here, through the collection of data relevant to IBD clinical management and service provision. These aspects should be considered strengths of this study and may be considered helpful in planning service recovery. However, our study also has limitations. Most notably, its retrospective nature in a single centre with a relatively small sample size. In addition, due to the lack of data regarding COVID-19, the changes in prescribing habits described here were largely made on an empirical basis and before the development of national guidance2, 21 and the possible changes in outcomes that may result are not yet clear. These decisions, whilst not entirely in line with NICE guidance, were made in an MDM with experienced IBD specialists on the basis of current understanding of the evolving pandemic and the inherent risk to patients.
To conclude, we observed alterations in prescribing habits during the COVID-19 pandemic that often included bypassing immunomodulators and/or anti-TNF therapy in favour of vedolizumab and greater use of monotherapy. We also observed a rapid reorganisation of service provision that included a shift towards telemedicine and online solutions. Follow-up studies are essential to understand the impact of these changes on patient outcomes and are currently being planned.
5 ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this work was carried out as part of a service evaluation exercise.
ACKNOWLEDGEMENTS
Declaration of personal interests: Francesca D'Errico, Polychronis Pavlidis, Raphael Luber, Sebastian Zeki, Katie Hill, Alexa Duff, Dearbhaile O'Hanlon, Sherill Tripoli, Anna Stanton, Andra Caracostea, Rebecca Reynolds, Simon Anderson, Shuvra Ray, Joel Mawdsley and Jeremy Sanderson have no conflicts of interests to declare. Sailish Honap has served as a speaker for Janssen, Pfizer and Takeda. Susanna Meade has received speaker fees from Dr Falk Pharma. Esha Sharma has served as a speaker for Pharmacosmos. Mark A Samaan has served as a speaker, a consultant and/or an advisory board member for Sandoz, Janssen, Takeda, MSD, Falk and Samsung Bioepis. Peter M Irving has served as a speaker for AbbVie, Celgene, Falk Pharma, Ferring, MSD, Janssen, Pfizer, Takeda, Tillotts, Sandoz, Shire and Warner Chilcott, an advisory board member for AbbVie, Arena, Genentech, Gilead, Hospira, Janssen, Lilly, MSD, Pfizer, Pharmacosmos, Prometheus, Roche, Sandoz, Samsung Bioepis, Takeda, Topivert, VH2, Vifor Pharma, and has received research funding from MSD, Pfizer and Takeda.




