Specific aspartate residues in FET3 control high-affinity iron transport in Saccharomyces cerevisiae
Abstract
Site-directed mutagenesis was performed on a set of six aspartate residues of Fet3, the multicopper ferroxidase involved in high-affinity iron transport in Saccharomyces cerevisiae, in order to comprehend the molecular determinants of the protein function. Asp312, Asp315, Asp319 and Asp320 were predicted by homology modelling to be located in a negatively charged surface-exposed loop of the protein. Other two aspartate residues (Asp278 and Asp279) are placed close to the type 1 copper- and iron-binding sites, possibly linking these sites to the negatively charged region. In vivo results showed that mutation of Asp319 and Asp320 to yield D319N and D320N derivatives strongly impairs the ability of the yeast to grow under iron-limiting conditions. In particular, substitution of Asp320 with asparagine essentially abolished the Fet3-dependent iron transport activity. All other mutants (D278Q, D279N, D312N and D315I) behaved essentially as the wild-type protein. The electron paramagnetic resonance spectrum of the soluble forms of D319N and D320N showed significant changes of the copper sites' geometry in D319N but not in D320N. At variance with the membrane-bound forms, soluble D319N and D320N derivatives were highly susceptible to proteolytic degradation, suggesting that replacement of Asp319 or Asp320 locally modifies the structure of Fet3, making the protein sensitive to proteolysis when it is not protected by the membrane environment. In turn, this might be evidence of a shielding role of the permease Ftr1, which could interact with Fet3 at the level of the aspartate-rich negatively charged region. Copyright © 2005 John Wiley & Sons, Ltd.
Introduction
The handling of transition metals is a critical issue for all living cells, due to the dual nature of these elements, which are essential but can become extremely toxic if their levels are not tightly regulated in the cell. Iron presents a further problem in that it is usually found in the environment in the ferric form, which is scarcely soluble.
High- and low-affinity iron uptake systems have been recently identified in yeast and these studies have begun to shed light also on iron homeostasis mechanisms in humans, since many pathways appear to be functionally conserved, although most of the proteins involved do not share obvious sequence similarity. In Saccharomyces cerevisiae, the first step in iron uptake is the reduction of the metal to its more soluble ferrous form, which is then translocated across the plasma membrane either by the low-affinity system (Km 30 µM) constituted by the protein Fet4, or by the high-affinity system (Km 0.15 µM), which requires the activity of an oxidase–permease complex formed by Fet3 and Ftr1 (Radisky and Kaplan, 1999; Kosman, 2003). According to the currently accepted model, the plasma membrane multicopper oxidase Fet3 catalyses the oxidation of Fe2+ to Fe3+, which then enters the cell via the action of the iron permease Ftr1. The requirement for a multicopper oxidase is dictated by the ability of these enzymes to couple the one-electron oxidation of substrate(s) to full reduction of dioxygen to water, by way of a functional unit formed by three types of copper binding sites with different spectroscopic and functional properties. Type 1 blue copper is the primary electron acceptor from the substrate, while a trinuclear cluster formed by type 2 copper and binuclear type 3 copper is the oxygen binding and reduction site (Solomon et al., 1996). Site-directed mutagenesis experiments have been employed to confirm the assignment of the ligands to the different copper atoms required for catalytic activity of S. cerevisiae Fet3 (Blackburn et al., 2000) and also to identify residues suggested to be involved in the binding of iron, the ferroxidase substrate, on the basis of homology modelling predictions (Bonaccorsi di Patti et al., 2000, 2001; Wang et al., 2003; Quintanar et al., 2004).
The iron permease Ftr1 is predicted to be a polytopic membrane protein with six or, more probably, seven transmembrane segments, which presents two REXLE and several EXXE sequence motifs that have been suggested to be involved in iron binding (Stearman et al., 1996; Severance et al., 2004). The existence of a complex between Ftr1 and Fet3 is hypothesized also by the fact that each of the two proteins is required during biosynthesis for correct targeting of both of them to the plasma membrane. Ftr1 appears to be necessary for copper loading of Fet3, which takes place in a post-Golgi compartment (Yuan et al., 1997), possibly because the presence of Ftr1 is required for exit of Fet3 from the ER (Sato et al., 2004).
In our search to define the molecular features relevant for the structure and function of Fet3 within the context of the permease–oxidase complex, we have chosen to perform site-directed mutagenesis on a set of six aspartate residues, four of which (Asp312, 315, 319 and 320) are predicted by homology modelling to be located in a negatively charged surface-exposed region of the protein (Bonaccorsi di Patti et al., 1999). The other two aspartate residues (Asp278 and 279) are placed close to the type 1 copper and iron-binding sites, possibly linking these sites to the negatively charged region.
Materials and methods
Yeast strains and culture media
S. cerevisiae strain Δfet3 DEY1397-6A (MATα, fet3::HIS3, ade2, can1, his3, leu2, trp1, ura3) was used in this study. Yeast cells were grown at 30 °C in YPD (1% yeast extract, 2% peptone, 2% glucose) or in minimal medium (0.67% yeast nitrogen base without amino acids, with the necessary auxotrophic supplements) with 2% glucose (MD) or 2% galactose (MGal). The medium was made iron-limited by addition of the iron chelator bathophenanthroline disulphonate (BPS) 20 µM.
Fet3 mutagenesis
-
D278Q rev 5′-ggtgtcttgaaatttctgcatg-3′
-
D279N rev 5′-ggtgttatcaaatttctgcatg-3′
-
D312Nfwd 5′-cgtgaattcaattgataacttc-3′
-
D315Irev 5′-gaagttgataattgaatccacg-3′
-
D319N fwd 5′-cttcttgaacgatttctacttgc-3′
-
D320Nfwd 5′-cttcttggacaatttctacttgc-3′
Fet3 expression, purification and analysis
Recombinant full-length and secreted Fet3 expression was driven by the Gal1/10 promoter in the multicopy expression vector pYeDP (Bonaccorsi di Patti et al., 2001). The yeast Δfet3 strain DEY1397-6A was transformed by the lithium acetate method according to standard procedures (Ausubel et al., 1988) with wild-type, and each mutant pYFet3 and cells were grown in MGal medium lacking uracil.
Cells were prepared for immunofluorescence according to Davis-Kaplan et al. (2004). For visualization of Fet3p, the rabbit anti-Fet3p antibody was used (1 : 500) followed by either an Alexa 594 or Alexa 488 conjugated goat anti-rabbit (1 : 500). All of the fluorescent secondary antibodies were obtained from Molecular Probes.
Total membrane extracts were obtained following lysis of cells with glass beads (Bonaccorsi di Patti et al., 2000). Full-length Fet3 was purified by passage on Sepharose derivatized with chloroethylamine, followed by affinity chromatography on ConA-Sepharose as described (Bonaccorsi di Patti et al., 1999), with the only modification that the protein was eluted from the derivatized Sepharose at 300 mM NaCl. Secreted Fet3 was purified from the medium of cells cultured in MGal containing 25 mM Tris–acetate, pH 6.5, and CuSO4 30 µM, by ion-exchange chromatography on DE-52 cellulose equilibrated in 25 mM Mops, pH 7.4. The resin was washed with 25 mM Mops, pH 7.4, containing 75 mM NaCl and Fet3 was eluted at 150 mM NaCl.
Total protein content was determined with the microBCA assay (Pierce). Non-denaturing SDS-PAGE and staining for Fet3 oxidase activity with o-dianisidine (oDA) were performed as described (Bonaccorsi di Patti et al., 2000). Immunodetection of Fet3 was performed with a polyclonal rabbit antibody directed against a peptide spanning Fet3 residues 164–178, as described (Bonaccorsi di Patti et al., 2001) or with a polyclonal antibody directed against soluble deglycosylated Fet3 (a generous gift of Professor J. Kaplan). FLAG-tagged Fet3 was also detected with the peroxidase-conjugated anti-FLAG M2 monoclonal antibody (Sigma).
Oxidase activity was measured spectrophotometrically at 540 nm with 0.1–5 mM p-phenylenediamine (pPD) in 100 mM sodium acetate pH 5 at 30 °C. Ferroxidase activity was measured with ferrous ammonium sulphate 1–100 µM in 100 mM sodium acetate, pH 5, either by direct determination of Fe3+ at 315 nm or by the indirect ferrozine assay (De Silva et al., 1997). Data analysis to determine the kinetic parameters Vmax and Km was performed with GraphPad (Prism) software. Kinetic constants and standard errors are from a direct non-linear fit of all initial velocity data to the Michaelis–Menten equation. Low-temperature X-band EPR spectra were recorded on a Bruker ECS 106 spectrometer equipped with an ER4111VT temperature controller. Paramagnetic copper content was measured by double integration of the spectra vs. a Cu–EDTA standard. Total copper content was measured by atomic absorption spectroscopy with a Perkin-Elmer 3030 instrument equipped with a graphite furnace.
Iron uptake assay
Cells were grown overnight in MGal medium, inoculated in MGal for 8–10 h and 20 µM BPS was added for further 15 h. Cells were harvested at OD600 = 0.5–1.0 and iron uptake was performed with 55FeCl3 (75.184 mCi/mg; NEN Life Sciences), essentially according to published procedures (Eide et al., 1992; Bonaccorsi di Patti et al., 2001). 55Fe uptake was measured at 30 °C for 10 min in 50 mM Mes, pH 6.1, containing 2% galactose in the presence of 1 mM ascorbate. The assay samples were chilled on ice, vacuum-filtered through Whatman GF/C glass filters and washed with 10 ml ice-cold 20 mM citrate, pH 5.5, containing 5 mM EDTA. When used above 0.2 µM, radioactive iron was diluted with cold iron and specific activity was adjusted accordingly. Background levels due to non-specific uptake were obtained on parallel samples kept on ice and were subtracted before calculation of uptake rates. Cell-associated radioactivity was measured by liquid scintillation counting with an LKB 1211 Rackbeta counter.
Results
In vivo characterization
S. cerevisiae Fet3 aspartate residues 278, 279, 312, 315, 319 and 320 were targeted for site-directed mutagenesis and initially two multiple mutants were produced, one in which all six residues were replaced (6Asp: D278Q, D279N, D312N, D315N, D319N, D320N) and one where only the four residues of the putative surface exposed loop were substituted with asparagine (4Asn: D312N, D315N, D319N, D320N). Both mutant Fet3 proteins were expressed from a multicopy plasmid under control of the GAL promoter in a Δfet3 S. cerevisiae strain and their ability to complement the growth defect of this strain in iron-limiting conditions was evaluated. Neither mutant Fet3 supported yeast growth (Figure 1A) or iron uptake (Figure 1B) in the presence of BPS, i.e. under conditions where high affinity metal transport is favoured. Also, no oxidase activity was detected by non-denaturing SDS-PAGE on membrane extracts; however, expression of the recombinant protein was confirmed by Western blot (Figure 1C). In order to ascertain whether the lack of iron transport activity was due to incorrect post-translational processing, immunofluorescence analyses were carried out on S. cerevisiae Δfet3 transformed with Fet3 wild-type, Fet3 6Asp or Fet3 4Asn. The results, reported in Figure 2, unequivocally showed that the mutant proteins were correctly localized on the plasma membrane of the cells.
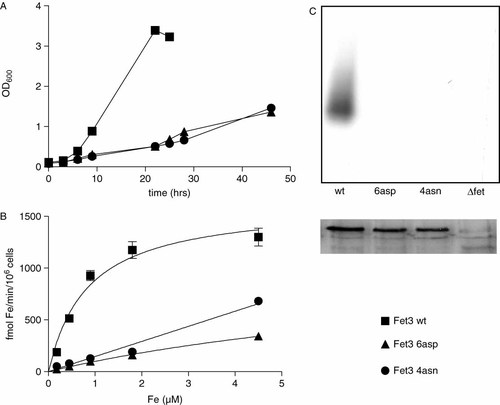
Growth curves in Mgal + BPS 10 µM (A), iron uptake (B) and SDS-PAGE analyses (C). The SDS-PAGE panel reports o-dianisidine staining (upper gel) and Western blot after deglycosylation with endo H (lower gel)
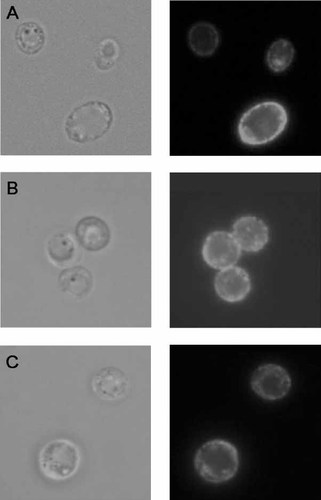
Immunofluorescence analysis of S. cerevisiae Δfet3 transformed with Fet3 wild-type (A), Fet3 6Asp (B) or Fet3 4Asn (C). The figure shows the clear membrane localization of all Fet3s
In the attempt to identify the aspartate residue(s) responsible for the loss of activity of Fet3, a set of six single mutants was produced and characterized. Fet3 D278Q, D279N, D312N and D315I were as efficient as Fet3 wild-type in supporting growth in the presence of BPS, while Fet3 D319N showed much slower growth and Fet3 D320N appeared to be unable to grow in iron-limited conditions (Figure 3A). Correspondingly, the kinetic parameters for high affinity iron uptake were comparable to those obtained with Fet3 wild-type for mutants D278Q, D279N, D312N and D315I, but iron transport was impaired for D319N and extremely low for D320N (Figure 3B, Table 1). Western blot SDS-PAGE analysis demonstrated that essentially equal amounts of recombinant protein were being synthesized (data not shown). On the other hand, staining for oxidase activity with o-dianisidine revealed important differences, since all mutants except D320N were positive, with D319N exhibiting less intense staining. This is shown in Figure 4, where representative ‘normally behaving’ mutants (D279N and D315I) are reported along with anomalous mutants D319N and D320N. If copper was omitted during lysis of cells, much lower amounts of oxidase-active Fet3 were detected both for wild-type and mutant proteins, this phenomenon being especially evident when membrane extracts were prepared from cells grown in the absence of BPS (Figure 4). Oxidase activity could be recovered by reconstitution of Fet3 with copper sulphate in the presence of ascorbate directly on the membrane extract. Fet3 D320N appeared to be devoid of oxidase activity, not only under any of growth and extraction conditions (Figure 4) but also after reconstitution (data not shown). Taken together, these results suggest that a fair amount of the recombinant Fet3 lacks copper, possibly because the protein is overexpressed compared to the endogenous permease Ftr1. It should be remembered that the presence of Ftr1 during biosynthesis of Fet3 has been repeatedly demonstrated to be essential for incorporation of copper in the ferroxidase, presumably because it leads to exit from the ER and correct processing through the secretory pathway (Stearman et al., 1996; Severance et al., 2004; Sato et al., 2004). This finding makes it possible to hypothesize that Ftr1 is limiting for complex formation in these conditions; therefore, since Ftr1 is induced by its own promoter, the amount of ferroxidase–permease complex should be the same in all cases (wild-type or mutant Fet3). This assumption is strengthened by the fact that reconstitution of surface Fet3 according to established procedures (Davis-Kaplan et al., 1998) did not increase iron uptake (Table 2), suggesting that only those Fet3 molecules ‘chaperoned’ out of the ER by/with Ftr1 are loaded with copper and reach the plasma membrane. The increase in oxidase-active protein clearly visible in Figure 4 would then be due to reconstitution of those Fet3 molecules trapped inside the cell, possibly in the ER. A direct consequence of this hypothesis would be that variations in Vmax for iron uptake would reflect alterations in ferroxidase catalysis and/or translocation of iron to Ftr1.
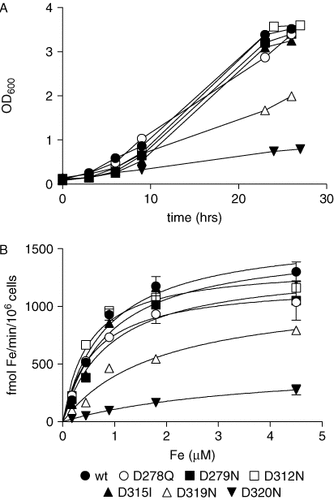
Growth curves in Mgal + BPS 20 µM (A) and iron transport (B) of S. cerevisiae ΔFet3 transformed with the following Fet3 derivatives: wild-type, D278Q, D279N, D312N, D315I, D319N and D320N

Oxidase activity staining of non-denaturing SDS-PAGE of Fet3 wild-type, Fet3 D279N, Fet3 D315I, Fet3 D319N and Fet3 D320N mutants grown in the presence of absence of 10 µM BPS, and extracted in the presence or absence of 50 µM CuSO4. Comparable amounts of protein extracts were loaded in each lane
| Fet3 | Km (µM Fe) | Vmax (fmol Fe/min/106 cells) |
|---|---|---|
| WT | 0.77 ± 0.16 | 1597 ± 115 |
| D278Q | 0.61 ± 0.08 | 1205 ± 51 |
| D279N | 0.89 ± 0.41 | 1335 ± 202 |
| D312N | 0.50 ± 0.16 | 1352 ± 127 |
| D315I | 0.82 ± 0.41 | 1526 ± 267 |
| D319N | 1.86 ± 0.52 | 1129 ± 151 |
| D320N | 3.68 ± 1.41 | 502 ± 108 |
| Fet3 | fmol Fe/min/106 cells | |
|---|---|---|
| −Cu | +Cu | |
| WT | 128 | 149 |
| D278Q | 152 | 179 |
| D279N | 144 | 144 |
| D312N | 126 | 130 |
| D315I | 71 | 104 |
| D319N | 102 | 119 |
| D320N | 82 | 82 |
In vitro characterization
In order to better characterize our Fet3 mutants, the soluble derivatives were produced by truncation of the protein at Gly555, essentially as described by Hassett et al. (1998). Wild-type and mutant proteins, tagged with a C-terminal FLAG epitope, were purified by anion exchange chromatography as detailed in the experimental section. In line with the results obtained in vivo, all mutants but D319N and D320N turned out to have catalytic and spectroscopic parameters statistically undistinguishable from those of wild-type secreted Fet3. Therefore, in the remaining part of this section we will focus our attention on D319N and D320N mutant proteins. SDS-PAGE analysis revealed that these mutant Fet3 proteins were partially degraded, as multiple bands were detected by the Fet3 polyclonal antibody (Figure 5A), fully reproducing the pattern obtained by Coomassie staining. As expected, detection with the anti-FLAG antibody yielded a more homogeneous electrophoretic pattern (Figure 5B). Oxidase activity staining confirmed that Fet3 D319N was less active than wild-type and D320N was practically inactive (Figure 5C). The copper content of the samples was low, with a stoichiometry of 1.25 Cu atoms/protein for wild-type, 3.44 for D319N and 2.07 for D320N. This result suggests that a significant amount of Fet3 wild-type and D320N is in the apo form, despite addition of copper during cell growth; however, this apo-protein could not be reconstituted by incubation with copper in reducing conditions, as judged by SDS-PAGE staining with oDA (data not shown). The EPR spectra of wild-type, D319N and D320N Fet3 are reported in Figure 6. The spectral lineshape of wild-type secreted Fet3 was found to be similar to that already reported (Hassett et al., 1998). In particular, features attributable to type 1 copper were clearly discernible, and the measured A// value (91.5 G) was in line with that in the literature (Hassett et al., 1998). The spectra of D319N and D320N mutants, on the other hand, appeared somewhat different, in particular the hyperfine structure in the parallel region was less defined, possibly due to linewidth broadening, and the  value was slightly higher. Nevertheless, features from type 1 copper were still unequivocally evident, at least in the D320N mutant, despite the unfavourable signal : noise ratio. Paramagnetic copper content was 41.5%, 35.5% and 49.1% for Fet3 wild-type, D319N and D320N, respectively, in line with the 50% expected value. Catalytic parameters obtained with iron and pPD as substrates are shown in Table 3. Fet3 D320N showed very low activity, thus catalytic parameters obtained for this mutant should be viewed with caution. The most prominent feature concerning the oxidase activity of the mutant Fet3 proteins was a large decrease in Vmax, while Km values for pPD appeared not to be affected, being even lower than wild-type. For both mutants the ferroxidase Vmax value was decreased too, but in this case Km for iron was also altered, particularly for Fet3 D319N, which showed an almost 10-fold increase compared to wild-type.
value was slightly higher. Nevertheless, features from type 1 copper were still unequivocally evident, at least in the D320N mutant, despite the unfavourable signal : noise ratio. Paramagnetic copper content was 41.5%, 35.5% and 49.1% for Fet3 wild-type, D319N and D320N, respectively, in line with the 50% expected value. Catalytic parameters obtained with iron and pPD as substrates are shown in Table 3. Fet3 D320N showed very low activity, thus catalytic parameters obtained for this mutant should be viewed with caution. The most prominent feature concerning the oxidase activity of the mutant Fet3 proteins was a large decrease in Vmax, while Km values for pPD appeared not to be affected, being even lower than wild-type. For both mutants the ferroxidase Vmax value was decreased too, but in this case Km for iron was also altered, particularly for Fet3 D319N, which showed an almost 10-fold increase compared to wild-type.
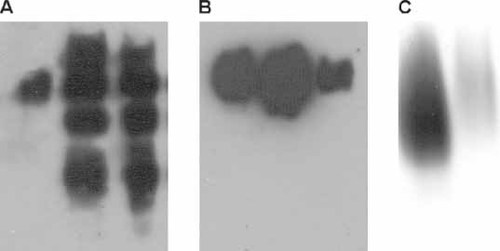
SDS-PAGE analysis of secreted Fet3 wild-type (left lanes), Fet3 D319N (middle lanes) and Fet3 D320N (right lanes). (A) Western blot with anti-Fet3; (B) Western blot with anti-FLAG; (C) oxidase activity staining with o-dianisidine. Amounts of loaded proteins were 1.5 µg, 2.5 µg, 6 µg (Western blots), and 2.5 µg, 12.5 µg, 40 µg (activity staining) for Fet3 wt, Fet3 D319N and Fet3 D320N, respectively
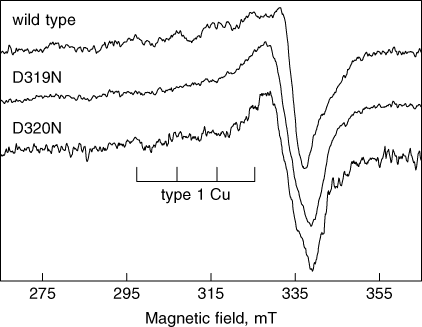
X-band EPR spectra of Fet3 wild-type, Fet3 D319N, Fet3 D320N. Experimental conditions: frequency, 9.5 GHz; power, 20 mW; modulation, 10 G; temperature 110 °C
| Fet3 | Vmax (pPD) (ΔA540/min) | Km (pPD) (mM) | Vmax (Fe) (µM Fe/min) | Km (Fe) (µM) |
|---|---|---|---|---|
| WT | 0.0605 ± 0.004 | 0.243 ± 0.07 | 3.982 ± 0.317 | 4.003 ± 1.28 |
| D319N | 0.0023 ± 0.0009 | 0.124 ± 0.05 | 0.760 ± 0.134 | 39.2 ± 12.85 |
| D320N | 0.00064 ± 0.00003 | 0.133 ± 0.03 | 0.108 ± 0.017 | 9.291 ± 4.92 |
Discussion
Since an experimentally determined three-dimensional structure of Fet3 is not available, many molecular details of the function of this protein remain unclear, particularly regarding the mechanism of iron translocation/channelling to the permease Ftr1 and the interactions between these two proteins. Much work has been done in the effort to identify the iron-binding site of Fet3 on the basis of homology modelling, with satisfactory results that have unambiguously assigned Glu185 as involved in iron binding (Bonaccorsi di Patti et al., 2000, 2001; Wang et al., 2003; Quintanar et al., 2004). Our model predicts the presence of a strongly negatively charged surface region, which might be important for iron handling and protein–protein interactions (Bonaccorsi di Patti et al., 1999). The presence of negatively-charged residues is a constant in iron-binding/trafficking proteins. As an example, five out of six residues in the putative iron coordination site of ceruloplasmin, the vertebrate homologue of Fet3, are Asp or Glu (Lindley et al., 1997). Also, Fet4 in S. cerevisiae—the protein supporting low-affinity iron uptake (Dix et al., 1994, 1997)—has iron-binding/trafficking elements rich in Asp and Glu, and their substitution with alanine abolishes Fet4-dependent iron uptake (Dix et al., 1997). Asp278 and Asp279 are located close to the active site of Fet3 and could link it to this region, which comprises Asp312, 315, 319 and 320. Only Asp278 and Asp320 are conserved among different yeast ferroxidases. A negatively charged residue (Asp or Glu) is found in position 312, while Asp279 is either substituted with Glu in S. cerevisiae Fet5, Arxula adeninivorans Fet3 and Schizosaccharomyces pombe Fio1 or with Thr in Candida albicans Fet3, Asp315 is replaced with Ile in A. adeninivorans Fet3 and Sz. pombe Fio1, and Asp319 is changed to Asn in S. cerevisiae Fet5 (Figure 7). These six aspartate residues have been substituted by site-directed mutagenesis to eliminate the negative charge and two multiple mutants (6Asp and 4Asn) and a set of six single mutants have been produced. Residues 279, 312, 319 and 320 have been changed to Asn; Ile was chosen to replace Asp315, since this residue is found in other two ferroxidases, and Asp278 was substituted with Gln to avoid introducing a potential N-glycosylation site. The results obtained for the multiple mutants indicate that both these proteins are no longer functional, as they are unable to support significant high-affinity iron uptake. Functional analysis of the six single mutants has allowed us to identify residues 319 and 320 as responsible for this result. As a matter of fact, Fet3 mutants D278Q, D279N, D312N and D315I were comparable to the wild-type protein in their ability to restore growth in iron-limited conditions and high affinity iron uptake in a Δfet3 strain. Our results with Fet3 D278Q are similar to those obtained by others with a D278A mutant (Wang et al., 2003), which showed a slight decrease in Vmax for iron transport and no significant change in Km. Moreover, a double mutant Fet3 D278Q/D279N essentially reproduced the results obtained with the D278Q protein (data not shown). Replacement of residues 312 and 315 had no apparent effect on the activity of Fet3, while substitution of aspartates in position 319 and 320 was clearly deleterious. Fet3 D319N showed a significantly altered growth in conditions of iron limitation and high-affinity iron uptake was impaired, with both a decrease in Vmax and an increase in Km. The impact of substitution D320N was even more severe, as the mutated ferroxidase reproduced the results obtained with the multiple mutants 6Asp and 4Asn: practically no growth in BPS or high-affinity iron transport, and no detectable oxidase activity on non-denaturing SDS-PAGE.
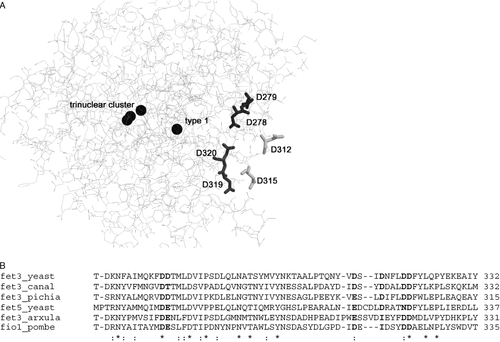
(A) Fet3 model showing the aspartate residues mutated in this study. Also shown are the four constitutive copper atoms. (B), sequence alignment in the region of mutated aspartates of Fet3 and Fet5 proteins from various sources
Mutations at Asp319 or Asp320 do not involve major changes of the immediate surroundings of the copper sites, as suggested by EPR spectra of Fet3 D319N and D320N. While the spectra of the mutants should be considered significantly different from that of the wild-type protein, in terms of both linewidths and some magnetic parameters (i.e. the  value), they still maintain the features typical of this multicopper oxidase (Wang et al., 2003; Hassett et al., 1998), with particular regard to the type 1 copper hyperfine structure. In this respect, it is worth noting that D320N Fet3, although being less active than D319N, appears spectroscopically more similar to the wild-type secreted protein, with a better resolved hyperfine structure (Figure 6). This result suggests that the loss of high-affinity iron transport observed with these two mutants is not related to gross misfolding of the mutated ferroxidase, but rather to subtle alteration of the electron pathways, leading to catalytic activity of the protein and/or of the translocation of iron to the permease Ftr1. The essentially normal EPR spectra of wild-type and D320N also indicate that the anomalous copper stoichiometries found for these secreted derivatives (1.25 and 2.07 Cu atoms/protein for wild-type and D320N, respectively) do not arise from partial saturation of the copper sites, but rather from the presence of apo- and holo-form mixtures.
value), they still maintain the features typical of this multicopper oxidase (Wang et al., 2003; Hassett et al., 1998), with particular regard to the type 1 copper hyperfine structure. In this respect, it is worth noting that D320N Fet3, although being less active than D319N, appears spectroscopically more similar to the wild-type secreted protein, with a better resolved hyperfine structure (Figure 6). This result suggests that the loss of high-affinity iron transport observed with these two mutants is not related to gross misfolding of the mutated ferroxidase, but rather to subtle alteration of the electron pathways, leading to catalytic activity of the protein and/or of the translocation of iron to the permease Ftr1. The essentially normal EPR spectra of wild-type and D320N also indicate that the anomalous copper stoichiometries found for these secreted derivatives (1.25 and 2.07 Cu atoms/protein for wild-type and D320N, respectively) do not arise from partial saturation of the copper sites, but rather from the presence of apo- and holo-form mixtures.
In vitro, Fet3 D319N and D320N showed Km values for pPD as substrate similar to that of wild-type Fet3; on the other hand, Km for Fe2+ was notably higher for Fet3 D319N (39.2 vs. 4 µM) and probably quite similar to wild-type for Fet3 D320N, due to the large error in the measure. A very large decrease in Vmax was evident, with residual pPD-oxidase Vmax of 4% and 1% and ferroxidase Vmax of 19% and 3% for Fet3 D319N and D320N, respectively, suggesting that catalysis is strongly perturbed. Since the mutants are particularly susceptible to proteolysis (see Figure 5A), the possibility exists that the real Vmax is much higher than the measured value, as probably only the fraction of undegraded protein contributes to the activity. This could be true at least for Fet3 D319N, as the semi-purified membrane-bound protein had a Vmax for iron oxidation similar to wild-type (3.20 vs. 3.99 µM Fe/min, data not shown). However, since neither of these mutants properly supported iron transport in vivo, i.e. when proteolytic cleavage did not occur, a role for proteolytic degradation in the poor performance of Fet3 D319N and D320N within the oxidase-permease complex appears unlikely. Therefore, decrease of high-affinity iron uptake Vmax would be due to other causes (see below). The Km value for iron oxidation of purified Fet3 D319N is much higher than that observed for iron transport in vivo, consistent with the general behaviour of the Fet3 system (Bonaccorsi di Patti et al., 2001; Wang et al., 2003; De Silva et al., 1997); however, in both instances this is higher than that observed with wild-type Fet3, suggesting that interaction with iron is also perturbed in this mutant. Fet3 D320N showed very low activity in vitro and in vivo it was also not very efficient in supporting high-affinity iron uptake.
From a molecular point of view, the lack of an experimentally determined structure of Fet3 prevents us from assessing exactly what the effects of mutations D319N and D320N can be; nevertheless, several important postulates can be made. According to homology modelling of Fet3, which proved to be a reliable approach to predict the role of residues involved in iron binding (Bonaccorsi di Patti et al., 2000, 2001; Wang et al., 2003; Quintanar et al., 2004), Asp319 and Asp320 are unlikely to be part of the iron-binding site. They would instead be located in a surface exposed loop and we can hypothesize that they may be involved in iron translocation to the permease Ftr1. Asp319 may be part of a pathway for iron on the surface of Fet3 and Asp320 (which is conserved among established yeast ferroxidases) may be part of an iron-binding (trafficking?) site shared between Fet3 and Ftr1. The possibility of the existence of a shared site has been put forward recently (Severance et al., 2004). The decrease in Vmax exhibited by the (mutated) ferroxidase–permease complex in vivo would then be due to inefficient translocation of oxidized iron within the complex. In accordance with this view, Km values for iron appear not to be strongly altered for Fet3 D320N both in vitro and in vivo (less than five-fold increase), while perturbation of Km is much higher in vitro for Fet3 D319N. It should be remembered that the affinity for iron of the ferroxidase has been suggested to be limiting for affinity of the whole complex (Bonaccorsi di Patti et al., 2001).
Finally, it is interesting to note that the secreted forms of D319N and D320N employed for this study were quite susceptible to proteolytic degradation, at variance with wild-type secreted Fet3. This lability was also noted for the secreted 6Asp and 4Asn mutants (data not shown), but it was not at all evident for the corresponding membrane-bound mutant proteins (see Figure 1). This result immediately suggests that replacement of Asp319 and/or Asp320 locally modifies the structure of Fet3, making the protein sensitive to proteolysis when it is not protected by the membrane environment. A possible explanation in this regard is that Asp319 and Asp320 reside at the Fet3–Ftr1 interface, and that the stability to proteolysis of the membrane-bound forms of D319N and D320N is physically due to the shielding action of the permease; however further work is needed to clarify this point.
Acknowledgements
We thank Dr Maurizio Minetti and Dr Donatella Pietraforte (Istituto Superiore di Sanità) for their kindness and support during EPR measurements, and Dr Jerry Kaplan for helping with the immunofluorescence experiments. This work was partially supported by University of Messina, Grant No. P.R.A 2002, to G.M.




