Long-Term Mortality in Acute Pancreatitis—A Population-Based Cohort Study
Funding: This work was supported by the Centre for Clinical Research Sörmland, Uppsala University, Sweden, grant number DLL-941252, and the Stockholm Research Council, Sweden, grant number FoUI-961115.
ABSTRACT
Background
Acute pancreatitis is a potentially life-threatening inflammation of the pancreas, with a rising incidence in most countries. Recent studies have suggested that acute pancreatitis is associated with increased long-term mortality. However, the extent to which this association is influenced by the development of chronic pancreatitis or comorbid conditions, such as malignant disease, remains unclear.
Objective
To assess the association between acute pancreatitis and long-term all-cause mortality.
Methods
The Swedish Pancreatitis Cohort (SwePan) was used, including all individuals with a first-time episode of acute pancreatitis in Sweden between 1990 and 2019 who survived the index hospital stay and 1:10 matched pancreatitis-free individuals from the general population. Multivariable conditional Cox proportional hazard models were used to compare mortality among individuals with acute pancreatitis compared with the matched pancreatitis-free control group.
Results
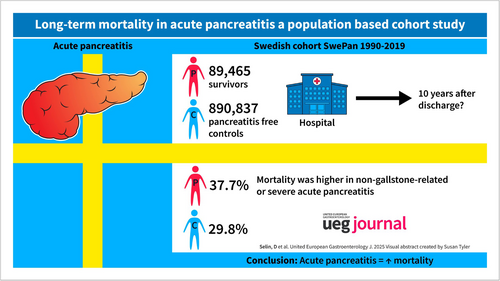
In total, 89,465 individuals discharged from hospital with acute pancreatitis and 890,837 matched pancreatitis-free individuals were followed up for 10,155,039 person-years (mean 10.0 years). There were 33,764 (37.7%) deaths among individuals with acute pancreatitis and 265,403 (29.8%) deaths among controls. In multivariable adjusted models, mortality was increased in individuals with acute pancreatitis throughout the follow-up period, particularly among those with severe and non-gallstone-related acute pancreatitis as compared to the matched controls. These results remained statistically significant after censoring the follow-up time for recurrent acute pancreatitis or a diagnosis of chronic pancreatitis.
Conclusions
Acute pancreatitis was associated with increased long-term mortality, even after adjusting for comorbidities, including cancer, and censoring for recurrent acute pancreatitis or chronic pancreatitis. Future research should assess causes of death and focus on understanding long-term morbidity to facilitate prevention through tailored follow-up strategies.
Graphical Abstract
SUMMARY
-
Summarise the established knowledge on this subject
- ◦
Previous studies suggest an association between acute pancreatitis and increased long-term mortality after hospital discharge.
- ◦
The extent to which comorbidities, recurrent acute pancreatitis, or chronic pancreatitis contribute to this association remains unclear.
- ◦
-
What are the new findings of this study?
- ◦
Acute pancreatitis was associated with increased long-term mortality, partly but not entirely explained by recurrent acute pancreatitis and chronic pancreatitis.
- ◦
Mortality was highest shortly after hospital discharge, declined over the first year, but remained elevated beyond 10 years of follow-up.
- ◦
Nongallstone-related acute pancreatitis and severe acute pancreatitis were associated with higher long-term mortality.
- ◦
Abbreviations
-
- CI
-
- confidence interval
-
- HR
-
- hazard ratio
-
- ICD code
-
- international classification of diseases code
1 Introduction
Acute pancreatitis is a potentially life-threatening inflammation of the pancreas. Its incidence is rising in many Western countries and is currently estimated at 20–40 cases per 100,000 person-years [1-3]. While short-term outcomes such as multiple organ failure, intensive care unit admissions, and in-hospital mortality have been extensively studied [4-10], less attention has been given to long-term morbidity following an episode of acute pancreatitis.
Acute pancreatitis induces an immense systemic inflammatory response, particularly in severe cases, resembling the response seen in sepsis [11-13]. Since sepsis is associated with an increased long-term mortality [14, 15], it is reasonable to hypothesize that acute pancreatitis may have a similar impact. Additionally, post-pancreatitis development of exocrine and endocrine dysfunction of the pancreas is well-described, conditions that are exacerbated by recurrent episodes of the disease [16, 17]. This dysfunction may also contribute to increased long-term mortality.
Some previous studies have reported on the long-term mortality after acute pancreatitis; however, the vast majority of these studies either lacked control groups, were of limited sample size, or were not designed to specifically study long-term mortality [3, 18-27]. However, recent work by Czapári et al. stands out as an exception [28]. They evaluated long-term mortality in a prospective multicentre cohort of 2613 individuals with acute pancreatitis. Their findings revealed an increased long-term mortality compared with the general population. However, the study had some limitations including the absence of a matched control group. Additionally, the methodology did not permit assessment of the isolated impact of a single episode of acute pancreatitis, excluding cases complicated by chronic pancreatitis, recurrent episodes, or coexisting malignant disease.
By using a large population-based cohort, the aim of this study was to provide a comprehensive assessment of the long-term mortality in adults with acute pancreatitis during the last 3 decades in Sweden.
2 Materials and Methods
2.1 Study Design and Setting
This was a nationwide matched cohort study based on the Swedish Pancreatitis Cohort (SwePan) database, designed to assess the long-term mortality in all Swedish individuals hospitalized for a first-time episode of acute pancreatitis between January 1, 1990, and December 31, 2019 compared to a matched pancreatitis-free population. The SwePan database has been described in detail elsewhere [29].
2.2 Ethical Permissions
The study was approved by the Central Ethical Review Board in Stockholm, Sweden (Ref. no. 2010/920-31/4 and 2015/0090-32). Informed consent is not required according to Swedish legislation regarding register-based research.
2.3 Assessment and Categorization of Pancreatitis
Individuals discharged from a Swedish hospital with a first-time diagnosis of acute pancreatitis between 1990 and 2019 were identified using the Swedish Patient Register. None of the patients had a history of acute or chronic pancreatitis prior to 1990. Episodes of acute pancreatitis were categorized as gallstone-related or non-gallstone-related. In addition, non-gallstone-related episodes were divided into two subgroups: (i) alcohol-related and (ii) non-alcohol-non-gallstone-related.
Gallstone-related acute pancreatitis was defined by (i) a diagnosis of biliary acute pancreatitis or (ii) a diagnosis of acute pancreatitis combined with a diagnosis of gallstone disease and/or gallstone-related surgery or intervention during the index hospital stay or within 90 days of discharge. All other episodes were classified as non-gallstone-related acute pancreatitis. Alcohol-related acute pancreatitis was defined as (i) a diagnosis of alcohol-induced acute pancreatitis or (ii) a diagnosis of acute pancreatitis combined with a diagnosis related to alcohol abuse at any time before or during the index hospital stay. All other non-gallstone-related episodes were classified as non-alcohol-non-gallstone-related acute pancreatitis. Individuals who fulfilled the criteria for gallstone-related acute pancreatitis were categorized as such, irrespective of any diagnosis of alcohol-related disease. (See Supporting Information S1: eTable 1 for the specific diagnosis codes and procedure codes used to categorize the subgroups of acute pancreatitis.)
Recurrent acute pancreatitis was defined as a new hospital admission due to acute pancreatitis at any time after the index hospital stay. Chronic pancreatitis was defined by a diagnostic code of chronic pancreatitis (ICD-10 K86, ICD-9 577B, 577W) at any time after the index hospital stay.
Severe acute pancreatitis was defined by either (i) admission to an intensive care unit, (ii) diagnosis codes or procedure codes indicating acute respiratory failure, acute renal failure, surgical-, endoscopic-, or percutaneous drainage of fluid collections, abdominal decompression, or drainage of pleural fluid collections, or (iii) inpatient care exceeding 14 days. The third criterion was used to capture individuals with prolonged hospital stay due to complications of acute pancreatitis, such as pseudocysts that were not drained and thus not accounted for by the first two criteria. Cardiovascular failure was not included as criteria for severe disease since ICD-10 does not separate acute from chronic heart failure, which could have introduced misclassification bias. All other episodes were classified as non-severe acute pancreatitis. (See Supporting Information S1: eTable 2 for the specific diagnosis codes and procedure codes used to categorize the severity of acute pancreatitis.)
2.4 Control Group
For each individual with acute pancreatitis, up to 10 control individuals from the Swedish background population were randomly selected based on age, sex, calendar period and municipality of residence. Survivor sampling was used; that is, individuals who developed acute pancreatitis at any time during the study period were not eligible as controls. The identification of controls was conducted by Statistics Sweden.
2.5 Exclusions
Individuals younger than 18 years at the index date were excluded as were individuals who died during the index hospitalization for acute pancreatitis (i.e., date of death on the day of hospital discharge or 1 day thereafter). To allow for isolation of the effects of a single episode of acute pancreatitis, individuals with a diagnosis of acute or chronic pancreatitis prior to the index date were excluded. Furthermore, individuals with pancreatic cancer prior to the index date or who developed pancreatic cancer within 1 year of the index date were excluded because of the possibility of misdiagnosis and reverse causation. Finally, individuals with a reused or erroneous personal identity number were excluded.
2.6 Outcome
Mortality after hospital discharge was obtained through the Swedish Cause of Death Register.
2.7 Covariates
The examined comorbidities were alcohol abuse, cancer, cardiovascular disease, chronic obstructive pulmonary disease, liver disease, dementia, connective tissue disease, diabetes mellitus (type 1 and 2), hypertriglyceridemia, moderate or severe chronic kidney failure, and obesity. Comorbidity status was determined by registered ICD-codes in the Swedish Patient Registry for all comorbidities except cancer status, which was determined by data from the Swedish Cancer Registry. Furthermore, comorbidity status was determined on the day before the index date for all comorbidities except for (i) cancer status, for which a diagnosis within 5 years of the index date was used and (ii) alcohol abuse, for which a diagnosis before or at the index date was considered. (See Supporting Information S1: eTable 3 for the specific diagnosis codes of each comorbidity.) Educational level was categorized as school attainment less than 10 years, 10–12 years, and more than 12 years. Disposable annual income was categorized into intervals of less than 5000 USD, 5001–10,000 USD, 10,001–15,000 USD, 15,001–20,000 USD, 20,001–25,000, 25,001–30,000 USD, and more than 30,000 USD. The country of birth was split into two categories: Sweden or other.
2.8 Statistical Analyses
Person-years of follow-up for all study participants were calculated from the date of hospital discharge until the date of death, the date of emigration, or the end of the study period (December 31, 2019). The survival functions of individuals with acute pancreatitis, stratified by type and severity, and the pancreatitis-free control population were estimated using Kaplan–Meier estimates.
Multivariable conditional Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) of mortality (after hospital discharge) among the cases with acute pancreatitis compared to the pancreatitis-free control population. Cox models were chosen for their ability to handle censoring, adjust for confounding, and provide easily interpretable results. Given that the proportional hazard assumption was unlikely to hold, two predefined approaches were applied to evaluate non-proportional hazards within the Cox framework: (i) time-stratified analyses with follow-up time categorized into 0–1 month, 1–3 months, 3–6 months, 6–12 months, 1–5 years, 5–10 years, and more than 10 years; and (ii) restricted cubic spline models with follow-up time split into 3 months intervals and using five knots (positioned at the 5th, 27.5th, 50th, 72.5th, 95th percentile of the follow-up time) [30].
Cox models with two different sets of covariates were constructed. The crude model included only the matching variables, that is, age (continuous, years), sex (male or female), and calendar period of index hospitalization (5-year intervals). The second model included the matching variables and educational level (< 10, 10–12, or more than 12 years), country of birth (Sweden or other), disposable annual income (categories defined above), alcohol abuse (no or yes), cancer (no or yes), cardiovascular disease (no or yes), chronic obstructive pulmonary disease (no or yes), connective tissue disease (no or yes), diabetes mellitus (no or yes), hypertriglyceridemia (no or yes), obesity (no or yes), dementia (no or yes), liver disease (no or yes) and moderate to severe chronic kidney disease (no or yes). The crude model was used for time-stratified analyses of the whole population only. The adjusted model was used for time-stratified analyses of the whole study population as well as for subgroups by the type and severity of acute pancreatitis. The restricted cubic spline model was used to compare subgroups of type and severity of acute pancreatitis and was based on the adjusted model.
The following sensitivity analyses were conducted: (i) time-stratified models restricted to the year 2000 and onward (to assess the effect of acute pancreatitis on mortality in a more recent healthcare setting); (ii) time-stratified models restricted to individuals without any known comorbidity (as defined above) at the index date and with an educational level of 9 years or more (to reduce residual confounding by lifestyle factors); (iii) time-stratified models restricted to individuals younger than 50 years at the index date (to investigate the effect of acute pancreatitis on mortality among individuals with lower expected mortality rates); and (iv) time-stratified models among individuals with non-alcohol-non-gallstone-related acute pancreatitis and alcohol-related acute pancreatitis (to investigate the effect of alcohol abuse on long-term mortality). All analyses were conducted with and without censoring the follow-up time for recurrent acute pancreatitis or chronic pancreatitis.
Due to pronounced effects of acute pancreatitis on long-term mortality in individuals younger than 50 years at the index date in preliminary analyses, the following post hoc analyses were performed: (i) Kaplan–Meier survival estimates restricted to individuals younger than 50 years at the index date; (ii) time-stratified models restricted to individuals younger than 50 years at the index date, with stratification for type and severity of acute pancreatitis and with and without censoring the follow-up time for chronic pancreatitis or recurrent acute pancreatitis. The model was adjusted for the same covariates as those listed above. Follow-up time in the stratified analyses was categorized to 0–6 months, 6–12 months, 1–5 years, 5–10 years and more than 10 years to accommodate the lower number of events in each subgroup.
All statistical analyses were performed using the statistical software Stata 17.0 (StataCorp LP, College Station, TX, USA).
3 Results
After exclusion of individuals who died during the index hospital stay (4422 individuals), the final cohort consisted of 89,465 individuals with a first-time episode of acute pancreatitis who were discharged from hospital alive and 890,837 matched controls (Table 1). Individuals were followed up for a mean of 10.0 years for a total of 10,155,039 person-years of follow-up. There were 33,764 deaths (37.7%) among individuals with acute pancreatitis and 265,403 deaths (29.8%) among controls. During follow-up, 17,522 individuals with acute pancreatitis (19.6%) experienced one or more episodes of recurrent acute pancreatitis and 6808 individuals (7.6%) developed chronic pancreatitis. The median time from hospital discharge to the first episode of recurrent acute pancreatitis was 249 days, while the median time to the development of chronic pancreatitis was 515 days. Notably, 3578 individuals (52.6%) who developed chronic pancreatitis had at least one prior episode of recurrent acute pancreatitis.
| Individuals with acute pancreatitis | Pancreatitis-free population | |||||
|---|---|---|---|---|---|---|
| Gallstone-related | Non-gallstone-related | Total (all causes) | ||||
| Total | Alcohol-related | Non-gallstone-non-alcohol-related | ||||
| Number of individuals, n (percent of total cases) | 41,454 (46.3) | 48,011 (53.7) | 7048 (7.9) | 40,963 (45.8) | 89,465 | 890,837 |
| Severe disease, n (percent of cases by cause) | 5601 (13.5) | 6650 (13.9) | 1093 (15.5) | 6771 (16.5) | 12,251 (13.7) | n/a |
| Male sex, n (percent) | 17,687 (42.7) | 29,248 (60.9) | 5443 (77.2) | 23,805 (58.1) | 46,935 (52.5) | 467,074 (52.4) |
| Median age, years (interquartile range) | 64 (48–76) | 60 (46–73) | 53 (44–62) | 62 (47–75) | 62 (47–75) | 62 (47–75) |
| Mean follow-up time, years | 9.0 | 9.5 | 9.4 | 9.5 | 9.3 | 10.1 |
| Person-years at risk, person-years | 372,662 | 455,027 | 65,998 | 389,028 | 827,689 | 9,327,350 |
| Deaths during follow-up, number of deaths (percent) | 13,766 (33.2) | 19,998 (41.7) | 3588 (50.9) | 16,410 (40.0) | 33,764 (37.7) | 265,403 (29.8) |
| Charlson comorbidity index ≥ 1, n (percent) | 15,005 (36.2) | 20,276 (42.2) | 3385 (48.0) | 16,891 (41.2) | 35,281 (39.4) | 249,828 (28.0) |
| Higher education (> 12 years), n (percent) | 8432 (20.1) | 7734 (16.1) | 804 (11.4) | 6930 (16.9) | 16,076 (18.0) | 214,263 (24.1) |
| Disposable annual income exceeding 30,000 USD, n (percent) | 5289 (12.8) | 5611 (11.7) | 578 (8.2) | 5033 (12.3) | 10,900 (12.2) | 141,731 (15.9) |
| Country of birth outside of Sweden, n (percent) | 6774 (16.3) | 7818 (16.3) | 1.246 (17.7) | 6572 (16.0) | 14,592 (16.3) | 121,929 (13.7) |
Individuals with acute pancreatitis had a higher prevalence of comorbidities, lower level of education and lower annual income compared with the control group. Among the acute pancreatitis cases, 45.2% were classified as gallstone-related, and 15.1% were classified as severe.
Kaplan–Meier estimates showed lower survival among individuals with acute pancreatitis compared with the pancreatitis-free control population (Supporting Information S1: eFigure 1). In the multivariable adjusted Cox model (Figure 1), the increased mortality was most pronounced during the first months of hospital discharge (HR 3.54 [95% CI 3.26–3.84] and 2.48 [95% CI 2.32–2.65] for the 0–1 and 1–3 monthtime strata, respectively). Thereafter, mortality declined but still remained significantly elevated throughout the study period (HR 1.27 [95% CI 1.24–1.30] in the long-term [> 10 years] time strata). The HRs were slightly attenuated after censoring for recurrent acute pancreatitis or chronic pancreatitis.
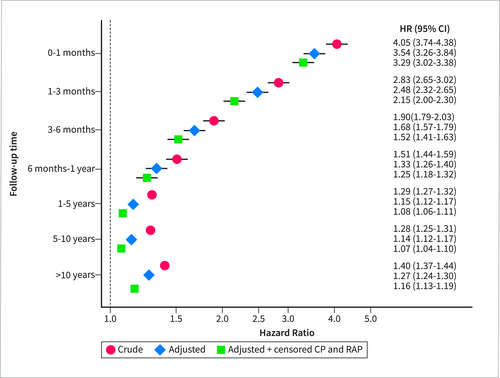
Hazard ratios (HR) and corresponding 95% confidence intervals (CI) for all-cause mortality in individuals with acute pancreatitis compared with matched individuals (without the disease), with and without adjustment for covariates (alcohol abuse, cardiovascular disease, chronic obstructive pulmonary disease, obesity, hypertriglyceridemia, liver disease, dementia, diabetes, renal failure, cancer, autoimmune disease, education level, country of birth and disposable income) and censoring for recurrent acute pancreatitis (RAP) and/or chronic pancreatitis (CP). All models were adjusted for the matching variables (year of birth, sex, municipality of residence and calendar period). Follow-up was started at the date of hospital discharge.
Subgroup analyses using time-stratified and restricted cubic spline models found increased HRs irrespective of cause and severity during the first 6 months after hospital discharge (Table 2, Figures 2 and 3). All causes and severities except non-severe gallstone-related acute pancreatitis were associated with increased mortality beyond 10 years of follow-up. HRs were generally higher in severe and non-gallstone-related episodes. After censoring for recurrent acute pancreatitis or chronic pancreatitis, the HRs attenuated towards 1 but remained increased in non-gallstone-related pancreatitis (HR 1.27 [95% CI 1.23–1.33] and HR 1.47 [95% CI 1.33–1.63] in the long-term [> 10 years] time strata for non-severe and severe episodes, respectively).
| All-cause mortality | ||||||||
|---|---|---|---|---|---|---|---|---|
| Follow-up time | Gallstone-related | Non-gallstone-related | ||||||
| Non-severe | Severe | Non-severe | Severe | |||||
| Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | |
| 0–1 month | 1.30 (1.07–1.58) | 1.19 (0.97–1.47) | 5.02 (3.92–6.44) | 4.91 (3.79–6.35) | 3.43 (3.02–3.90) | 3.17 (2.77–3.62) | 11.95 (9.94–14.38) | 11.28 (9.33–13.64) |
| 1–3 months | 1.51 (1.32–1.72) | 1.32 (1.14–1.52) | 4.18 (3.45–5.07) | 3.53 (2.85–4.36) | 2.22 (2.00–2.46) | 1.92 (1.72–2.15) | 6.45 (5.49–7.57) | 5.84 (4.92–6.93) |
| 3–6 months | 1.24 (1.10–1.40) | 1.17 (1.03–1.33) | 2.65 (2.19–3.22) | 2.40 (1.94–2.96) | 1.57 (1.42–1.73) | 1.42 (1.28–1.58) | 3.42 (2.90–4.03) | 2.97 (2.47–3.57) |
| 6 months–1 year | 1.01 (0.92–1.10) | 0.99 (0.90–1.08) | 1.50 (1.26–1.78) | 1.33 (1.10–1.61) | 1.41 (1.31–1.52) | 1.33 (1.22–1.44) | 2.25 (1.94–2.58) | 2.01 (1.72–2.34) |
| 1–5 years | 0.94 (0.90–0.97) | 0.91 (0.87–0.94) | 1.31 (1.21–1.41) | 1.31 (1.21–1.42) | 1.21 (1.18–1.25) | 1.13 (1.09–1.18) | 1.65 (1.54–1.76) | 1.61 (1.49–1.74) |
| 5–10 years | 0.94 (0.91–0.98) | 0.93 (0.89–0.97) | 1.08 (0.99–1.18) | 1.04 (0.94–1.15) | 1.28 (1.24–1.33) | 1.18 (1.13–1.23) | 1.49 (1.38–1.62) | 1.41 (1.28–1.55) |
| > 10 years | 1.04 (1.00–1.08) | 1.01 (0.97–1.05) | 1.16 (1.07–1.27) | 1.18 (1.07–1.30) | 1.44 (1.39–1.49) | 1.27 (1.23–1.33) | 1.61 (1.49–1.75) | 1.47 (1.33–1.63) |
- Note: Model 1: Time-stratified Cox regression models adjusted for the matching variables (year of birth, sex, municipality of residence and calendar period), alcohol abuse, cardiovascular disease, chronic obstructive pulmonary disease, obesity, hypertriglyceridemia, liver disease, dementia, diabetes mellitus, renal failure, diagnosis of cancer within 5 years of the index date, autoimmune disease, education level, country of birth and disposable income. Model 2: Time-stratified Cox regression models adjusted for covariates included in Model 1. In addition, the follow-up time was censored at time of diagnosis of RAP and CP.
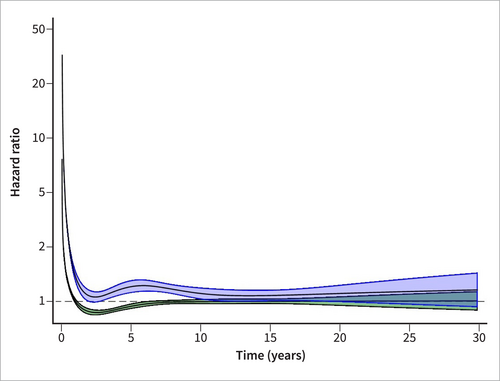
Restricted cubic spline model of mortality after hospital discharge for gallstone-related acute pancreatitis. Hazard ratios and corresponding 95% confidence intervals based on multivariable Cox regression models adjusted for the matching variables (year of birth, sex, municipality of residence and calendar period), alcohol abuse, cardiovascular disease, chronic obstructive pulmonary disease, obesity, hypertriglyceridemia, liver disease, dementia, diabetes mellitus, renal failure, diagnosis of cancer within 5 years of the index date, autoimmune disease, education level, country of birth and disposable income. Severe gallstone-related acute pancreatitis (blue) and non-severe gallstone-related acute pancreatitis (green).
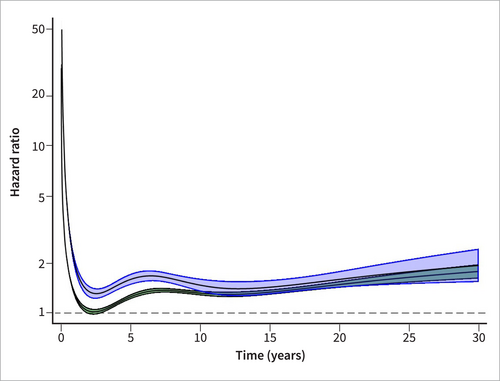
Restricted cubic spline model of mortality after hospital discharge for non-gallstone-related acute pancreatitis. Hazard ratios and corresponding 95% confidence intervals based on multivariable Cox regression models adjusted for the matching variables (year of birth, sex, municipality of residence and calendar period), alcohol abuse, cardiovascular disease, chronic obstructive pulmonary disease, obesity, hypertriglyceridemia, liver disease, dementia, diabetes mellitus, renal failure, diagnosis of cancer within 5 years of the index date, autoimmune disease, education level, country of birth and disposable income. Severe nongallstone-related acute pancreatitis (blue) and nonsevere nongallstone-related acute pancreatitis (green).
In sensitivity analyses, the HRs were comparable to those in the main model when (i) restricting the study period from 2000 to 2019 (Supporting Information S1: eTable 4) and (ii) restricting the analyses to individuals without comorbidities or low educational level (Supporting Information S1: eTable 5). In individuals younger than 50 years, there were 3326 (12.8%) deaths after hospital discharge among individuals with acute pancreatitis and 9346 (3.6%) deaths among the control population. Kaplan–Meier estimates showed lower survival among individuals younger than 50 years with acute pancreatitis, particularly for severe nongallstone-related episodes, compared to the pancreatitis-free population (Supporting Information S1: eFigure 2). The trends in HRs in time-stratified Cox models among individuals younger than 50 years were comparable to those in the whole population (Supporting Information S1: eTable 6). After censoring for recurrent acute pancreatitis or chronic pancreatitis, the HRs were attenuated towards 1 for gallstone-related acute pancreatitis (HR 1.09 [95% CI 0.92–1.30] and HR 1.49 [95% CI 0.95–2.34] in the long-term [> 10 years] time strata for non-severe and severe acute pancreatitis, respectively). The HRs for non-gallstone-related acute pancreatitis remained increased when censoring for recurrent acute pancreatitis or chronic pancreatitis (HR 1.95 [95% CI 1.76–2.16] and HR 2.51 [95% CI 1.93–3.25] in the long-term [> 10 years] time strata for non-severe and severe episodes, respectively).
When comparing subgroups of non-gallstone-related acute pancreatitis, there was a trend of higher mortality in non-severe alcohol-related episodes compared with nonsevere nongallstone–nonalcohol-related episodes. However, after censoring the analysis for recurrent acute pancreatitis or chronic pancreatitis, the HRs for non-severe alcohol-related acute pancreatitis attenuated to the same levels as the HRs for non-severe non-gallstone-non-alcohol-related episodes (Supporting Information S1: eTable 7). An increased long-term mortality was observed for both nonalcohol–nongallstone-gallstone- and alcohol-related acute pancreatitis. In a long-term setting (> 10 years), after censoring for recurrent acute pancreatitis or chronic pancreatitis, there was no statistically significant association between alcohol-related acute pancreatitis and mortality compared with the control population (HR 1.29 [95% CI 0.88–1.89]). The corresponding HR for nonalcohol–nongallstone-related episodes was statistically significantly increased (HR 1.46 [95% CI 1.32–1.62]).
4 Discussion
In this population-based cohort study, we found that acute pancreatitis was associated with an increased mortality after hospital discharge that remained increased even after more than 10 years of follow-up. Mortality was higher in non-gallstone-related and severe episodes and seemed to be mediated in part via recurrent acute pancreatitis and development of chronic pancreatitis as well as via higher prevalence of comorbidities and lower socioeconomic status.
The current study had a number of strengths, including its nation-wide coverage and use of validated registers, the large number of acute pancreatitis cases and the use of a matched pancreatitis-free control population. This provided a good internal validity and sufficient statistical power to conduct detailed subgroup and sensitivity analyses. The results are likely to have a high generalizability to other populations in Western countries that have comparable demographics and access to health care.
One of the study weaknesses was the lack of detailed data on certain confounders, such as smoking, alcohol consumption, physical activity and body mass index [31]. We attempted to reduce the impact of residual confounding from these lifestyle factors by adjusting the analyses for educational level and annual income, which are highly correlated to the missing confounders [32-34]. Furthermore, the estimates remained virtually unchanged in sensitivity analyses that (i) were restricted to healthier individuals with a lower risk of mortality (by excluding individuals with comorbidities and those with a low level of education) and (ii) were stratified nongallstone-related acute pancreatitis by alcohol abuse. Finally, the analyses were censored for recurrent acute pancreatitis or chronic pancreatitis, two factors highly associated with smoking [35] and alcohol abuse [36]. An additional limitation is that the diagnosis but not the cause of acute pancreatitis has been validated in the Swedish Patient Register [37]. In particular, alcohol-induced acute pancreatitis was likely to be underreported in our study. The diagnostic code for alcohol-related acute pancreatitis (K85.2) was not introduced until 2006 and seems to have been underutilized since then. One study found that 59% of all acute pancreatitis cases were classified as “unspecified” (K85.9) and that only 3.5% were classified as alcohol-induced in the Swedish Patient Register [37]. To address this, we included diagnostic codes for alcohol abuse in the definition of alcohol-induced acute pancreatitis. However, the Swedish Patient Register does not capture diagnoses from primary care and many individuals with high alcohol consumption do not seek care or receive a diagnosis of alcohol abuse. Consequently, it is likely that our definition of alcohol-induced acute pancreatitis underestimated the true proportion of alcohol-induced acute pancreatitis in the study population. On the other hand, the positive predictive value of the used definition was most likely very high, and the large sample size ensured sufficient power to assess mortality in this subgroup.
Further, the current dataset lacked the components necessary to apply the Revised Atlanta classification for severity. Therefore, the variables used to define disease severity were selected to approximate the Atlanta classification as closely as possible. Notably, it was not possible to differentiate between moderately severe and severe episodes without substantial risk of misclassification bias; as a result, these categories were combined and collectively referred to as “severe”. Also, the register-based data collection constitutes a risk of misclassification bias with respect to covariate status. The Swedish Patient Register does not contain diagnoses from the primary health care, which may contribute to under diagnosis of comorbidity status. However, such misclassification would most likely be non-differential with respect to the future occurrence of acute pancreatitis. Also, though not a limitation per se, it should be noted that the exclusion of individuals who died during the index hospital stay affected many baseline characteristics of the cohort, including a lower proportion of severe disease in the current study compared to the entire SwePan population (13.7% vs. 18.3%). Therefore, the findings should not be used to infer such characteristics of the overall acute pancreatitis population.
To our knowledge, the work by Czapári et al. is the only previous population-based assessment of long-term mortality in acute pancreatitis compared with a control population. They found an increased long-term mortality in acute pancreatitis and, importantly, noted that the first year post-discharge mortality was higher than the in-hospital mortality (5.5% vs. 3.5%). The leading cause of death during the first year post-discharge was end-stage cancer, which suggests an overrepresentation of malignant disease in individuals with acute pancreatitis. The second leading cause of death in their study was pancreatitis-related sepsis, indicating recurrent acute pancreatitis or development of chronic pancreatitis. In our study, interestingly, the long-term mortality remained increased when adjusting for malignant disease and when censoring for development of recurrent acute pancreatitis or chronic pancreatitis. These findings suggest that the association between acute pancreatitis and long-term mortality is mediated through multiple pathways.
Previous research on long-term mortality in acute pancreatitis is less comparable to the current study. For example, a single-center retrospective study from Finland by Karjula et al. found a 4 times higher mortality rate among in acute pancreatitis compared to a matched control population with a median follow-up time of 9.5 years [27]. However, the study was limited by the sample size (1644 cases) and the single-center study design. The external validity was further limited by the high proportion of cases with alcohol-related acute pancreatitis (71.4%). A recent large Danish population-based study found a trend of decreasing long-term (31–365 days) mortality in acute pancreatitis during 1988–2018 (adjusted mortality rate ratio 0.64 [95% CI 0.56–0.74] comparing 2013–2017 to 1988–1992) [3]. The study design did not include a pancreatitis-free control population, and thus, the results are not directly comparable to our study.
Chronic pancreatitis is associated with an increase in long-term mortality [38]. In the current study, the development of chronic pancreatitis was found to contribute to the increased long-term mortality after a first-time episode of acute pancreatitis. However, the exposure-outcome association remained after censoring for chronic pancreatitis, indicating that acute pancreatitis is associated with long-term mortality independent of future occurrence of chronic pancreatitis, particularly if the episodes are severe or non-gallstone-related. Importantly, the associations observed in our study were stronger when the analyses were limited to individuals under 50 years of age. This does not necessarily indicate an age-specific effect on mortality. Instead, the higher HRs estimates most likely reflect the lower number of expected deaths in the younger control group.
There are several potential mechanisms through which acute pancreatitis may lead to increased long-term mortality. In addition to the cancer-related mortality and the directly pancreatitis-related mortality noted by Czapári et al. development of post-pancreatitis diabetes mellitus is likely to contribute to the long-term increase in mortality [17]. Further, the systemic inflammatory stress caused by acute pancreatitis may in some individuals result in persistent organ dysfunction [39], non-resolved inflammatory responses [40], and acceleration of pre-existing conditions [41]. Our study highlights the need for further research to better understand the causes of death and the development of chronic diseases across different time periods following acute pancreatitis. Improved understanding of these factors is likely to support the development of tailored follow-up of patients with acute pancreatitis to treat or prevent long-term consequences of the disease.
In conclusion, in this population-based cohort study, we found increased long-term mortality after an episode of acute pancreatitis. The association was in part, but not fully, explained by comorbidities, recurrent acute pancreatitis, and development of chronic pancreatitis. The results stress the need for patient-tailored follow-up to prevent or treat sequelae following acute pancreatitis. Future studies should assess the cause-specific death and development of chronic diseases in patients with acute pancreatitis.
Author Contributions
Concept and design: All authors; Data analysis: D.S., O.S.-A., V.O.; Manuscript preparation: D.S., O.S.-A.; Manuscript revision: All authors; Final approval: All authors.
Ethics Statement
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (Ref. no. 2010/920-31/4 and 2015/0090-32).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data from the current study cannot be shared outside of the research group due to legislation regarding the management of register data handed out by the record-holding authorities to researchers in Sweden. The authors do however encourage contact from colleagues interested in the data to evaluate future research collaborations.