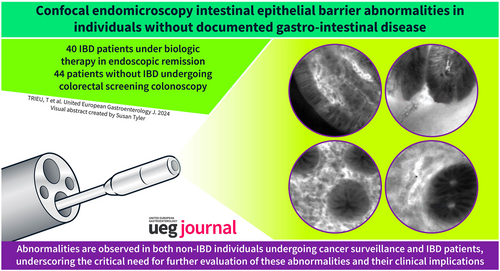
Confocal Endomicroscopy Intestinal Epithelial Barrier Abnormalities in Individuals Without Documented Gastro-Intestinal Disease
Funding: The authors received no specific funding for this work.
ABSTRACT
Background and aims
Probe-based confocal endomicroscopy (pCLE) allows real-time microscopic visualization of the intestinal mucosa surface layers. Despite remission achieved through anti-tumor necrosis factor or vedolizumab therapy, anomalies in the intestinal epithelial barrier are observed in inflammatory bowel disease (IBD) patients. Our study aimed to assess these abnormalities in non-IBD individuals and compare them with IBD patients in endoscopic remission to identify the associated factors.
Methods
The study involved 84 patients, 40 with IBD under biologic therapy for over 6 months and in endoscopic remission, and 44 without IBD or irritable bowel syndrome (IBS) undergoing colorectal screening colonoscopy. White light endoscopy and probe-based confocal laser endomicroscopy were performed in the ileum, right colon, transverse colon, left colon, and rectum. Demographic, clinical, biological, and morphological factors were examined.
Results
pCLE revealed abnormalities in both non-IBD individuals and those with IBD in endoscopic remission, such as fluorescein leakage, blood vessel dilatation, and hypervascularization across all segments, as well as epithelial gaps in the ileum, and crypt dilatation in the colon. Comparing the two groups, IBD patients exhibited slightly more gaps in the ileum, increased fluorescein leakage in the transverse colon, and fewer vessel dilatation in the transverse colon. Abnormalities were more frequent in cases of hypertension (p = 0.03), dyslipidemia (p = 0.02), female gender (p = 0.02), selective serotonin reuptake inhibitor (p = 0.03), and family history of IBD (p = 0.04) or colorectal cancer (p = 0.03).
Conclusion
Confocal endomicroscopy abnormalities are present in both non-IBD individuals undergoing colorectal cancer screening colonoscopy as in those with IBD in endoscopic remission. Further research is needed to understand the pathophysiological mechanisms of these abnormalities and their clinical impact.
Graphical Abstract
1 Introduction
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders of the gastrointestinal tract, primarily consisting of two clinicopathological entities: ulcerative colitis (UC) and Crohn's disease (CD). The incidence of IBD has continued to rise over the past 50 years, particularly in developing countries, suggesting a significant role of environmental factors such as diet and lifestyle in the pathogenesis of IBD [1]. The intestinal epithelial barrier, located between the luminal contents and the lamina propria, plays a crucial role in intestinal homeostasis, particularly in innate defense against pathogenic microorganisms and subsequent exposure to immune cells of the lamina propria. It is composed of an epithelium lined with enterocytes, Paneth cells secreting antimicrobial peptides, mucus-producing goblet cells, and enteroendocrine cells that are interconnected by tight junctional protein complexes [2]. Along with mucosal healing and histological or transmural remission, restoring intestinal barrier function has emerged as a potential therapeutic target in future IBD treatment strategies to achieve prolonged clinical remission, increased survival without surgical resection, and reduced hospitalization [3].
Probe-based confocal laser endomicroscopy (pCLE) is an endoscopic imaging technique that provides real-time microscopic imaging of the superficial layer of digestive mucosa, including the epithelium, and the surrounding connective tissue, immune cells, and blood vessels. Specific features that can be characterized by confocal endomicroscopy include crypt lumen dilatation and architecture, size of blood vessels, and integrity of the epithelial barrier, which is assessed by fluorescein extravascular leakage and epithelial gaps [4].
In IBD, the applications of pCLE are manifold: detecting colonic dysplastic lesions during colorectal cancer screening [5], discriminating active disease from quiescent disease [6], predicting the occurrence of disease relapse in the following 12 months [7], and predicting therapeutic response to anti-TNFα [8] or anti-integrin α4β7 [9] therapy. In these indications, pCLE has demonstrated excellent correlation with histology. However, a recent study [10] showed the persistence of confocal endomicroscopy abnormalities in most of 37 IBD patients endoscopically treated with anti-TNFα or vedolizumab and that these abnormalities were not correlated with risk of relapse after 33 months of follow-up. The clinical significance of these anomalies remains to be clarified. Abnormalities in confocal endomicroscopy have also been demonstrated in other conditions such as Barrett's esophagus, pancreatic diseases, and patients with irritable bowel syndrome (IBS) [5, 11].
The aim of our study was to assess the presence of anomalies in confocal endomicroscopy among subjects without documented colonic pathology and undergoing colorectal cancer screening and compare them with IBD patients with clinical, biological, and endoscopic remission.
2 Materials and Methods
2.1 Patient Population
From March 2017 to November 2023, patients undergoing coloscopy for colorectal cancer screening at CHU Liège University Hospital were randomly screened for participation in our prospective study. These patients did not have documented gastro-intestinal disease. They were studied together with a previously recruited cohort of IBD patients in clinical and endoscopic remission [10]. These IBD patients were treated with biological therapy (anti-TNF or vedolizumab) for more than 6 months and were in clinical and biological remission. The cohort of IBD patients was previously described [10]. Briefly, clinical remission was defined by a Harvey-Bradshaw Index ≤ 4 and a full Mayo Score ≤ 3 without any score more than 1 and a bleeding score of 0 in CD and UC, respectively; biological remission was defined by a C-reactive protein (CRP) level ≤ 5 mg/L and fecal calprotectin ≤ 250 μg/g; endoscopic remission was defined by the absence of ulcers and erosions and a Mayo endoscopic score of 0 in CD and UC, respectively. Patients were excluded if they had an allergy to fluorescein, inadequate colonic preparation (Boston bowel preparation scale score ≤ 2 in any segment of the colon), or endoscopic findings during the procedure (such as erythema and loss of vascular pattern in UC, or erosions in CD). Additionally, no patient had a recent history of anti-inflammatory medication usage within 6 months. The patient flow chart is shown in Figure 1.
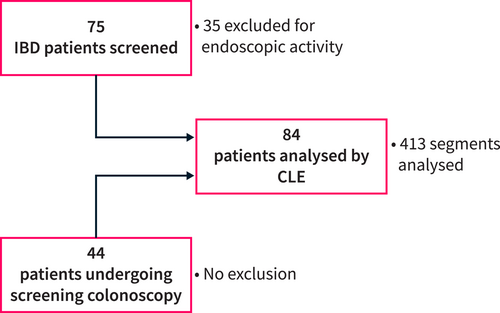
Patient flow chart. CLE, confocal laser endomicroscopy; IBD, inflammatory bowel disease.
2.2 Procedure
An endoscopic procedure was performed by an experienced endoscopist (J.P.L.) under deep sedation with propofol after colonic preparation with macrogol 3350 only. The endoscopic equipment used was Olympus CF 185 or CF 190. The ileum and all colonic segments were systematically evaluated.
The pCLE procedure was performed using a probe inserted into the accessory channel of the endoscope using a ColoFlex mini-probe from Mauna Kea Technologies Cellvizio. In each patient, video sequences of optical biopsies were recorded using the Cellvizio system in 5 consecutive segments, five minutes post-intravenous injection of 2.5 mL of fluorescein sodium 10% (500 mg/5 mL from SERB) at the ileal intubation of the examination: ileum, right colon, transverse colon, left colon, and rectum. The recording video was performed on the most stable segments possible, without the use of any spasmolytic agents. All video sequences were read offline. In our study, each video was reviewed with an analysis of qualitative and non-quantitative variables. Qualitative variables (fluorescein leakage, epithelial micro-erosion, presence of epithelial gap, vessel dilatation) for the ileum and (fluorescein leakage, crypt dilatation, vessel dilatation) for the colon were assessed by two gastroenterologists (J-P.L. and S.V.) and binary coded by abnormality present or absent. The second investigator was blinded to patient characteristics.
Micro-erosions were defined as erosion that were not visible on standard endoscopy but on endomicroscopy. Epithelial gaps were quantified and classified into two categories: absence or presence of gaps noted during a one-minute examination. Compared to the previous study conducted by Loly et al. [10], we decided to combine the categories 1–10 and more than 10 to increase statistical power. Fluorescein leakage was defined as the visualization of a whitish fluid between the cells, which disappears over time, and it was not necessarily correlated with the presence of an epithelial gap. The use of fluorescent agents facilitates visualization of mucosal structures and highlights features such as capillary networks, architectural details, and cellular patterns. The diameter of the vessels and crypts was measured at their widest point and compared to the 20-micron scale constantly displayed on the screen. The diameter of the blood vessels was considered elevated if it exceeded 10 μm, and crypt dilatation was noted when the diameter exceeded 5 μm. These are illustrated in Figures 2 and 3. At the time of inclusion, there was no validated scale. The study published by Loly et al. [10] was initially carried out on a population in deep endoscopic, clinical and biological remission, and these factors were arbitrarily defined as those that were reproducible. The factors used in our study are those most validated in the ENHANCE index study (confocal laser ENdomicroscopy for histological HeAliNg in ulCErative colitis), which is the only scale where intra- and inter-observer correlation coefficients have been studied [12], and which was published during our study, reinforcing our hypotheses. The presence of epithelial gaps and micro-erosion anomalies in the colon were not studied because no such anomalies were observed in the patients included in our study.
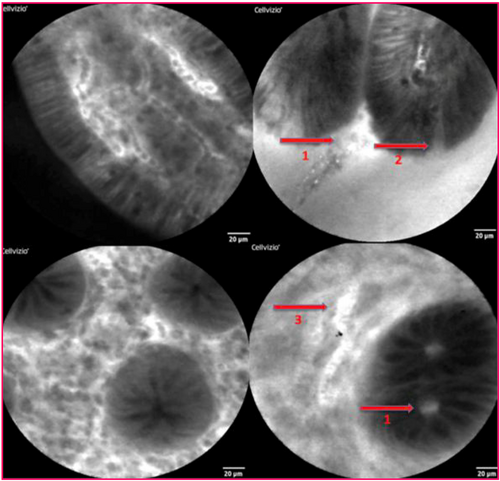
Anomalies found on pCLE imaging: 1. Fluorescein leakage: 2. Presence of epithelial gap; 3. Vascular dilatation.
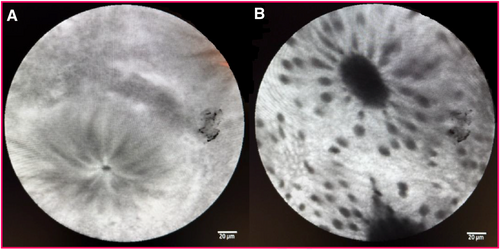
(A) Normal crypt. (B) Crypt dilated.
2.3 Data Collection
The demographic, clinical, and morphological characteristics of all patients were collected including: age, sex, ethnicity, weight, height, body mass index, smoking status, alcohol consumption (> 10 units/week), consumption of ≥ 5 servings of fruits and/or vegetables per day, consumption of ultra-processed foods ≥ 5 per day (such as processed meat, sodas, refined sugary foods, salted snacks), physical activity ≥ 3 times/week, chronic abdominal pain or diarrhea, rectal bleeding in the months preceding the examination, use of biological therapy, corticosteroids, immunosuppressants, all medications taken by the subject concurrently with the examination were recorded., antibiotic use ≥ 1x/6 months, non-steroidal anti-inflammatory drug use ≥ 1x/6 months, history or presence of hypertension ≥ 140/90 mmHg at the time of endoscopic procedure, documented diabetes, hepatic steatosis (based on ultrasound, CT scan, or elastography), confirmed colonic diverticulitis by CT scan, colonic or extra-colonic surgical history, first- or second-degree family history of IBD or digestive cancer, personal history of digestive or extra-digestive cancer, presence of atopic disease (atopic dermatitis, allergic asthma, allergic rhinitis), cardiovascular disease (cardiac arrhythmia, coronary artery disease, arteriopathy), symptoms of non-celiac gluten sensitivity, IBS diagnosis according to the Rome IV criteria, celiac disease.
Biological parameters were collected based on blood tests performed within 6 months prior to the examination and included: levels of CRP, hemoglobin, neutrophils, creatinine, hypokalemia, lipid disorder (total cholesterol > 190 mg/dL, LDL-cholesterol > 116 mg/dL), and hepatic cytolysis (GOT > 34 U/L or GTP > 55 U/L).
2.4 Statistics
The results are presented as means and standard deviations (SD), medians (quartiles), and ranges (minimum, maximum) for quantitative parameters, and as frequencies (%) for qualitative variables. Parameters were compared between the two groups using Fisher's exact test for qualitative variables and Student's t-test (or Kruskal–Wallis test) for quantitative variables. To investigate endomicroscopic abnormalities (by segment location) based on characteristics, multivariate logistic regression models were utilized. Odds ratios (OR), confidence intervals, and p-values are reported. Some variables underwent logarithmic transformation to normalize their distribution. Firth's correction was applied in certain cases. Results are considered significant at a 5% level of uncertainty (p < 0.05). Calculations were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
2.5 Ethical Considerations
All patients were aged over 18 years and provided written informed consent to participate in the study, which was approved by the Ethics Committee of the University of Liège (B707201630687; January 19, 2017).
3 Results
3.1 Patients Characteristics
A total of 84 patients were included. 40 (47.6%) suffered from IBD, 23 (27.4%) with CD and 17 (20.2%) with UC, while 44 (52.4%) had no documented gastro-intestinal disease. The main characteristics in these two patient groups are shown in Table 1. No non-IBD patient was diagnosed with IBD in the 6 years following pCLE examination. No endoscopic lesions were found in IBD patients, whether Mayo one in UC or aphthous ulceration in CD.
| n | Non-IBD (N = 44) | IBD (N = 40) | p-value | |
|---|---|---|---|---|
| Variable | ||||
| Age at colonoscopy (years, mean ± SD) | 84 | 54.39 ± 11.24 | 41.00 ± 13.26 | < 0.001 |
| Female, n (%) | 38 | 17 (38.6) | 21 (52.5) | 0.27 |
| Male, n (%) | 46 | 27 (61.4) | 19 (47.5) | 0.27 |
| BMI (kg/m2, mean ± SD) | 84 | 26.27 ± 3.62 | 24.50 ± 3.28 | 0.022 |
| CD/UC | 40 | — | 23(57.5)/17(42.5) | NA |
| Disease duration at time of colonoscopy (y) | 40 | — | 13.00 (9.50; 20.50) | NA |
| Duration since biological initiation t (y) | 40 | — | 3.73 (1.63; 6.83) | NA |
| Biologic treatment | 40 | — | 40 | NA |
| Anti-TNFα, n (%) | — | 22 (55.0) | NA | |
| Vedolizumab, n (%) | — | 18 (45.0) | NA | |
| Active smoking at time of colonoscopy, n (%) | 84 | 4 (9.1) | 10 (25.0) | 0.027 |
| Alcohol > 10 units per week, n (%) | 84 | 7 (15.9) | 2 (5.0) | 0.16 |
| Hypertension, n (%) | 84 | 10 (22.7) | 4 (10.0) | 0.15 |
| CRP (mg/L) | 66 | 1.50 | 0.80 | 0.99 |
| Diabetes, n (%) | 81 | 8 (19.5) | 4 (10.0) | 0.35 |
| Dyslipidemia, n (%) | 81 | 25 (61.0) | 12 (30.0) | 0.007 |
| Ultra-processed food ≥ 5/day, n (%) | 76 | 4 (10.0) | 7 (19.4) | 0.33 |
| Metformin, n (%) | 84 | 7 (15.9) | 3 (7.5) | 0.32 |
| NSAID > 1X/6 months, n (%) | 84 | 13 (29.5) | 3 (7.5) | 0.012 |
| SSRI/SNRI, n (%) | 84 | 6 (13.6) | 10 (25.0) | 0.43 |
| Non-celiac gluten sensitivity, n (%) | 79 | 5 (12.2) | 3 (7.9) | 0.71 |
| Family history of IBD, n (%) | 83 | 1 (2.3) | 18 (46.1) | 0.011 |
| Family history of colorectal cancer, n (%) | 82 | 26 (59.1) | 13 (34.2) | 0.009 |
- Note: Results are considered significant at a 5% level of uncertainty (p < 0.05).
- Abbreviations: CD, Crohn's disease; CRP, C-reactive protein; IBD, inflammatory bowel disease; NA, non-applicable NSAID, nonsteroidal anti-inflammatory; SSRI, Selective serotonin reuptake inhibitors; SNRI, Serotonin-norepinephrine reuptake inhibitor; TNFα, tumor necrosis factor; UC, ulcerative colitis; Y, years.
3.2 Confocal Endomicroscopic Assessment
The average duration of the endoscopic and pCLE procedures were 21 min, with an ileal intubation rate of 100%. The transit time from the anus to the ileum was approximately 4 min on average, followed by 1–2 min of video recording per segment (5 × 1–2 min = 5–10 min) with 12 images/s. The withdrawal time included the endoscopic examination, endomicroscopic video recordings, and any necessary polypectomies, with an average duration of 17 min. Polyps were detected in 12 out of 44 patients undergoing colorectal cancer screening (27.27%), with a total of 26 subcentimetric polyps, all of which were resected using a cold snare without complications. Among the polyps, 22 were adenomatous with low-grade dysplasia, and the remaining 4 were hyperplastic.
Confocal endomicroscopy lesions in the various segments in IBD and non-IBD patients are shown in Table 2, while fluorescein leakage is illustrated in Figure 4. The interobserver correlation rate of pCLE assessment was calculated as a percentage of agreement by taking all binary values of all variables. Ninety-one percent of these values were identical (Table 3). When assessments were divergent between the two investigators, the video was reviewed, and an agreement was found. The diameter of blood vessels and hypervascularization could not be interpreted in 4 patients in the rectum and three in the left colon due to artifacts reducing the quality of these videos, which is not statistically significant.
| Variable | Group | Ileum | Right colon | Transverse colon | Left colon | Rectum |
|---|---|---|---|---|---|---|
| Fluorescein leak | IBD | 27 (67.5) | 15 (38.5) | 17 (42.5) | 13 (32.5) | 8 (20.0) |
| Non-IBD | 33 (75.0) | 11 (25.0) | 4 (9.1) | 6 (13.6) | 6 (13.6) | |
| Micro-erosion | IBD | 12 (30.0) | NA | NA | NA | NA |
| Non-IBD | 8 (18.2) | NA | NA | NA | NA | |
| Epithelial gaps | IBD | 25 (59.5) | NA | NA | NA | NA |
| Non-IBD | 26 (59.0) | NA | NA | NA | NA | |
| Vessel dilatation | IBD | 25 (62.5) | 29 (82.9) | 21 (61.8) | 27 (75.0) | 20 (57.1) |
| Non-IBD | 39 (88.6) | 40 (90.9) | 37 (86.0) | 31 (73.8) | 31 (70.5) | |
| Crypt dilatation | IBD | NA | 22 (56.4) | 23 (57.5) | 23 (57.5) | 25 (62.5) |
| Non-IBD | NA | 23 (52.3) | 18 (40.9) | 18 (40.9) | 34 (77.3) |
- Abbreviations: IBD, inflammatory bowel disease; NA, non-applicable; pCLE, probe-based confocal laser endomicroscopy.
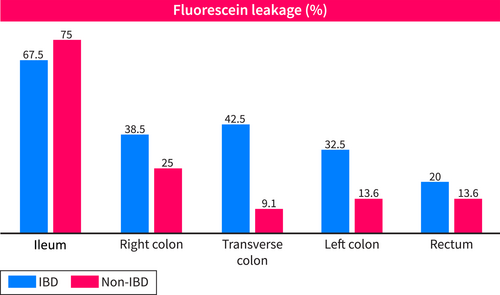
Proportion of segments with fluorescein leakage by intestinal location and by IBD (n = 40) and non-IBD (n = 44) patients.
| Segment | Inter-observer correlation (%) |
|---|---|
| Ileum | 89% |
| Right colon | 90% |
| Transverse colon | 92% |
| Left colon | 91% |
| Rectum | 93% |
| Overall agreement | 91% |
3.3 Multivariate Analysis of Risk Factors for Confocal Endomicroscopy Abnormalities
Multivariate analysis for each segment and each anomaly was performed on all patients, and significant results are shown in Table 4. The presence of colonic polyps (benign) was not significantly correlated with the presence of endomicroscopic abnormalities in non-IBD patients.
| Effect | OR | Low lim OR | Sup lim OR | p-value |
|---|---|---|---|---|
| ILEUM fluorescein leakage N = 73 | ||||
| IBD yes versus no | 0.849 | 0.261 | 2.758 | 0.785 |
| Dyslipidemia yes versus no | 4.957 | 1.233 | 19.931 | 0.0241 |
| ILEUM vessel diameter > 10 μm N = 83 | ||||
| IBD yes versus no | 0.314 | 0.083 | 1.187 | 0.0878 |
| RIGHT COLON fluorescein leakage N = 75 | ||||
| IBD yes versus no | 1.42 | 0.545 | 3.705 | 0.473 |
| RIGHT COLON crypt dilatation N = 83 | ||||
| IBD yes versus no | 0.961 | 0.381 | 2.423 | 0.9324 |
| SSRI/SNRI versus none | 9.379 | 1.098 | 80.14 | 0.0321 |
| RIGHT COLON vessel diameter > 10 μm N = 64 | ||||
| IBD yes versus no | 6.516 | 0.412 | 103.139 | 0.1835 |
| Anti-TNFα yes versus no | 0.040 | 0.002 | 0.805 | 0.0355 |
| Ultra-processed foods yes versus no | 0.053 | 0.006 | 0.493 | 0.0099 |
| TRANSVERSE COLON fluorescein leakage N = 82 | ||||
| IBD yes versus no | 14.509 | 2.814 | 74.806 | 0.0014 |
| History of hypertension yes versus no | 7.586 | 1.239 | 46.443 | 0.0284 |
| 2nd degree familial history of colon cancer yes versus no | 4.195 | 1.124 | 15.655 | 0.0328 |
| TRANSVERSE COLON crypt dilatation N = 76 | ||||
| IBD yes versus no | 1.858 | 0.715 | 4.829 | 0.2035 |
| Sex male versus female | 0.327 | 0.125 | 0.853 | 0.0223 |
| TRANSVERSE COLON vessel diameter > 10 μm N = 69 | ||||
| IBD yes versus no | 0.602 | 0.135 | 2.689 | 0.5058 |
| 2nd degree familial history of IBD yes versus no | 0.064 | 0.005 | 0.876 | 0.0395 |
| LEFT COLON fluorescein leakage N = 78 | ||||
| IBD yes versus no | 1.544 | 0.353 | 6.763 | 0.5643 |
| Sex male versus female | 0.143 | 0.028 | 0.738 | 0.0202 |
| 2nd degree familial history of IBD yes versus no | 14.991 | 1.9 | 118.263 | 0.0102 |
| Non-celiac gluten sensitivity yes versus no | 5.977 | 1.046 | 34.153 | 0.0444 |
| LEFT COLON crypt dilatation N = 84 | ||||
| IBD yes versus no | 3.001 | 1.133 | 7.953 | 0.0271 |
| LEFT COLON vessel diameter > 10 μm N = 69 | ||||
| IBD yes versus no | 1.679 | 0.519 | 5.437 | 0.3873 |
| RECTUM fluorescein leakage N = 84 | ||||
| IBD yes versus no | 1.153 | 0.339 | 3.914 | 0.8199 |
| BMI (kg/m2) | 0.806 | 0.664 | 0.979 | 0.0299 |
| RECTUM vessel diameter > 10 μm N = 68 | ||||
| IBD yes versus no | 0.818 | 0.3 | 2.231 | 0.6949 |
- Note: Results are considered significant at a 5% level of uncertainty (p < 0.05).
- Abbreviations: Anti-TNFα, anti-tumor necrosis factor α; BMI, body mass index; IBD, inflammatory bowel disease; Lim, limit; NA, non-applicable; OR, odds ratio; SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin-norepinephrine reuptake Inhibitor.
4 Discussion
In IBD patients, several studies have revealed confocal endomicroscopy abnormalities with various applications of pCLE in clinical practice, such as distinguishing active disease from quiescent IBD [6], predicting disease relapse within 12 months [7], and predicting therapeutic response to anti-TNFα [8] or anti-integrin α4β7 therapy [9]. Our previous study [10] highlighted the persistence of confocal endomicroscopy abnormalities in IBD patients, even when they were in clinical, biological, endoscopic, and even histological remission, regardless of whether they were treated with anti-TNFα or anti-integrin α4β7 therapy. These abnormalities included fluorescein leakage, vessel dilatation across all segments, epithelial gaps and micro-erosions in ileal segments, and crypt dilatation in colonic segments. Interestingly, in the current study, similar irregularities were also detected in non-IBD individuals undergoing colorectal cancer screening, as 18.2% of them exhibited ileal micro-erosions in pCLE assessment without any endoscopic lesions observed. Is there a tight junction dysfunction, subclinical microinflammation, or another underlying pathophysiological mechanism? We do not yet have an answer. Standard histology does not provide insights into cell junction analysis, but this paves the way for further studies on epithelial barrier function, as highlighted by Iacucci et al. [4].
IBD management relies on the concept of treat-to-target, as outlined in the updated Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) guidelines [13]. Along with mucosal healing and histological or transmural remission, the restoration of intestinal barrier function is increasingly recognized as a potential therapeutic goal in future IBD treatment strategies [3]. However, according to the present study, this target remains to be confirmed, considering the confocal anomalies observed in non-IBD patients. The description of mucosal changes in the lower gastrointestinal tract in individuals without diagnosed gastrointestinal disease has already been studied but on relatively small sample sizes in terms of patient numbers, and comparing “healthy” patients with patients either with active inflammatory bowel disease [6, 7]. A systematic study of endomicroscopic anomalies in all segments (ileum, right, transverse and left colon, rectum) has not been previously documented in the literature [6], and its clinical and mechanistic significance remains uncertain. No previous study has investigated the associated risk factors. Our analysis uncovered some exploratory leads. Hypertension represents not only a global public health challenge but also the most significant risk factor for cardiovascular diseases. Research by Kim et al. [14] has shown that the biomarkers of intestinal permeability are higher in patients with hypertension than in those without hypertension. On one hand, some studies have found that spontaneously hypertensive rats exhibit decreased intestinal mucosal thickness and blood flow, reduced glandular goblet cells, shortened intestinal villus height, decreased tight junction proteins such as occludin and zonula occludens-1, and increased intestinal permeability. These findings suggest that hypertension may contribute to impaired intestinal barrier function. On the other hand, dysbiosis can also lead to impaired gut barrier function, resulting in bacterial endotoxin translocation into the bloodstream through the mesentery. This process triggers chronic inflammation and vascular endothelial damage, leading to decreased vasodilator factors and increased vasoconstrictor factors. Ultimately, the increased peripheral resistance may contribute to the development or exacerbation of hypertension [15]. Whether hypertension results in elevated gut permeability or vice versa remains unclear. There may be a bidirectional relationship between the two factors rather than a unidirectional causal link. A more comprehensive understanding of the role of gut permeability in the development of hypertension may benefit targeted interventions aimed at preventing and delaying the onset of elevated blood pressure.
Dyslipidemia may disrupt the composition of the intestinal mucus layer, and thereby the epithelial barrier. The mucus is composed of proteins, carbohydrates, lipids, and a significant amount of water, forming a sticky gel that coats the mucosa. In addition to its surface hydrating role, mucus serves as a physical protective barrier against the invasion of microbes or other harmful agents, including mechanical injuries or destructive enzymes. It also acts as a chemical barrier by containing epithelial antimicrobial peptides and secretory IgA, which help against luminal invasion of microorganisms [2]. Alterations in phospholipid concentration were also found in the intestinal mucus barrier of patients with UC [16]. The association of hypertension and dyslipidemia may correspond to a wider spectrum, such as metabolic syndrome.
BMI level correlates with a protective effect against fluorescein leakage in the rectum, in contrast to a study that indicated a slightly higher but statistically insignificant permeability in individuals with obesity (BMI: 35.04 ± 3.98 kg/m2), while other studies found no discernible difference between individuals with overweight/obesity and lean controls [17]. Our data lack interpretability because the average BMI of our sample did not exceed 26 in non-IBD subjects and 25 in patients with IBD. The association between obesity and compromised gut barrier in humans remains to be clarified.
The consumption of ultra-processed foods appears to be a protective factor against vascular dilatation, but this finding must be questioned because our study was performed without a validated food questionnaire. In the literature, food additive emulsifiers such as carboxymethylcellulose and polysorbate-80 are rather known to decrease the thickness of the mucus layer and the diversity of the microbiome, with some emulsifiers potentially increasing bacterial expression of flagellin and lipopolysaccharide, thereby enhancing their epithelial translocation capacity and leading to a pro-inflammatory state and intestinal permeability disorder, with a higher risk of IBD [18].
Non-celiac gluten sensitivity, also known as wheat sensitivity, has been the focus of a prospective study in 2021 [19], where fluorescein leaks on pCLE were also observed, but in the duodenum in that instance. The underlying pathophysiology of food-induced alterations in the gastrointestinal tract remains largely unknown but may include an increase in paracellular or transcellular permeability due to an increase in claudin-2, a decrease in occludin, and eosinophil degranulation, or an impairment of the gut vascular barrier with downregulation of the endothelial barrier [20]. Non-celiac gluten sensitivity was found in 10% of our cohort.
Sexual dimorphism is a common feature observed in various prevalent disorders, including autoimmune, metabolic, cardiovascular, and psychiatric diseases. Microbiota analysis conducted on 341 female and 348 male mice from 89 inbred strains revealed significant differences in gut microbiota composition between male and female mice under controlled conditions. Moreover, gonadectomy and hormone replacement led to a shift in microbiota composition driven by sex hormones, but the mechanisms involved remain unidentified [21]. Changes in gut microbial composition may explain the higher prevalence of increased intestinal permeability observed in women in our study, as dysbiosis can affect intestinal permeability in patients with inflammatory bowel disease [2]. Further research is necessary to elucidate the precise role of sex hormones in epithelial barrier function.
Intestinal permeability may be affected by antidepressants such as SSRI and SNRI, as demonstrated in a study on healthy adult rats in 2018. In this study, escitalopram, venlafaxine, and fluoxetine were found to increase permeability in the ileum [22], while sertraline and citalopram improved intestinal epithelial function by blocking the pro-inflammatory signal mediated by Toll-like receptor 3 in another research [23]. In our study, the most used antidepressants were escitalopram and venlafaxine, and they were associated with crypt dilatation. Although we have no explanation for this anomaly, it is interesting to link it to the clinical use of antidepressants in the treatment of IBS [24].
Our study has several limitations. Some criteria in pCLE may be seen as subjective, such as vessel dilatation. Certain pCLE factors might also be regarded as imprecise or non-specific; however, we opted to evaluate only the most reproducible and less subjective features in line with the recent ENHANCE index study [12]. Our results could have been affected by external factors, including intestinal preparation with macrogol that involves mechanical or osmotic stress, anesthesia agents, or the time lapse between the intravenous administration of fluorescein and the pCLE video recording of the distal segments of the colon. The interpretation may have been imprecise in certain segments of the colon due to its peristalsis and the absence of a spasmolytic agent used. However, several of them still present with endomicroscopic abnormalities, particularly fluorescein leakage, which is an objective parameter commonly used in various studies with validated scales [4]. Additionally, the lack of available information in medical records represents a limitation of our study. The restricted diversity of the study population is also significant, as there were no patients of Asian, Hispanic, or African descent, no obese patients, and limited variety in medical histories due to the recruitment of young IBD patients and non-IBD patients without specific comorbidities, coming for colorectal cancer screening. The sample size is constrained because the recruitment occurred in a single center, which may introduce certain biases and confounding factors, limiting the statistical power and generalizability of the findings. It would be interesting to compare pCLE abnormalities with IBD patients who are not in endoscopic remission, which was not conducted in our study.
5 Conclusion
Confocal endomicroscopy abnormalities are found in both patients with IBD in remission and non-IBD subjects undergoing colorectal cancer screening. In the treat-to-target strategy for IBD, the benefit of achieving complete confocal endomicroscopic remission beyond endoscopic and histologic remission is still to be confirmed. Other factors such as hypertension, dyslipidemia, diet, hormonal influence, medications, dysimmune process, and genetic predispositions may contribute to this epithelial barrier dysfunction. Exploring larger-scale cohorts, incorporating genomic, proteomic, and intestinal microbiota analyses, could provide deeper insights into the underlying pathophysiological mechanisms of intestinal barrier integrity and identify new therapeutic targets.
Author Contributions
Thanh-Hien Trieu: drafting of the article, analysis, and interpretation of the data. Sophie Vieujean: investigator of confocal endomicroscopy. Nicolas Delhougne: investigator of colonoscopy. Laurence Seidel: analysis and interpretation of the data. Edouard Louis: critical revision of the article for important intellectual content. Jean-Philippe Loly: investigator of colonoscopy and confocal endomicroscopy, conception and design, final approval of the article.
Acknowledgments
The authors have nothing to report.
Conflicts of Interest
S.V. has received speaker fees from AbbVie, Celltrion, Ferring, Janssen, Lilly and Takeda; and served on the Speakers Bureau for Janssen. E.L. has received education and research grant support from Takeda, Pfizer, Janssen, AbbVie, and Fresenius-Kabi; received speaker fees from AbbVie, Ferring, Falk, Takeda, Janssen, Pfizer, Celgene, Galapagos, and BMS; served on the advisory board for AbbVie, Ferring, MSD, Takeda, Celgene, Janssen, Gilead, Galapagos, Arena, Pfizer, Eli Lilly, and BMS; and served as a consultant for AbbVie. All co-authors have seen and agreed with the contents of the manuscript.
Abbreviations
-
- BMI
-
- body mass index
-
- CD
-
- Crohn's disease
-
- CRP
-
- C-reactive protein
-
- ENHANCE
-
- confocal laser Edomicroscopy for histological HeAliNg in ulCErative colitis
-
- IBD
-
- inflammatory bowel disease
-
- IBS
-
- irritable bowel syndrome
-
- NSAID
-
- non-steroidal anti-inflammatory drugs
-
- OR
-
- odds ratio
-
- pCLE
-
- probe-based confocal laser endomicroscopy
-
- SNRI
-
- serotonin-norepinephrine reuptake inhibitor
-
- SSRI
-
- selective serotonin reuptake inhibitors
-
- TNFα
-
- anti-tumor necrosis factor alpha
-
- UC
-
- ulcerative colitis
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.