Diagnosis and assessment of disease severity in patients with nonalcoholic fatty liver disease
Abstract
Nonalcoholic fatty liver disease (NAFLD) includes simple steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and eventually cirrhosis and hepatocellular carcinoma (HCC). The diagnosis of NAFLD is based on the detection of excess fat disposition in the liver, which is the first step to trigger further evaluation of NAFLD, including necroinflammation and fibrosis. In this review, we discuss non-invasive biomarkers and imaging tools that are currently and potentially available for different features (steatosis, necroinflammation and fibrosis) and disease severity assessment of NAFLD. In the past 2 decades, advances in non-invasive tests of fibrosis have transformed the management of NAFLD. Blood and imaging biomarkers have already been evaluated in multiple studies for the diagnosis of fibrosis and cirrhosis. Among the various histological features of NAFLD, the degree of fibrosis has the strongest correlation with liver-related morbidity and mortality. Non-invasive tests of fibrosis have been shown to predict liver-related outcomes, both in the general population and among patients with NAFLD. What is lacking, however, is good data to support the use of non-invasive tests as monitoring and response biomarkers. With the conclusion of several large phase 3 studies in the next few years, the availability of paired liver biopsy, non-invasive test and clinical outcome data will likely advance the field and shed light on new biomarkers and the way to use various non-invasive tests in a longitudinal manner.
Graphical Abstract
INTRODUCTION
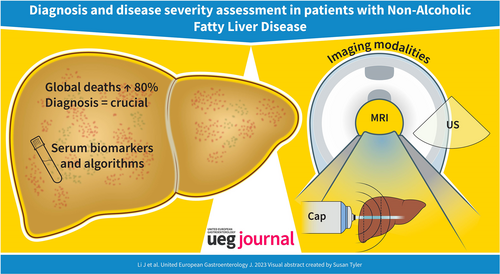
Nonalcoholic fatty liver disease (NAFLD) affects 29.8% of the global adult population and is the most common chronic liver disease worldwide.1 Its spectrum includes simple steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and eventually cirrhosis and hepatocellular carcinoma (HCC). The prevalence of HCC has increased by 11.5 times in NASH patients from 2002 to 2016, making it one of the leading causes of liver transplantation in the United States,2 and the number of global deaths attributable to NAFLD has increased by 80.2% in the last 3 decades, reaching 0.17 million in 2019.3 However, the underdiagnosis of NAFLD and underestimation of the disease severity have resulted in a heavier burden on public health. Considering the ongoing epidemic of NAFLD, characterizing the severity of NAFLD is essential for risk stratification and optimizing the management plan.
The diagnosis of NAFLD is based on the detection of excess fat disposition in the liver (≥5% hepatic steatosis), which is the first step to trigger further evaluation of the severity of NAFLD. To date, liver biopsy is still the gold standard for diagnosing NAFLD and assessing its severity, with the key histological features including the degree of steatosis, necroinflammation and fibrosis.4 However, the disadvantages of liver biopsy include sampling variability, intra- and inter-observer variability and the invasiveness of the procedure, limiting its use in routine practice, not to mention repeated assessment of disease severity.5, 6 Data on non-invasive tests (NITs) in a dynamic context to signal treatment response are still evolving. Improvements in FIB-4 or serum biomarkers such as ELF, liver stiffness, or combination parameters have been associated with histologic response, but the exact thresholds of improvement remain to be validated in large multicenter studies within this context of use.7 As a result, several serum biomarkers and non-invasive imaging tools have been developed to meet the clinical need for the diagnosis and severity assessment of NAFLD.
In this review, we discuss non-invasive biomarkers and imaging tools that are currently and potentially available for different features (steatosis, necroinflammation and fibrosis) and disease severity assessment of NAFLD.
DIAGNOSIS OF STEATOSIS
Serum biomarkers and algorithms
Over the past 2 decades, several algorithms have been explored to identify steatosis in patients with NAFLD, including fatty liver index (FLI),8 hepatic steatosis index (HSI),9 NAFLD liver fat score,10 SteatoTest11 and NAFLD ridge score.12 Although these methods were derived from different studies, they typically comprise similar components that reflect liver function, metabolic parameters, the status of metabolic disease and the sex difference, which were given exclusive weighting factors. However, these approaches have inconsistent diagnostic accuracy of steatosis, with area under the receiver-operating characteristics curves (AUROCs) ranging from 0.79 to 0.87.4
In terms of FLI, great variations in diagnostic accuracy were observed in different cohorts. A meta-analysis showed that when FLI <30 was used to exclude non-alcoholic fatty liver (NAFL), the pooled sensitivity and specificity were 81% and 65%, respectively.13 When FLI ≥60 was used to confirm NAFL, the pooled sensitivity was only 44%, while the specificity reached 90%,13 showing modest discriminatory performance. A cross-sectional study recruiting 135 patients with biopsy-proven NAFLD showed poor diagnostic accuracy of FLI, with an AUROC of less than 0.60.14 The relatively small sample size and high metabolic burden in this study cohort may have potentially influenced the diagnostic performance.14 A study conducted in China, involving over 130,000 individuals, established population-specific cut-off points of FLI, which were stratified by gender, waist circumference and body mass index (BMI),15 reaching AUROC of 0.856 in males and 0.909 in females, overall displaying a sound diagnostic performance.
In addition, some new biomarkers and related panels have been explored. Cen et al.16 developed and validated a clinical and laboratory-based nomogram to diagnose NAFLD, which included BMI, diastolic blood pressure, uric acid, fasting blood glucose, triglyceride, and alanine aminotransferase (ALT), showing a high AUROC of 0.857. Machine-learning has also been employed. Yip et al.12 developed the NAFLD ridge score, which incorporates ALT, high-density lipoprotein cholesterol, triglyceride, haemoglobin A1c, white blood cell count and the presence of hypertension, achieving an AUROC of over 0.8 in both the training and validation cohort.12 The dual cut-offs of 0.24 and 0.44 for corresponding ruling out and ruling in NAFLD resulted in an overall diagnostic accuracy of 87% in both cohorts.12 Of note, the heterogeneity in different populations and reference standards for NAFLD diagnosis have led to variations in diagnostic accuracy and limited widespread use across the world. Therefore, more high-quality longitudinal studies are required for further investigation.
Imaging modalities
Conventional ultrasonography is the most common method for detecting steatosis because of its ease of availability and low cost. It can detect steatosis with >2.5%–20% liver fat content.17 In contrast, ultrasound-based controlled attenuation parameter (CAP) and magnetic resonance imaging-proton density fat fraction (MRI-PDFF) are more sensitive and accurate in diagnosing steatosis.
CAP measurement by transient elastography has gradually become the preferred tool due to its relatively high diagnostic accuracy and point-of-care assessment. A meta-analysis reported that AUROCs of CAP were 0.924, 0.794 and 0.778 for ≥S1, ≥S2 and = S3,18 verifying its best performance in mild steatosis. However, various cut-off points determined in different studies pose a dilemma for CAP in steatosis diagnosis. Currently, the best cut-offs are reported by Karlas et al.,19 using the M probe, with values of 248, 268, and 280 dB/m for ≥S1, ≥S2, and = S3, respectively. A study recruiting patients with morbid obesity obtained higher cut points of 300, 328 and 344 dB/m for ≥S1, ≥S2, and = S3, respectively.20 The choice of probe21 and the different characteristics of population, including BMI, diabetes, sex and aspartate aminotransferase, can influence CAP values22 and potentially bring about diagnostic inconsistency for steatosis.
MRI-PDFF is currently the most accurate test to quantify hepatic steatosis,23 considered as a promising surrogate for liver biopsy, being more accurate than CAP in detecting all grades of steatosis in NAFLD patients (AUROC 0.99).24 However, the type of field strengths, the option of vendors and phantoms, a high cost, the requirement for radiological expertise and a lack of standardized cut-off values for steatosis stage relatively limit the use of PDFF. In a retrospective study, cut-off values of PDFF for ruling-in and ruling-out of hepatic steatosis were identified based on chemical shift imaging and high-speed T2-corrected multi-echo magnetic resonance spectroscopy,25 which is an exploration of optimal cut-off points among healthy individuals. To evaluate the efficacy of drugs, a >30% relative reduction in MRI-PDFF between baseline and end of treatment is the objective.
Moreover, some new techniques, such as ultrasound hepatorenal index26 and ultrasound-derived fat fraction,27 have also emerged in the clinic, showing good diagnostic performance, but their reproducibility and diagnostic value need further validation.
INFLAMMATION
The current reference method for the diagnosis of inflammation is the histological examination of liver biopsy with a diagnosis of NASH. NASH is defined by the presence of steatosis with lobular inflammation and hepatocyte ballooning. NASH is the aggressive form of the disease, compared to patients without NASH, with a higher rate of fibrosis progression, progression to cirrhosis, liver-related complications and hepatocellular carcinoma, as well as a higher mortality rate.28 There is currently no biomarker available for the non-invasive diagnosis of inflammation, especially ballooning. Common markers, including transaminases, have insufficient specificity and sensitivity.7 As for liver fibrosis, blood tests combining several blood markers have been developed to diagnose NASH, but the studies involved small patient numbers and validation of the results in large representative populations is lacking in the literature.29 Determination of CK-18 fragments in the blood could predict histological NASH and severity of disease.30 However, at this time, the performance of this test is insufficient for its use in clinical practice. Recently, it was shown that neither single marker nor multimarker scores achieved the predefined acceptable AUC 0·80 for replacing biopsy in detecting people with both NASH and clinically significant fibrosis (ie, at-risk NASH; NAFLD Activity Score ≥4 and F ≥ 2).31
FIBROSIS
There are two main families of non-invasive methods for the diagnosis of liver fibrosis: blood tests, which combine blood markers in approximately complex mathematical algorithms, and devices that measure liver stiffness calculated from the speed of an elastic wave generated by a probe applied to the skin.4 All these non-invasive tests are tools to diagnose the severity of the liver disease. However, their performance is heavily affected by the clinical context. for example, by enriching the patient population based on age, gender, alcohol use and metabolic risk factors, one may increase the pretest probability of advanced liver disease and thus improve the positive predictive values of non-invasive tests.
Simple blood tests have the advantage of using clinical parameters (age, sex, body mass index…) and blood biomarkers (transaminases, platelets, albumin…) that are routinely used by physicians.32 The cost is low and their calculation is accessible through free websites or smartphone applications. The two most validated simple tests in NAFLD are the NAFLD Fibrosis Score (variables: age, body mass index, hyperglycaemia/diabetes, AST, ALT, platelets, albumin) and the Fibrosis-4 index (FIB-4) (variables: age, AST, ALT, platelets). Specialised blood tests use biomarkers directly linked to liver fibrosis derived from the latest knowledge of the pathophysiological mechanisms underlying fibrogenesis and fibrolysis in chronic liver diseases. These direct biomarkers (hyaluronic acid, a2-macroglobulin…) correspond to proteins that constitute the extracellular matrix or to enzymes involved in its remodelling.7 The most validated test is the Enhanced Liver Fibrosis (ELF) score. This test is a marker set consisting of tissue inhibitor of metalloproteinases 1 (TIMP-1), amino-terminal propeptide of type III procollagen (PIIINP) and hyaluronic acid (HA) showing good correlations with fibrosis stages in chronic liver disease.33
Numerous devices can be used to measure liver stiffness. Few investigations have directly compared the performances of the different liver stiffness measuring devices for the non-invasive diagnosis of liver fibrosis in NAFLD. FibroScan is the most evaluated device for liver stiffness measurement (LSM) in NAFLD.34 The two diagnostic thresholds for ruling out or ruling in advanced liver fibrosis with FibroScan are <7.9 kPa and >9.6 kPa, respectively. Recently, it has been shown that two novel non-invasive scores, combining liver stiffness measurement and AST/ALT ratio, platelets, sex, age and diabetes status, Agile 4 and Agile 3+ scores, outperformed FIB-4 and LSM in terms of AUROC, percentage of patients with indeterminate results and positive predictive value to rule-in cirrhosis or advanced fibrosis.35
All NAFLD patients should have an assessment of liver fibrosis using a blood test and/or measurement of liver stiffness. A non-invasive evaluation with a low result (NAFLD Fibrosis Score below −1.455, or FIB-4 below 1.30, or liver stiffness below 7.9 kPa) is sufficient to confirm the absence of advanced chronic liver disease. A non-invasive evaluation with a high result (NAFLD Fibrosis Score above 0.676, or FIB-4 above 2.67, or liver stiffness above 9.6 kPa) is insufficient alone to affirm the presence of advanced chronic liver disease (Figure 1). However, the accuracy of biomarkers in type 2 diabetes is decreased and specialized tests should be used first-line to diagnose advanced liver fibrosis in these patients. 36
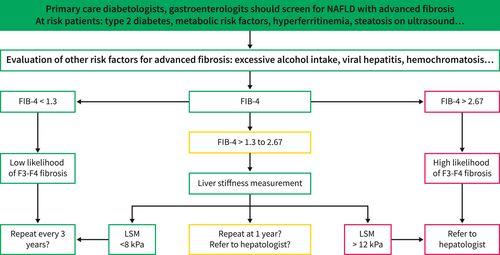
This result must be confirmed by another method: liver stiffness measurement if the initial method was a blood test, and vice versa. Liver biopsy is not required when both methods are in agreement. Specialised blood tests (Fibrotest, Fibrometer, ELF, N-terminal propeptide of type 3 collagen (PRO-C3)) are more effective than simple blood tests. The measurement of liver stiffness included in standard ultrasound devices is as effective as FibroScan measurement and the measurement of liver stiffness by magnetic resonance elastography could be the gold standard in the future.37
Data on NITs in a dynamic context to signal treatment response are still evolving. Improvements in FIB-4 or serum biomarkers such as ELF, liver stiffness, or combination parameters have been associated with histologic response, but the exact thresholds of improvement remain to be validated in large multicenter studies within this context of use.7 Additional data are needed to determine whether changes in NITs that correlate with the treatment response are mechanism-specific or treatment agnostic. The validation of existing biomarkers as measures of treatment response will accelerate the development and approval of therapeutic agents and justify their adoption into clinical practice.
ASSESSMENT OF DISEASE SEVERITY
Portal hypertension
Portal hypertension is central in the development of essentially all cirrhotic complications (e.g., ascites, variceal haemorrhage, hepatic encephalopathy and hepatorenal syndrome). In the landmark PREDESCI trial, the use of propranolol or carvedilol in patients with clinically significant portal hypertension (CSPH, defined by a hepatic venous pressure gradient [HVPG] ≥10 mm Hg) significantly reduced the incidence of hepatic decompensation from 27% in the placebo group to 16% in the treatment group.38 However, HVPG measurement is an invasive procedure largely restricted to expert centres and research settings. Thus, the latest Baveno VII consensus recommends the use of non-invasive tests to diagnose CSPH.39 Essentially, clinicians can exclude CSPH in patients with LSM <15 kPa and platelet count ≥150 × 109/L. In contrast, one can assume that a patient has CSPH when LSM exceeds 25 kPa (Figure 2).
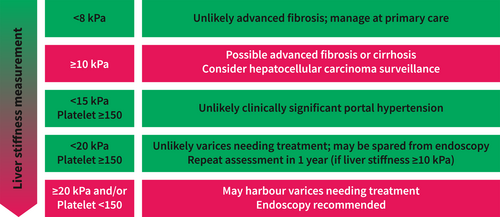
However, the 25 kPa LSM threshold to rule in CSPH only applies to patients with chronic viral hepatitis, alcohol-related liver disease and non-obese NASH as obesity can confound LSM.40 To complicate matters further, a recent study suggests that patients with NASH might develop hepatic decompensation at a lower HVPG.41 On another note, based on the Baveno VII criteria, a significant proportion of patients with compensated advanced chronic liver disease will be classified in the grey zone (i.e., LSM 15–25 kPa and/or platelet count <150 × 109/L), for which the risk of CSPH is unclear. In this context, it is possible to add spleen stiffness measurement (SSM) for better patient characterization.42
Patients with CSPH tend to have splenomegaly through increased pressure in the splenic vein. Thus, compared with LSM, SSM may even reflect portal hypertension more directly. SSM <21 kPa and >50 kPa are proposed cut-offs to exclude and rule CSPH, respectively, in patients with chronic viral hepatitis.39 The performance of SSM should be validated in the NAFLD population. Moreover, the existing literature is largely based on the old 50 Hz probe. The manufacturer has prepared a new 100 Hz probe dedicated to SSM. Because the frequency of ultrasound influences the measurement of shear wave velocity and therefore tissue stiffness, the performance of SSM using the 100 Hz probe must be independently validated.
Another practical role of non-invasive assessment of advanced liver disease is to determine the risk of varices and the need for screening endoscopy. The Baveno VI criteria state that patients with LSM <20 kPa and platelet count ≥150 × 109/L have <5% chance of harbouring varices needing treatment and can be spared from endoscopy.43 The criteria have since been validated in patients with NASH-related cirrhosis.44 A randomised controlled trial in patients with hepatitis B-related cirrhosis also confirmed that LSM and SSM were non-inferior to routine endoscopy in detecting varices needing treatment and preventing future variceal haemorrhage.45
Predicting clinical outcomes
Among the various histological features of NAFLD, the degree of fibrosis has the strongest correlation with liver-related morbidity and mortality.46 Not surprisingly, non-invasive tests of fibrosis have been shown to predict liver-related outcomes both in the general population and among patients with NAFLD.4 In fact, even simple fibrosis scores such as FIB-4 have been shown to have a high negative predictive value in excluding future development of cirrhosis, hepatic decompensation and hepatocellular carcinoma.47, 48 Accumulating data in liver biopsy cohorts also suggest that non-invasive tests are as good as, if not better than, histological fibrosis stage in predicting liver-related events.49 In a secondary analysis of 4 clinical trials of simtuzumab and selonsertib in patients with NASH and F3-F4 fibrosis, ELF ≥11.3, NAFLD fibrosis score ≥0.67 or FIB-4 ≥3.25 were cut-offs associated with reduced event-free survival.
In routine practice, clinicians see patients repeatedly and have access to serial test results. Furthermore, it is important to assess the response to treatment over time and understand the clinical significance of changes in non-invasive tests. The latter is limited by a lack of effective treatments in routine practice. That being said, several therapeutic agents (e.g., obeticholic acid, semaglutide, resmetirom and lanifibranor) have demonstrated positive effects on NASH resolution and/or fibrosis improvement in the past few years.50-53 Emerging data suggest that these therapeutic agents improve non-invasive tests along with liver histology.54 Proving the prognostic significance of non-invasive test response will be an important next step.
CONCLUSIONS
In the past 2 decades, advances in non-invasive tests of fibrosis have transformed the management of NAFLD. To diagnose steatosis, MRI-PDFF is the gold standard but is usually reserved for research. CAP is useful as a point-of-care technique to identify steatosis. No NIT is accurate enough for the diagnosis of inflammation. To diagnose liver fibrosis, liver stiffness measurement is the best method in clinical practice. What is lacking, however, is good data to support the use of non-invasive tests as monitoring and response biomarkers. With the conclusion of several large phase 3 studies in the next few years, the availability of paired liver biopsy, non-invasive test and clinical outcome data will likely advance the field and shed light on new biomarkers and the way to use various non-invasive tests in a longitudinal manner.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
Vincent Wong served as a consultant or advisory board member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions and Visirna; and a speaker for Abbott, AbbVie, Gilead Sciences, Novo Nordisk and Unilab. He has received a research grant from Gilead Sciences, and is a co-founder of Illuminatio Medical Technology Limited. Victor de Lédinghen served as a consultant or advisory board member for AbbVie, Echosens, Gilead Sciences, Intercept, Novo Nordisk, Pfizer, Escopics, Alfasigma, Janssen, BMS, GSK, and Orphalan. No disclosure for the two other authors.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable (no new data generated).