The Workgroup Serrated Polyps and Polyposis (WASP) classification for optical diagnosis of colorectal diminutive polyps with iScan and the impact of the revised World Health Organization (WHO) criteria
Abstract
Background and aims
The Workgroup Serrated Polyps and Polyposis (WASP) developed criteria for optical diagnosis of colorectal polyps. The aims of this study were: (1) to improve optical diagnosis of diminutive colorectal polyps, especially SSLs, after training endoscopists in applying WASP criteria on videos of polyps obtained with iScan and (2) to evaluate if the WASP criteria are still useful when polyps are pathologically revised according to the World Health Organization (WHO) 2019 criteria.
Methods
Twenty-one endoscopists participated in a training session and predicted polyp histology on 30 videos of diminutive polyps, before and after training (T0 and T1). After three months, they scored another 30 videos (T2). Primary outcome was overall diagnostic accuracy (DA) at T0, T1 and T2. Polyps were histopathologically classified according to the WHO 2010 and 2019 criteria.
Results
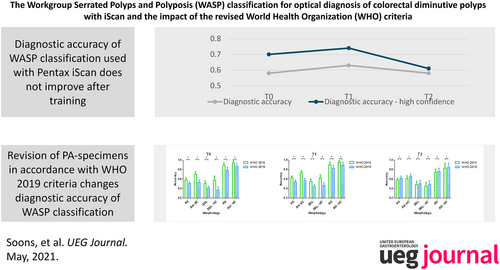
Overall DA (both diminutive adenomas and SSLs) significantly improved from 0.58 (95% CI 0.55–0.62) at T0 to 0.63 (95% CI 0.60–0.66, p = 0.004) at T1. For SSLs, DA did not change with 0.51 (95% CI 0.46–0.56) at T0 and 0.55 (95% CI 0.49–0.60, p = 0.119) at T1. After three months, overall DA was 0.58 (95% CI 0.54–0.62, p = 0.787, relative to T0) while DA for SSLs was 0.48 (95% CI 0.42–0.55, p = 0.520) at T2. After pathological revision according to the WHO 2019 criteria, DA of all polyps significantly changed at all time points.
Conclusion
A training session in applying WASP criteria on endoscopic videos made with iScan did not improve endoscopists' long-term ability to optically diagnose diminutive polyps. The change of DA following polyp revision according to the revised WHO 2019 criteria suggests that the WASP classification may need revision.
Graphical Abstract
INTRODUCTION
Key summary
Summarise the established knowledge on this subject
-
In 2016, the Workgroup Serrated Polyps and Polyposis (WASP) developed a classification system for endoscopic differentiation of adenomas (ADs), hyperplastic polyps (HPs) and sessile serrated lesions (SSLs).
-
Prospective studies on its diagnostic use are promising, although the classification has never been tested using other virtual chromoendoscopy techniques than narrow band imaging (NBI), nor has it been updated since the revised World Health Organization (WHO) criteria for digestive system tumors in 2019.
What are the significant and/or new findings of this study?
-
A training session in applying WASP criteria on endoscopic videos made with iScan did not improve endoscopists' long-term ability to optically diagnose diminutive polyps.
-
The recently revised WHO criteria for histopathological diagnosis of colorectal tumors significantly changed the diagnostic value of the WASP classification.
The majority of polypectomies are performed on polyps that are innocent at the time of removal and up to 80% of all detected polyps during colonoscopy are diminutive (≤5 mm), of which 50% are non-neoplastic (e.g., hyperplastic or inflammatory). Moreover, cancer prevalence is ≤0.1% in diminutive polyps.5, 6 Following European and American guidelines, removal and pathological examination of all polyps is common practice.7-10 However, because of the low rate of neoplasia in diminutive polyps, removal of these innocent diminutive polyps results in additional costs.
In an attempt to reduce costs, various classification systems for optical differentiation between neoplastic and non-neoplastic polyps have been proposed. One of these classification systems was developed in 2016 by the Workgroup Serrated Polyps and Polyposis (WASP) by combining the Narrow Band Imaging (NBI) International Colorectal Endoscopic (NICE) classification with the criteria proposed by Hazewinkel et al. to distinguish SSLs from ADs and hyperplastic polyps (HPs).11-13 This differentiation is important because the optical difference between SSLs and HPs is subtle with marked clinical implications. In 2018, the WASP classification was prospectively evaluated and found to meet both thresholds as stated in the Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI)-statement from the American Society for Gastrointestinal Endoscopy (ASGE).14, 15 By meeting both PIVI criteria, correct use of the WASP classification could result in the implementation of the resect & discard strategy, as well as the diagnose & leave in strategy. Current studies have focused on validating the WASP classification with the use of NBI. However, it remains unclear if the WASP classification can also be used with different electronic chromoendoscopy techniques such as iScan-Optical Enhancement (OE) (PENTAX Europe GmbH).
Recently the World Health Organization (WHO) published renewed criteria for pathological classification of digestive system tumors.16 According to these criteria only one unequivocal aberrant crypt, rather than two or three, is already sufficient to diagnose a SSL. It is estimated that following these criteria, HPs will be re-diagnosed as SSLs in approximately 7% of cases.17, 18 To date it is unknown what the effect of these revised WHO 2019 criteria is on the optical diagnosis of SSLs.
Pentax iScan-OE combines optical and digital image processing technologies providing real-time image enhancement. Three different modes for real-time image processing are available, that is, iScan mode 1 for the detection of lesions, iScan mode 2 for mucosal pattern characterization and iScan-OE for characterization of blood vessels, glandular ducts and mucosa.19, 20 As the OE-mode mimics the characteristics of NBI, we hypothesized that the WASP classification can also be applied using iScan-OE. Previous studies have already shown that endoscopists are fairly good in differentiating ADs from HPs but find it more difficult to optically diagnose SSLs.21
The primary aim of this study was to improve the optical diagnosis of diminutive colorectal polyps, especially SSLs, after participating in an interactive training session to train participants in applying the WASP classification using the iScan-OE system. The secondary aim of this study was to evaluate if optical diagnosis using the WASP classification is still feasible after implementation of the revised WHO 2019 criteria.
METHODS
Study design
This prospective study was conducted to compare the optical diagnosis of endoscopists with the pathological assessment of polyps. The study consisted of two phases: a training phase and a re-evaluation phase (see Figure 1). This study was performed in accordance with the Standards for Reporting Diagnostic Accuracy (STARD statement).22
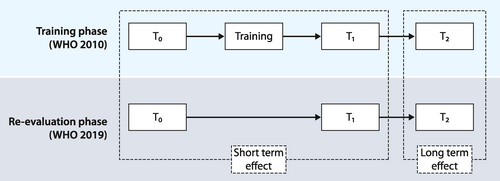
Study design. WHO World Health Organization criteria to diagnose gastrointestinal lesions; T0 Test 0; T1 Test 1; T2 Test 2
Training phase
The training phase was performed to measure the short- and long-term effects of training in the WASP classification on the optical diagnosis of diminutive colorectal polyps using iScan-OE digital chromoendoscopy.
Development of interactive training module
-
Clinical importance of optical diagnosis of diminutive colorectal polyps, including the PIVI criteria15;
-
Introduction and breakdown of the WASP classification into the NICE criteria and the criteria formulated by Hazewinkel et al11-13;
-
Differentiation between type 1 (HP or SSL) and type 2 (AD or SSL) polyps using the NICE criteria, illustrated with short videos;
-
Differentiation of ADs and/or HPs versus SSLs using the Hazewinkel criteria, illustrated with short videos.
The videoclips were retrieved from a prospectively collected database of short (≤10 s) videoclips of diminutive polyps in patients who had a colonoscopy following a positive immunochemical fecal occult blood test (iFOBT), or in patients who were known with serrated polyposis syndrome (SPS) during surveillance colonoscopy. Only videos of diminutive polyps were included in the study because we specifically aimed to evaluate our results with regard to the PIVI criteria which are restricted to polyps ≤5 mm. In all videoclips, the lesion was consecutively shown using iScan 1, iScan 2 and iScan OE. Two expert gastroenterologists (TB & MvK) evaluated the training module for comprehensibility and feasibility. Unclear elements were corrected, and missing information was added. The training module was made using Microsoft PowerPoint (Microsoft Corporation). The training had an approximate duration of 20 min.
Short-term effects of training
To evaluate the short-term effects of the training on the accuracy of optical diagnosis of SSLs, senior endoscopists and endoscopists in training were invited to participate in a live training session that was organized in January 2020. Preceding the training session, a set of 30 non-magnified endoscopic videoclips of diminutive colorectal polyps (nine ADs, nine HPs and 12 SSLs) were presented (T0). The videos were only shown once. For each videoclip, the participant predicted polyp histology, and stated whether prediction was done with high or low confidence level, with high confidence meaning that participants were ≥90% confident of their diagnosis. Neither patient characteristics, nor polyp location or size were presented. Participants were unaware of the number of videos per category. No feedback was given afterwards. After the training session, the same set of 30 videoclips was presented again but in a different random sequence and assessed by the participants under identical circumstances (T1). Videos of polyps that were used during the training module were excluded from use in the first part of the training phase.
Long-term effects of training
To evaluate the long-term effects of the training on the accuracy of optical diagnosis of SSLs, all participants were invited again to participate in the second part three months later. Participants predicted polyp histology and again stated whether prediction was done with high or low confidence on a new set of 30 non-magnified videoclips of diminutive polyps, including nine ADs, five HPs and 16 SSLs (T2). Neither patient characteristics, nor polyp location or size were presented. Again, participants were unaware of the number of videos per category. No additional training was offered prior to this phase. No videos of polyps that were used in the training module nor the first part of the training phase were used in this phase.
For the second part, an online platform (LimeSurvey GmbH) was used. Participants could log in on the platform at any time for 20 days. In the module, participants could only move forward, therefore it was impossible for participants to go back and see a previous video again or to change a previous answer.
Re-evaluation phase
The re-evaluation phase was performed to evaluate the effect of the revised WHO 2019 criteria for pathological diagnosis on the diagnostic accuracy of optical diagnosis using the WASP classification. For this, the same optical diagnoses from the training phase were used in the analyses. However, this time they were compared to the revised pathological diagnosis, in accordance with the WHO 2019 criteria.16
Histopathological diagnosis
The histopathological diagnosis was used as reference standard in all cases. All polyps used in this study were assessed by two dedicated gastrointestinal (GI) pathologists. All pathological diagnoses were based on morphological features on hematoxylin and eosin-stained slides. During the training phase all polyp specimens were initially assessed in accordance with the WHO 2010 criteria, as opposed to the re-evaluation phase in which all polyp specimens were assessed in accordance with the WHO 2019 criteria.23 During both the training- and re-evaluation phase all polyp specimens were revised by two expert GI pathologists (CvdP and IN).16 Any disagreement was resolved by discussion. Pathologists were unaware of patient characteristics, endoscopic appearance of the lesions and diagnosis made by the first pathologist.
Ethical approval
Ethical approval for the assembling of the prospective database and hence the performance of this study was waived by the Institute Review Board Committee on Research Involving Human Subjects Arnhem-Nijmegen (reference number: 2018-4514). The study was performed in accordance with the Helsinki Declaration. This study was registered at the Dutch Trial Register (reference number: NL8340).
Statistical analysis
This study was powered on an expected improvement in diagnostic accuracy after participating in the training. An improvement in diagnostic accuracy of 10% was expected. With 15% discordant pares, a significance level op 5% and a power of 90%, this resulted in a calculated sample size of 154 videos to be analyzed. Since a selected group of senior endoscopists and endoscopists in training could score the same set of videos, a high inter-cluster correlation was expected. Therefore, an inflation factor was calculated using the formula 1+(n-1)ᵨ.24 An assumed intra-rater correlation of 0.05 together with 30 observations per participant resulted in an inflation factor of 2.45 and 378 observations (13 participants) needed for paired analysis.
Descriptive statistics were reported as median values with interquartile ranges (IQR), mean values with 95% confidence intervals (95% CI) and frequencies or percentages, when appropriate. Histopathological diagnosis was used as reference standard to calculate the diagnostic accuracy of optical diagnosis. To compare diagnostic performance before and after training the Paired Sample t-Test was used. This test was also used to compare the diagnostic accuracy before and after revision of the polyp specimens. To investigate whether the level of experience of the participant (senior vs. in training endoscopist) influenced the outcomes an Independent Samples t-Test was used.
Analyses were performed in IBM SPSS Statistics, version 25 (SPSS Inc.). A p-value <0.05 was considered statistically significant.
RESULTS
Polyp characteristics
Characteristics for all 60 polyps included in the three tests are shown in Table 1. The videos were made during colonoscopies of 38 patients, with 37 (97.3%) having a positive iFOBT. A total of 34 videos (56.7%) was made of polyps located in the rectosigmoid. Mean polyp diameter was 2.9 mm, and most polyps (55%) were sessile.
| Polyps Included in tests (n = 60) | |
|---|---|
| Number of patients, n | 38 |
| Indication for colonoscopy, n (%) | |
| Positive iFOBT | 37 (97.3) |
| SPS surveillance | 1 (2.6) |
| Location, n (%) | |
| Coecum | 5 (8.3) |
| Ascending colon | 4 (6.7) |
| Transverse colon | 14 (23.2) |
| Descending colon | 3 (5.0) |
| Sigmoid | 13 (21.7) |
| Rectum | 21 (35.0) |
| Diameter (mm), mean (95% CI) | 2.9 (2.6–3.3) |
| Polyp morphology, n (%) | |
| Pedunculated | 1 (1.7) |
| Sessile | 33 (55.0) |
| Flat elevated | 26 (43.3) |
- Abbreviations: 95% CI, 95% confidence interval; iFOBT immunochemical faecal occult blood test; mm, millimetres; SPS, serrated polyposis syndrome.
Participants
Sixty-two eligible participants were contacted, but finally 11/37 senior endoscopists and 10/25 endoscopists in training agreed to participate in the study. Table 2 shows the characteristics of participating endoscopists. Participants had a median colonoscopy experience of 5 years, with a median annual colonoscopy volume of 300 procedures per year. Eight of the 11 senior endoscopists were accredited to perform colonoscopies in the Dutch population screening program for CRC.
| Total cohort (n = 21) | |
|---|---|
| Senior endoscopist, n (%) | 11 (52.4) |
| Working in a regional hospital, n (%) | 17 (81.0) |
| Colonoscopy experience, y, median (IQR) | 5 (1.25–16.5) |
| Annual colonoscopy volume, median (IQR) | 300 (225–450) |
| Accredited to perform colonoscopies in the Dutch population screening program for CRC, n (%) | 8 (38.1) |
| Familiar with WASP criteria before training, n (%) | 7 (33.3) |
- Abbreviations: CRC, colorectal cancer; IQR, interquartile range; WASP, workgroup serrated Polyps and Polyposis.
Training phase
Short-term effects
Mean improvement in optical diagnosis after the training is presented in Table 3. The mean score for all 30 optical estimations before training was 0.58 (95% CI 0.55–0.62) which significantly increased to 0.63 (95% CI 0.60–0.66) after training (mean improvement 0.05, 95% CI 0.02–0.08, p = 0.004). Thirteen of 21 participants showed improved accuracy post-training, while five participants showed a decreased accuracy. The accuracy of three participants remained unchanged (Figure 2). When only taking the estimations with high confidence into consideration, the mean pre- and post-training accuracies were 0.70 (95% CI 0.64–0.75) and 0.74 (95% CI 0.69–0.78), respectively. Ten participants showed improved accuracy post-training, whilst nine participants showed a decreased accuracy. The accuracy of two participants remained unchanged.
| T0, accuracy (95% CI) | T1, accuracy (95% CI) | Improvement, mean (95% CI) | p-value | T2, accuracy (95% CI) | Improvement, mean (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| Overall | 0.58 (0.55–0.62) | 0.63 (0.60–0.66) | 0.05 (0.02–0.08) | 0.004* | 0.58 (0.54–0.62) | −0.01 (−0.06–0.05) | 0.787 |
| Overall – HC | 0.70 (0.64–0.75) | 0.74 (0.69–0.78) | 0.04 (−0.01–0.09) | 0.166 | 0.61 (0.55–0.67) | −0.10 (−0.20–0.00) | 0.076 |
| SSL versus non-SSL | 0.51 (0.46–0.56) | 0.55 (0.49–0.60) | 0.04 (−0.01–0.08) | 0.119 | 0.48 (0.42–0.55) | −0.02 (−0.09–0.05) | 0.520 |
| SSL versus non-SSL – HC | 0.57 (0.48–0.66) | 0.62 (0.56–0.69) | 0.03 (−0.04–0.11) | 0.383 | 0.45 (0.38–0.53) | −0.11 (−0.23–0.01) | 0.083 |
| AD versus non-AD | 0.89 (0.84–0.94) | 0.90 (0.85–0.96) | 0.02 (−0.03–0.06) | 0.480 | 0.74 (0.67–0.81) | −0.16 (−0.24–−0.09) | 0.001* |
| AD versus non-AD – HC | 0.94 (0.91–0.98) | 0.95 (0.90–0.99) | 0.00 (−0.03–0.04) | 0.804 | 0.83 (0.73–0.93) | −0.11 (−0.22–0.00) | 0.063 |
- Note: * significant improvement respective to T0.
- Abbreviations: 95% CI, 95% confidence interval; AD, adenoma; HC, high confidence; SSL, sessile serrated lesion.
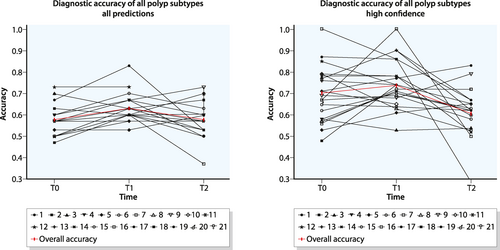
Individual and overall improvement of diagnostic accuracy of sessile serrated lesions, before (T0) and after (T1 and T2) participating in a training. Data are presented for all optical diagnosis (top) and high confidence diagnosis only (bottom)
For the 12 SSLs, no significant improvement was found. Mean accuracy pre-training was 0.51 (95% CI 0.46 – 0.56), which improved to 0.55 (95% CI 0.49–0.60, p = 0.119) post-training. Eight participants improved their scores post-training, whereas the score of four participants deteriorated. The accuracy of nine participants remained unchanged (Figure S1). The accuracy of estimations performed with high confidence was 0.57 (95% CI 0.48–0.66) pre-training, which improved to 0.62 (95% CI 0.56–0.69, p = 0.383) post-training. Twelve participants improved their scores, while five participants showed decreased scores post-training. The accuracy of two participants remained unchanged.
For the adenomas, also no significant improvement was seen, although the mean accuracy was already relatively high pre-training, that is, mean accuracy pre-training was 0.89 (95% CI 0.84–0.94) and post-training 0.90 (95% CI 0.85–0.96, p = 0.480). Mean accuracy of high confidence estimations was 0.94 (95% CI 0.91–0.98) pre-training, and 0.95 (95% CI 0.90 –0.99, p = 0.804) post-training.
No significant differences in accuracy rates nor differences in improvement between senior endoscopists and endoscopists in training were seen at T0 or T1.
Long-term effects
Seventeen participants (81%) participated in the evaluation of long-term effects. Table 3 shows the long-term mean improvement regarding the diagnostic accuracy. The mean score for all 30 optical estimations decreased to 0.58 (95% CI 0.54–0.62) after three months, compared to T0 (mean deterioration −0.01, 95% CI −0.06–0.05, p = 0.787). Ten participants showed improved overall accuracy after three months, while six participants showed a decreased accuracy. For one participant the accuracy remained stable. When focusing on high confidence estimati ons, accuracy decreased to 0.61 (95% CI 0.55–0.67) after three months, relative to T0 (mean deterioration −0.10, 95% CI −0.20–0.00, p = 0.0076). Long term accuracy with high confidence improved in six participants and deteriorated in 11 participants (Figure 2).
For SSLs, mean accuracy deteriorated to 0.48, compared to T0 (95% CI 0.42–0.55, p = 0.520). Eleven participants improved their long-term accuracy, while the accuracy of six participants deteriorated (Figure S1). The accuracy of optical diagnoses of SSLs performed with high confidence deteriorated to 0.45 (95% CI 0.38–0.53, p = 0.083) after three months, compared to T0. In seven participants the long-term accuracy improved, whereas the accuracy of eight participants deteriorated. Two participants did not rate any of the SSL videos with high confidence.
Diagnostic accuracy for adenomas significantly decreased to 0.74 (95% CI 0.67–0.81, p = 0.001). Mean accuracy of high confidence estimations was 0.83 (95% CI 0.73–0.93, p = 0.063) at T2.
No difference in accuracy rates nor difference in improvement between senior endoscopists and endoscopists in training were seen at T2.
Re-evaluation phase
After revision of all polyp specimens, the diagnoses of two ADs, one SSL and eight HPs were changed (Table 4). This resulted in eight ADs, three HPs and 19 SSLs as a reference for the short-term effect tests (T0 and T1). For the long-term effect test (T2) 10 ADs, four HPs and 16 SSLs served as reference standard.
| Initial diagnosis | ||||
|---|---|---|---|---|
| Diagnosis after revision | AD | SSL | HP | |
| AD | 16 | 1 | 1 | |
| SSL | 1 | 27 | 7 | |
| HP | 1 | - | 6 | |
- Abbreviations: AD, adenoma; HP, hyperplastic polyp; SSL, sessile serrated lesion.
Figure 3 shows the difference in diagnostic accuracy according to the WHO 2010 and WHO 2019 criteria, respectively. In all cases the diagnostic accuracy changed significantly. The overall diagnostic accuracy at T0 dropped from 0.58 (95% CI 0.55–0.62) according to WHO 2010, to 0.51 (95% CI 0.48–0.54, p < 0.001) according to WHO 2019. At T1, the diagnostic accuracy dropped from 0.63 (95% CI 0.60–0.66) to 0.54 (95% CI 0.51–0.58, p < 0.001). At T2 the diagnostic accuracy increased from 0.58 (95% CI 0.54–0.62) to 0.62 (95% CI 0.59–0.66, p < 0.001). For high confidence estimations the diagnostic accuracy at T0 dropped from 0.70 (95% CI 0.64–0.75) according to WHO 2010, to 0.53 (95% CI 0.49–0.58, p < 0.001) according to WHO 2019. At T1 it dropped from 0.74 (95% CI 0.69–0.78) to 0.57 (95% CI 0.54–0.61, p < 0.001). Finally, at T2 the diagnostic accuracy increased from 0.61 (95% CI 0.55–0.67) to 0.66 (95% CI 0.60–0.72, p < 0.001). For detailed information on other polyp subtypes, see Table S1.
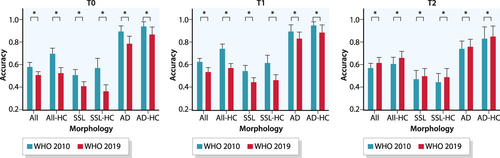
Differences in diagnostic accuracy before (pre) and after (post) histopathological review of polyps according to the WHO 2010 and 2019 criteria, respectively. AD, adenoma; HC, high confidence; SSL, sessile serrated lesion; * significant difference
DISCUSSION
After participating in an interactive training to apply the WASP classification using iScan, the diagnostic accuracy of endoscopists to correctly classify colorectal polyps significantly improved from 0.58 to 0.63 (p = 0.004). However, this improvement was no longer present after 3 months, as the diagnostic accuracy was again 0.58. Furthermore, no difference in diagnostic accuracy was observed over time when only optical diagnoses of colorectal polyps made with high confidence were considered. Despite our efforts to improve the optical diagnosis of SSLs, no significant improvement from 0.51 pre-training to 0.55 after training (p = 0.119) was observed. After three months the diagnostic accuracy further deteriorated to 0.48 (p = 0.520) for SSLs. After revision of all polyp specimens according to the WHO 2019 guideline, the pathological diagnoses of 2/18 ADs and 8/14 HPs changed. This considerable number of revisions resulted in an inevitable change of diagnostic accuracy rates during the re-evaluation phase.
The results of our training phase conflict with previously published results on the implementation of the WASP classification in terms of accuracy. When the WASP classification was introduced in 2016, a comparable study was conducted to validate the classification.11 The accuracy rates were higher than we found in our study. In the original study, the accuracy was 0.63 at baseline, improved to 0.79 after the training, and remained high after six months: 0.76. The accuracy for SSLs was also better than in our study; 0.74 at baseline, 0.86 after training and 0.87 after six months. These accuracy rates were even higher when diagnoses made with high confidence were considered. In that study, two images (high-resolution white light endoscopy and NBI) rather than videos of 45–50 small and diminutive polyps were shown simultaneously for an undefined period of time to evaluate the diagnostic accuracy. In addition, only senior endoscopists participated in that study. Although we did not find differences in accuracy rates between senior endoscopists and endoscopists in training in our study, one could argue that this resulted in a higher overall accuracy in the previous study.11
Based on promising results in 2016, the WASP classification was also assessed in real-life in 2018. High accuracy rates above 97% were found for diminutive SSLs in the proximal colon and rectosigmoid.14 Nonetheless, this real-life study was performed by highly skilled and dedicated endoscopists. Of the 39 endoscopists that were invited to participate, only 27 (69%) passed a test that qualified them to participate in the final real-life phase of the study. This rather strict selection contrasts with our population of which almost half consisted of endoscopists in training.
In 2018, the first endoscopic classification system for colonoscopy with iScan was established: the Simplified Identification Method for Polyp Labeling during Endoscopy (SIMPLE). The outcomes of the validation phase of this classification system were better than our results using the WASP classification in combination with iScan, with accuracy rates of 94% and 91% after training for all diagnoses and high confidence diagnoses, respectively.25 Although 21 videos were used rather than still images, the duration of these videos was approximately 10 times longer than the videos in our study (90–120 s vs. ±10 s). Moreover, polyps were <10 mm, which is larger than the ≤5 mm polyps we included in our study. Again only expert endoscopists participated in the study.
So, our overall accuracy rates were remarkably lower than previous, comparable studies have shown. Yet, these studies differ from our study because larger (<10 mm) polyps were displayed for a longer period of time, the interval to the long-term follow up was longer and only experienced endoscopists were enrolled in these studies. Additionally, to validate the WASP classification, still images were used rather than videos and to validate the SIMPLE classification only 21 videos were shown.
Interestingly, in our study the accuracy rates for optical diagnosis of ADs at T0 and T1 were higher than in previous studies. In the two studies on the WASP classification above, the accuracy rates for all optical AD diagnoses and diagnoses made with high confidence were 76% and 82%–89%, respectively.11, 14 As these high accuracy rates were already present before our training, it seems plausible that since the publication of both studies in 2016–2018, endoscopists have become increasingly aware on the importance of optical diagnosis and how to perform it.
To our knowledge, we are the first to report the effect of the implementation of the WHO 2019 criteria for histopathological classification on optical diagnosis of diminutive colorectal polyps. A significant difference in diagnostic accuracy rates for diminutive colorectal polyps was noted at all time points. These differences in diagnostic accuracy using the WHO 2019 criteria compared to the WHO 2010 criteria may well indicate that the WASP classification in its current form may be less useful. It should be taken into consideration that participants in our study were trained with videos of polyps that were classified according to WHO 2010 rather than WHO 2019 criteria. Additional studies, using only (images or videos of) polyps classified according to WHO 2019 criteria, are needed to assess the real effect of the revised WHO criteria on the diagnostic value of the WASP classification. Furthermore, it needs to be determined whether it is possible to differentiate HPs and SSLs based on endoscopic criteria only.
Initially, the Hazewinkel criteria to endoscopically identify SSLs were validated on a dataset of images of 150 polyps with a median diameter of 5 mm, of which 34 were at least 10 mm.13 The mean diameter of polyps in this study was 2.9 mm, with a maximum of 5 mm. Possibly, some endoscopic features (e.g., clouded surface characteristic for SSLs) are more difficult to determine from such small lesions. This might at least partially explain the relatively low accuracy rates of SSLs in our study.
It has been proposed that the optical diagnosis of colorectal polyps might improve significantly due to the implementation of computer-aided diagnosis (CADx)-systems. Various prospective studies, evaluating different CADx-systems in combination with different imaging modalities, have shown accuracy rates and negative predictive values (for diagnosing neoplastic vs. non-neoplastic lesions) of 74.4%–93.2% and 73.5%–96.5%, respectively.26-31 Although these results seem promising, they still need to be evaluated for the implementation of the ‘diagnose & leave in strategy’ and/or the ‘resect & discard strategy’. Moreover, only half of the studies evaluating CADx-systems included SSLs which makes it even more difficult to extrapolate these results to real-world clinical practice.
Our study has several strengths. First, we used videos rather than images in order to maximally resemble daily clinical practice. Second, all pathological diagnoses were re-reviewed by two dedicated GI-pathologists. Third, by providing an online final test after 3 months, the majority (81%) of participants were able to participate in this part of the study regardless of the COVID-19 pandemic. Nevertheless, the lower accuracy rates at T2 might imply that this method of testing and/or the COVID-19 pandemic may have made the participants less dedicated.
Some limitations also need to be addressed. First, senior endoscopists and endoscopists in training participated in the study, which allowed us to include a relatively large cohort of endoscopists, compared to other studies. However, it may limit the ability to compare our results with previous studies as they exclusively included expert endoscopists. Additionally, the duration of 10 s per video might be too short for less experienced endoscopists to be able to correctly classify colorectal polyps. Second, after re-reviewing all histopathological diagnoses by two dedicated GI-pathologists one AD was revised to a HP and vice versa. This cannot be attributed to the WHO 2019 criteria as no changes on this part have been introduced. It can therefore not be ruled out that, at least partly, some inter- and intra-observer variability in pathological diagnoses was introduced during the review process.
In conclusion, the findings of this study show that the overall optical diagnosis of diminutive colorectal polyps initially improved after participating in an interactive training to educate participants on the use of the WASP classification using the iScan-OE system, but this improvement was no longer present after three months. No short- or long- term improvement could be observed for optical diagnosis of SSLs. Thus, according to our study results we would argue against the optical diagnosis of diminutive polyps, especially SSLs. Additionally, after changing the gold standard from the WHO 2010 to the WHO 2019 criteria for histopathological classification, all accuracy rates changed significantly. We therefore suggest to revise the WASP classification in accordance to the WHO 2019 criteria. In the future, CADx-systems hold the promise to support the endoscopist in optical diagnosis, thereby reducing or even excluding the human factor in the optical diagnosis in daily endoscopic practice.
CONFLICT OF INTEREST
Dr. Siersema currently receives research support from Pentax (Japan) and is on the advisory board of Boston Scientific (USA). The authors have no other relevant conflicts of interest to disclose.
AUTHOR CONTRIBUTION
Elsa Soons designed and performed the study, collected, analyzed and interpreted the data and wrote the paper. Peter Siersema, Tanya Bisseling and Mariëtte van Kouwen contributed to the conception and design of the study, interpretation of the data and critically reviewing the manuscript. Yark Hazewinkel contributed to the interpretation of the data and critically reviewed the manuscript. Chella van der Post and Iris Nagtegaal re-reviewed the biopsies, interpreted the data and critically reviewed the manuscript. All authors gave final approval of this version to be published.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.