Electrocatalysis: Prospects and Role to Enable an E-Chemistry Future
Abstract
Electrocatalysis is a crucial technology that will enable future low-carbon chemical production and energy beyond fossil fuels. Notwithstanding the intense and growing research in the area, the potentialities of the field are largely unexplored. We provide case examples and discuss emerging possibilities that have still not been investigated enough but are necessary to exploit this potential and enable future e-chemistry. Starting from defining trends and setting the scene, as well as clarifying the difference between electrochemistry and electrocatalysis, some elements of this vision to foster innovation in the field are discussed. The aim is to stimulate discussion and reflection rather than review the state-of-the-art. Aspects discussed regard i) passing from electro to photoelectrocatalytic approaches, ii) the possibilities of making chemicals from the air, iii) the exploitation of both anodic and cathodic reactions, as well as tandem/paired electrocatalytic reactions, and iv) emerging possibilities for anodic selective oxidation and mediated synthesis. Priorities and strategies to enable an e-chemistry future are discussed. Intensifying research in these directions and extending the still-too-limited current approaches, including in modelling and design, is the necessary effort to accelerate the realisation of a distributed future e-chemistry.
1 Introduction
Electrocatalysis defines the area of electrochemistry and related technologies where the use of a catalyst significantly alters the electrochemical redox processes in terms of rate and/or selectivity. In electrocatalytic (EC) technologies, the potential to be applied to the electrodes is provided by an external source. In photoelectrocatalytic (PEC) processes, it is instead either generated by an integrated photovoltaic (PV) module or using photoactive electrode(s).
Electrocatalysis is a rising star in the area of catalytic technologies. In the last decade, the number of publications containing the term catalysis has increased by about 63 % from nearly 19.400 in 2014, according to the Web of Science. Still, the share of articles containing the term electrocatalysts has increased from 6 % to about 14 %. Even more impressive, the number of reviews on electrocatalysis increased from 33 in 2024 to 776 in 2024. This rising scientific interest is related to the push to develop technologies based on renewable energy sources (RES)1. Among those using direct RES (photo-, electro-, and plasma catalysis, mainly), electrocatalysis is considered closer to industrial application2.
Electrocatalysis represents a shift from traditional thermal methods2a, 3 that uses heat (derived from fossil resources) to drive the catalytic transformation. The electrification of chemical reactors,3c, 4 e. g. providing the heat through heating via electrical current (direct or indirect), is an alternative. It does not change the intrinsic catalytic mechanism but only affects heat transfer aspects. However, effective progress beyond the use of fossil fuels5 requires a more innovative effort and a change in the catalytic process technologies,4c, 5a as offered by electrocatalytic technologies3c, 6. In addition, electrocatalysis is important in chemical energy conversion, a necessary technology for long-distance transport and long-term storage of energy.7
1.1 Trends and Setting the Scene
There are two well-established electrocatalytic technologies: i) to make H2 from water electrolysis, a century-old technology but with a fast increase of interest also industrially in relation to the H2 economy8 and ii) electrocatalytic organic synthesis9 for speciality chemicals. The latter are used in typically small-scale processes. At the same time, a few industrial examples exist for commodity chemicals, such in the adiponitrile process10 or the dimethoxylation of 4-tert-butyltoluene to produce a protected benzaldehyde, a step in the lysmeral synthesis11 used as a fragrance. A pillar for electrocatalysis is the chlor-alkaline industry, which is the industrial technology to produce Cl2 and NaOH. Even with the progress in this area, from mercury to diaphragm electrolytic cells and finally to ion exchange membrane (IEM) electrolytic cells, there are still several challenges12. However, research effort is limited due to the scarce industrial interest in developing further this established technology. There are, however, elements of novelty to mention in this area, such as the development of novel single-atom catalysts for electrosynthesis of chlorine from seawater-like solution13, the coproduction of H2 in chlor-alkali process14 or the use of chlor-alkali technology to power environmental electrochemical treatment technologies15.
Electrocatalysis is also used in organic pollutant degradation16. However, besides the scientific interest, the industrial applications are limited to niche uses because the technology is not typically cost-competitive to alternatives. It may be used as an anodic reaction alternative to oxygen evolution reaction (OER) to improve kinetics and reduce overpotential in water electrolysis17 or other reactions like CO2 reduction (CO2RR)18. However, the use of more valuable anodic oxidations, as described later, is preferable. Thus, there are no relevant industrial examples of using organic pollutant degradation as anodic oxidation in electrocatalytic processes, notwithstanding the many research studies.
The water electrolysis area, although still presenting active research on the development of improved electrocatalysts, is mainly focused on improving the technological aspects to decrease the cost because the H2 produced in this way is still uncompetitive. There are plans for large industrial production under policy pressure. Still, without subsidies and/or external drivers for deploying this technology on a large scale, it may be questioned whether water electrolysis will become a pillar of future sustainable energy and chemical production19. There are, however, contrasting opinions on this indication.
One of the critical points regards the storage/transport of hydrogen to overcome temporal and geographical mismatches between locations for the use of green H2 and for its low-cost production from RES. Using green H2 to produce e-fuels is an option to overcome this gap20, but there are strong concerns about the costs and the effective cost advantage (for society). Rather than passing through the intermediate production of H2 by electrolysis, (photo)electrocatalytic (PEC) technologies to produce e-fuels (in one step) offer a clue to overcome the limits of e-fuels technologies21. We may call solar fuels those synthesised in this way to differentiate from those passing through intermediate H2 formation21, 22, e. g. e-fuels. Solar fuel processing incorporates a step of renewable energy capture to avoid dependence on the grid and become independent of external energy supplies, an important step for a resilient development that minimises market dependence and thus enables the concept of net-zero communities for a circular economy23. Often, these technologies are called artificial leaves or trees because they mimic nature's capability to use sunlight, carbon dioxide, and water to produce chemicals24.
It is not relevant here to discuss further pros/cons. Still, there is often a consensus that the large-scale deployment of H2 production by electrolysis will not depend on the possible future improvements in the electrocatalysts. Even if minimisation in the use of critical raw materials (CRM) and the design of the electrode to improve mass and charge homogeneous distribution, minimise H2 bubble contact, etc., will improve performances, these aspects will have a minor impact on industrial deployment of H2 electrolysis technology, in its different technological declinations (alkaline and proton-exchange membrane cells, high-temperature solid-oxide electrolysers, etc.).
Many improvements are instead still possible in electrocatalytic organic synthesis to improve selectivity, activity, and stability. However, the (often) small-volume applications, the short-term time to market, and the high added value of the products often hinder dedicated research.
There are instead emerging directions in electrocatalysis, which combine a large potential for application and a relevant need to improve electrocatalysts/electrodes in terms of Faradaic efficiency, selectivity, stability and productivity, e. g. current density2a, 3c, 5a. These areas are related to the electrocatalytic i) CO2RR25, ii) N2 fixation26 and iii) electrocatalytic conversion of biomass-derived chemicals to produce large-volume chemicals27. We should also add the electrocatalytic production of H2O228, a valuable clean oxidant in industrial-relevant processes (propylene oxide, caprolactam), besides relevant uses as bleaching agents (particularly, but not only pulp and paper industry), water treatment, Na-perborate and -percarbonate production, etc. However, the industrial potential of the three above areas is higher.
As reported by Liu et al29 in their roadmap of electrocatalysts for green catalytic processes, the research on electrocatalysis (in terms of the number of papers up to March 2020) is largely dominated by water electrolysis and related aspects (OER and oxygen reduction – ORR: hydrogen evolution – HER). CO2RR and N2 reduction (NRR) account for about 4 % and 1 %, respectively, with respect to the sum of papers on water electrolysis and related aspects. This roadmap does not consider the electrocatalytic production of biobased chemicals and H2O2.
The aim here is not to overview the recent progress in these areas, being already several additional reviews of the few cited above. As an example, limiting to CO2RR, about 500 reviews (in English using the keywords “CO2 and electro” in SciFinder) have been published in the last decade. The number of papers is impressively higher. The question is whether there is a correspondence between this massive research effort and technological improvements towards application.
For example, analysing selected recent reviews on the rational design of CO2RR electrocatalysts,30 it may be concluded that despite the scientific advances, a generalised approach, e. g. not valid for the specific set of results, is missing31. There is a lack of effective progress towards industrialisation, i. e. overcoming current limits for reliable electrode sizes (above at least 10 cm2) and effective practical operative conditions. There is a gap between scientific advances and progress in the industrial application of electrocatalytic technologies, which is not only related to the usual effort necessary in scaling chemical processes.
In terms of practical challenges and limitations associated with making large-volume chemicals from CO2 through electrocatalytic processes, there are other aspects to consider besides the scientific and technological challenges, including scalability, mentioned above. The first is the availability and cost of RES to perform the process on a large scale. It is necessary to avoid or minimize the use of external RES, which are critical elements in costs and dependence on externalities. It is necessary to develop approaches which directly integrate the use of solar energy, as the PEC approaches discussed in the next section. A main issue discussed later in more detail is that this implies the change to a distributed model of production, which is, thus, a radical change with respect to the current modalities of chemical production. Avoiding the cost of separate units for capturing and purifying CO2 is another main issue. The current approaches in this direction are unsatisfactory in terms of cost and energy use. Fully novel approaches are needed. Downstream operations are often not considered, but the key issue is applicability.
A general question is that the fixed and operative costs related to electrocatalytic technology are still too high. While the focus of research is often on performance, cost-effectiveness (considering the whole technology, including ancillary elements) has to be the goal. The design of both electrocatalysts/electrodes and electrocatalytic cells has to be revised to minimize these costs. In addition, operative conditions have often been unsuitable in terms of exploitability. While improving the electrocatalysts/electrodes is a worthy exercise as well as a better mechanistic understanding, the critical issue in accelerating the applicability of electrocatalytic technologies is related to the engineering of the systems. However, the largest majority of the publications are on the mechanistic aspects and design of the electrocatalysts, with limited studies and a lack of effective new ideas on system engineering. In other words, the requirements for introducing electrocatalytic technologies on a large scale are the opposite.
Many will disagree with these statements and claim a higher perspective for electrocatalytic applications. There are examples, although still limited, of pilot units and efforts in scaling up electrocatalytic technologies.32 In addition, some research projects aim to construct and test electrocatalytic bench/pilot scale units. For example, the EU projects i) RECODE (grant 768583) for CO2 to formic acid as a part of recycling carbon dioxide in the cement industry, ii) OCEAN (grant 767798) also for CO2 to formic acid but as a part of a value chain to produce C2 monomers33, iii) PERFORM (grant 820723) for a platform pilot unit for biobased chemicalsì electrochemical production, with two showcases of maleic acid from furfural paired with the production of valeric acid from levulinic acid, and adipic acid production from glucose) and iv) CELBICON (grant 679050), turning CO2 into carbon monoxide and hydrogen. Avantium is a Netherlands company actively investing in these directions.
From an industrial perspective, the results are still far from the conditions for exploitability, or they are insufficiently reliable to invest in further development. There are a few start-up companies in the area (for example, i) CERT Systems to convert electrocatalytically CO2 into carbon-based fuels and chemicals, such as ethanol and ethylene, ii) Jupiter Ionics for an electrochemical process to produce green ammonia, iii) CERT for CO2 electrolysis to ethylene, iv) Oxylys Energy to convert CO2 into green e-methanol, v) NitroVolt for electrochemical Li-mediated ammonia synthesis, vi) HPNow for the electrochemical production of hydrogen peroxide. However, the effective exploitability of these technologies, based on the claims, is not sufficiently proven. Major companies are instead reluctant to invest in novel electrocatalytic processes, which are still considered a long-term possibility (2050 or beyond).
The introduction of radically new technologies always requires significant time to market. In addition, there are other external constraints, such as the availability of cost of renewable electrical energy, which determine exploitability. However, the time to market is potentially shorter in the case of electrocatalysis because the implementation does not depend on scaling by size as in traditional chemical processes but mainly on numbers. Furthermore, some novel technologies, such as 3D printing, may accelerate the scalability of electrodes and reactors34. Nevertheless, the transition is slower than usual, even when compared to the deep transformation that occurred about 70 years ago in chemical production35 due to the change in feedstocks.
The reflection, which is the aim of this personal account, is whether the current approach in electrocatalysis is appropriate to make a fast transition or instead it is necessary to revise it. In this respect, the first point to clarify is the concept of electrocatalysis vs electrochemistry36.
1.2 Electrocatalysis vs Electrochemistry
These two terms (electrocatalysis and electrochemistry) are often used as synonymous and considered equivalent, but they underpin different physicochemical phenomena and mechanisms. Rarely is there sufficient proof and evidence to discriminate among them, as shown for water photoelectrolysis.37
Electrochemistry refers to the redox process occurring at the surface of the electrode upon application of a potential. In organic electrosynthesis, the processes are also often mediated by redox transfer agents present in solution9e, 38. The electron transfer occurs at the interface, typically through an inner- or outer-sphere mechanism as described, for example, by Marcus theory39, still remaining at the core of the description of the electrochemical mechanisms40. Electron transfer occurs at the intersection of the two potential energy surfaces. It is thus determined from the reorganisation energy, which includes the reorganisation of the solvent (outer sphere) and ligands or strongly coordinated molecules (inner sphere).
The theory was developed for single electron transfer, but two relevant cases requiring adaptation of the theory are multiple electron transfer processes and proton-coupled electron transfer41. In these electrochemical approaches, the electrode surface is not explicitly involved in the mechanism. However, for simultaneous multiple electron transfer, the activation energy increases significantly. Thus, it may be more convenient energetically to form intermediate species for a cascade sequence of single electron transfer reactions. These intermediates may chemisorb or be stabilised at the catalyst surface. For the classical reaction of hydrogen evolution reaction (HER), the first electron transfer occurs on a proton, forming a chemisorbed hydrogen species (Hads) that, through a concerted proton-electron transfer or alternatively reacting with a second Hads species, generates H2 (Volmer, Heyrovsky, and Tafel steps). Thus, some aspects of catalysis are included in electrochemical approaches and modelling. Still, there is no effective description of many catalytic aspects, particularly nanostructure, and its implications in terms of reaction mechanism.
In electrocatalysis, instead, a surface catalytic mechanism is used to model and understand the process, including the so-called Sabatier principle and its variations, e. g. that a volcano plot exists in correlating reaction rate and an activity descriptor42. This approach has been extensively used in electrocatalysis to identify optimal electrocatalysts following the pioneering studies by Nørskov and coworkers43. Although widely used by many researchers, there are limitations in the approach, particularly for relevant multielectron electrocatalytic reactions44. More relevant is that the computational approach does not effectively differentiate between catalysis and electrocatalysis. More aspects will be discussed later.
There is thus a gap on both sides in developing a unified electrochemical and electrocatalysis theory that is able to include both words and can be effectively used to design novel electrocatalysts for the emerging field mentioned above45. Many scientists probably do not agree with this statement. Still, the absence of proper recognition of this critical gap is one of the crucial factors slowing the progress in the field. There is also a lack of proper distinction between catalysis and electrocatalysis, which should be approached differently, including from a theoretical perspective.
While the concepts discussed are heterodox and far from having a consensus, it is surprising that there is an absence of discussion on them as well as regarding the limits in current electrochemistry vs electrocatalysis and electrocatalysis vs catalysis approaches. Thus, this personal account aims to stimulate a discussion and reflection that is considered crucial to accelerate the deployment of electrocatalytic technologies for energy and chemical production.3c, 5a, 21c
2 A Vision to Foster Innovation
In front of the impressive research activities on electrocatalysis, as commented before, the topic appears from a bird's eye view as focused on a few methodological directions (concisely described as attempts to identify the nature of the active sites and reaction mechanism or to report top performances independently whether they are relevant from an application perspective), leaving not sufficiently explored other crucial aspects for exploitation.
2.1 From Electro to Photoelectro-Catalysis
We are in a deep transition46, e. g., one that radically changes the energy and chemical production scenario towards a sustainable, circular and resilient future47. There are discording ideas on whether and when this may occur, but overcoming current centralised production models for a decentralised48 production is one of the disruptive directions. Decentralised production offers many advantages in terms of minimised impact, use of local resources, integration within communities, faster time to market, greater flexibility, avoided or strongly reduced dependence on externalities, etc. Realising net-zero communities requires the development of such a type of decentralised production.
Integration of the direct capability of harnessing solar light is necessary, e. g., to realise photoelectrocatalytic (PEC) devices. Figure 1 illustrates schematically the type of PEC devices where the photoactive unit is integrated into the anodic part (PECa) or is present externally as a photovoltaic unit (PV/EC), eventually integrated into the cell. Detailed comments on the differences between these (and other) typologies of PEC cells were reported elsewhere21b. In brief, in PECa design, the photoactive functionalities are integrated into one of the electrodes, typically the anode, as in PECa design. There is the possibility of having both anode and cathode photoactive, but there are many difficulties to overcome in a practical realization, making this approach ineffective. The design scheme of the PECa compact cell differs from the first because the cathode and anode are directly located at the two sides of a membrane. This design allows for the reduction of transport limitations, which greatly determine the performance. In addition, as outlined in Figure 2, it is possible to eliminate the electrolyte and realize zero-gap cells using gas-diffusion electrodes. More details elsewhere.49 Instead, the PV/EC configuration is based on a separate PV cell that drives the electrocatalytic (EC) unit.
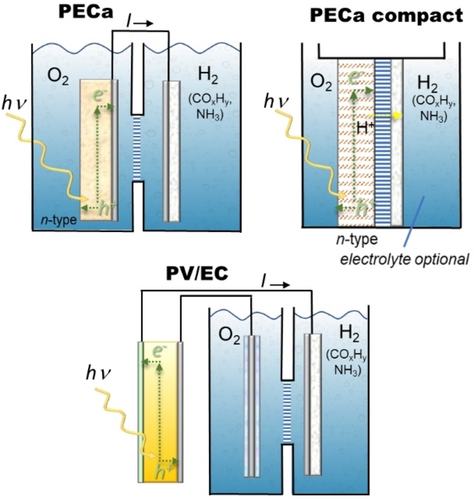
Simplified schemes of photoelectrocatalytic (PEC) devices: (top) when the photoactive unit also acts as the anode (PECa), including the compact version or (bottom) a photovoltaic-driven electrocatalytic unit (PV/EC).
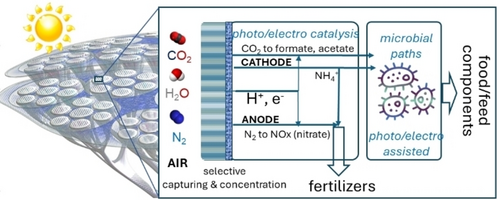
Schematic presentation of the conceptual approach to making chemicals from the air in a hybrid artificial-tree-type integrated device. Adapted with permission from ref.52 Copyright RSC, 2024.
However, compared to electrocatalytic devices, where the current density is dictated by the cell design and operations, in a PEC system, the current density is associated with that provided by the photoactive element (an external photovoltaic unit, or a photo-cathode or -anode), the resistance of the cells, and coupling between the photo and electro components. While typical current densities of industrial interest in electrocatalytic processes are above 500 mA/cm2, they are over one order of magnitude lower in PEC devices. Furthermore, while electrocatalytic devices operate continuously (24 h), even if the carbon footprint of the available electrical energy may negatively impact the effective greenhouse (GHG) emissions, PEC devices need to operate when sunlight is available. This would require rethinking design in terms of low manufacturing cost for discontinuous operations rather than in terms of conventional aspects such as solar-to-chemical conversion efficiency21b.
In terms of how photoelectrocatalytic approaches differ in effectiveness compared to traditional electrochemical methods, it may be indicated that essentially, in all the three schemes of PEC devices presented in Figure 1, e. g. including when the photoactive elements are integrated into the electrodes (PECa design), the mechanism of the generation of a photocurrent which drives the electrocatalytic processes. Thus, essentially, the effectiveness and mechanism are the same for EC and PEC approaches. Still, the latter has limitations in terms of potential and (internal) current densities, which are associated with the characteristics of the photoactive elements. In the EC approach, they can be externally modulated to the optimal value, while this is more difficult to achieve in PEC design. Thus, in contrast to EC devices, where current densities can be up to 1 A/cm2, PEC devices typically have up to two orders of magnitude lower current densities.21b
A distributed production also requires minimising downstream operations (conversion and separation) as much as possible, making them eventually compatible with the PEC production unit (as productivity, pressure, etc.). These aspects significantly influence the choice and design criteria even during the initial evaluation. It is thus surprising that there is scarce attention given to these aspects in the literature. Similarly, PEC devices are typically studied as independent elements from their integration in the value chain. They could also be used to design novel approaches, as outlined in the following sub-section.
2.2 From Air to Chemicals
The PEC devices presented in Figure 1 are also called artificial leaf or artificial photosynthesis devices21b, 24b-24f because, as in natural photosynthesis, they can use CO2, water and solar light to make chemicals. Often, the same definition is used for PEC-type devices for water splitting. However, except in some cyanobacteria, H2 production is not a target product for natural photosynthesis. Note also that artificial photosynthesis is different from natural one in several important aspects: i) the solar-to-chemical efficiency is significantly higher, already over 10 % or above, 24d, 50 while it is typically below 1 % for natural photosynthesis, ii) the target products (formic acid, methanol, C2 chemicals, etc.) are not the products obtained in natural photosynthesis, and often they can be toxic for natural systems, iii) the photosynthesis machinery is less complex and sophisticated (for example, self-regulation systems are not present), but more robust and easier to realise. Thus, mimicking natural processes is a commonly used term, but effectively, the artificial leaf has to be conceptually different. Differently, in artificial photosynthesis, mimicking natural processes is an effective goal, but these systems have significantly lower efficiencies than the PEC devices. Also, photosynthesis particulate-type devices have significantly lower solar-to-chemical efficiencies in CO2 conversion.21b, 51
An important difference between natural and artificial leaves is that the former are able to capture CO2 and water from the air directly. Thus, realising an artificial tree, e. g., the further extension of the concept of an artificial leaf, requires incorporating the possibility of capturing CO2 and water from the air, and eventually also N2, and using them in an integrated PEC device. A further extension regards the possibility of making not only simple chemicals but also the complex molecules we need, such as carbohydrates, lipids, proteins, etc. Enzymes are capable of such syntheses, but the slow step is to convert CO2 by the photosynthesis machinery to the C-substrate used further in the enzymatic cycles. By coupling a PEC device to convert CO2 to such a type of C-substrates (acetate, in particular) to be fed to enzymes, it is possible to intensify the overall rate by over three orders of magnitude. Synthetic foods produced in this way would thus require much less resources and land compared to agriculture, drastically reducing the environmental impact at the same impact. This is thus a potentially disruptive technology for a sustainable future.
Centi and Perathoner52 discussed this possibility in a perspective paper. The concept they presented is graphically shown in Figure 2. It represents a vision for the future, while the paper discusses the current status of the research on the specific elements of this technology and the gaps to overcome. There is progress in the specific sub-topics, but the attempt to integrate them into a single unit has not yet been investigated.
This device combines i) the capabilities of functionalised membranes (or equivalent systems) in capturing and concentrating H2O, CO2 and eventually N2 to the surface of an electrocatalyst while preventing contact with molecules that may inhibit the activity such as O2; ii) the possibility of a PEC device to use sunlight to convert CO2 on the cathodic side (to acetate preferably), while fixing N2 to nitrate on the anodic side; the cathodic sides is a feed for microbial further conversion, while the anodic side can be used as a fertiliser; iii) the abilities of microbial processes in synthesising complex molecules such as carbohydrates or proteins, eventually assisted by photo/electro components to bypass limitations by using co-enzymes (NADH and ATP).
While the cited paper52 reports the status of research on the single components of the hybrid artificial-tree-type integrated device presented in Figure 2, specific examples or case studies on the integrated device are still not present in the literature. Thus, it is necessary to foster research in this direction.
2.3 Exploit Both Anodic and Cathodic Reactions
(Photo)electrocatalytic devices have separate zones for oxidation and reduction reactions. This fact offers creative possibilities for process intensification and the design of innovative solutions53. Instead, literature studies typically focus on a single electrode with minor attention on the whole device enough and exploiting both cell sides.3a, 54 The research focus of electrocatalysis for the target reactions is on the cathodic development, with OER being the typical anodic reaction used (with conventional catalysts, although often it is the limiting process).54 OER is a 4e− redox process with a slugging kinetic55 that requires a relatively high overpotential, e. g. lower energy efficiency. It was estimated that OER may account for up to 90 % of the total electricity input in CO2 electrolysers.56 There is increasing attention, but not systematic, to using different anodic reactions than OER to improve the kinetics and efficiency. At the same time, they could produce an added value compared to O2 production.
A general remark, however, is that the anodic reaction, from an industrial perspective, should be carefully chosen to combine with the value chain of the cathodic reaction in terms of market, volumes of production, field of interest, etc. A typical example is the use of wastewater purification as the anodic reaction,57 which typically does not result in practical applications (as coupling to CO2RR, for example), while it is one of the advanced oxidation processes (AOP) in use as such (e. g. without valorisation of the cathodic reaction). Electrocatalysts typically used are boron-doped diamond (BDD), PbO2, SnO2 and Ti-based anode (e. g., Ti4O7, blue titanium oxide).
However, possible useful integration can be identified, even if not still studied. In biogas production, the CO2 produced (typical biogas composition is two-thirds CH4 and one-third CO2) could be photoelectrocatalytically converted to methane at the cathode (thus lowering carbon footprint and increasing productivity). Instead, the wastewater produced in the digestor (where the biogas is produced) could be fed to the anodic part of the electrocatalytic cell to reduce the chemicals that inhibit the wastewater treatment by active sludges. The added value derives from the improved wastewater treatment operations.
The OER alternatives proposed to reduce costs and improve sustainability range from raw chemical generation (e. g. H2O2) to waste oxidation and molecule upgrade.58 Apart from hydrogen peroxide, which could be preferably produced at the cathode by oxygen reduction (ORR) than at the anode by water oxidation (WOR)28, 59 and requires high overpotentials, the other anodic reactions proposed in the literature (apart from waste total oxidation) include unsafe (even if established) inorganic reactions (such as NaCl to Cl2), or organic chemicals conversion which have a difficult coupling (as value chain) to CO2RR cathodic reaction (for example ethanol to acetate, glycerol or 1,2-propandiol to lactic acid).58 However, they can be used in some biorefineries. There is still a need to explore new possibilities, but having a precise vision of the industrial context in which the co-valorisation (cathode and anode) technology would be located.
However, an aspect often underestimated is that the reaction rates (and mass/electron balance) at anodic and cathodic sides should match when pairing two electrocatalytic reactions. An electrocatalytic cell is a closed circuit where both reactions are mutually influenced. While separate reactions are typically investigated even when co-valorisation of anodic/cathodic reactions is proposed, only results with the full cell, e. g. when both reactions are simultaneously investigated, provide the right results even in terms of optimal electrocatalysts. There is a lack of studies in this direction. When a photoactive element is present, e. g. PEC devices, a further critical aspect and variable is added. Experience has proven that the electrocatalyst performances (and selection) in a PEC cell are different from those in an electrocatalytic cell with an externally applied bias.60
Typically, electrocatalytic reactions are studied at room temperature without analysing the effect of the reaction temperature. In addition, the same temperature is used at both the cathodic and anodic sides. However, it is technically feasible to have cells with a temperature difference up to 50–100 °C that allows a better match between the reactions at the cathode and anode sides. This possibility was explored in the frame of an EU project (TERRA, grant 677471) and offers new clues to design advanced electrocatalytic processes.
Pairing CO2RR with the electro-oxidation of organic (biobased) chemicals (OOR) is an emerging direction. The oxidation of 5-hydroxymethylfurfural (HMF, a biobased platform molecule61) to 2,5-furan-dicarboxylic acid (FDCA, a monomer for alternative biobased polymers substituting terephthalic acid62) is an example of coupling CO2RR with OOR. The substitution of OER with this anodic oxidation decreases the overpotential, as shown in Figure 3, besides producing a valuable added-value chemical.
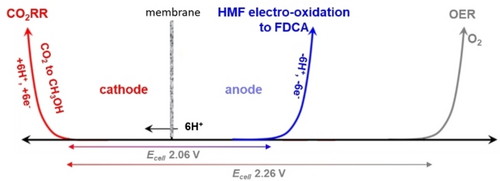
Current density versus applied voltage and minimum potentials for paired CO2 electrolysis (to methanol) and HMF oxidation to FDCA, compared to OER. Based on data reported in ref.63
A techno-economic assessment (TEA) of various possibilities (about 300) for coupling CO2RR with the selective electro-oxidation of organics (OOR) has been reported by Na et al63. They developed an automatic framework approach for process simulations, although with limits in accounting for some process systems, such as solidification, acid treatments, and GDL-type electrolyser devices. The approach was used to estimate the levelized costs of chemicals, concluding that for coupling with CO2RR, formic acid, n-propanol, acetaldehyde, allyl alcohol, glycolaldehyde, and ethylene glycol are preferable. Other chemicals such as FDCA, 2-furoic acid, ethyl acetate, lactic acid, formic acid, glycolic acid, and oxalic acid were also indicated as suitable. The analysis, besides some limitations in the TEA approach, does not account for the relevance of the value chain, both in terms of costs of raw materials (including the critical issue of renewable electrical energy cost) and the use of the products, including the difference in the market size. Nevertheless, it shows pairing CO2RR with OOR is an important direction deserving more effort in finding optimised devices and reaction combinations and testing them in pilot plants.
While the industrial exploitability and integrability in biorefineries still need to be proven more convincingly64, these indications show that it is possible to consider (in perspective) a distributed model of chemical production where (photo)electrocatalysis becomes the pillar for low-carbon chemical production beyond fossil fuels, i. e. an e-chemistry based on carbon circularity and biobased resources.3c, 4c, 5a, 5b Improving the effort to exploit both anodic and cathodic reactions is a must to go in this direction.
A requirement, however, is the possibility of designing ad-doc electrocatalysts given a target reaction, as current mechanistic and theoretical approaches are still unable to provide a general toolbox of electrocatalysts suitable for novel OOR.
While, in principle, the same considerations valid for CO2RR-ORR pairing could also be applied for NRR (N2 fixation to ammonia),26, 65 the still insufficient performances have not yet stimulated the investigation of suitable pairing reaction. There is, however, an important difference to the previous cases. An attractive possibility is to pair NRR with N2 oxidation to NOx (nitrate, in particular) (NOR) to develop in a single step the production of ammonium nitrate,54 i. e. allow a distributed production of green fertilisers. The concept is shown in Figure 4.
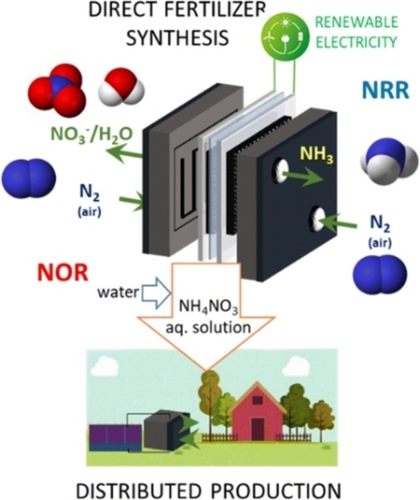
Concept of a direct electrocatalytic device for a distributed production of fertilisers (ammonium nitrate aqueous solution). NOR: nitrogen oxidation reaction. NRR: nitrogen reduction reaction. Reproduced with permission from ref.54. Copyright RCS, 2023.
This technology of direct electrocatalytic production of ammonium nitrate offers several potential advantages: i) it is well suited for distributed production (at farmer or small district level) using locally available renewable electricity sources (or can be coupled to a photoactive unit as presented in Figure 1), and ii) it produces an ammonium nitrate aqueous solution for direct local use as a fertiliser lowering the carbon footprint, avoiding long-distance transport of the fertiliser (and related externalities), decreasing cost (being avoided the energy-intensive step producing solid ammonium nitrate, with also associated safety issues). However, studies on NRR are still far from exploitability, and those on NOR are still very limited.66 No results have yet been reported on an integrated device, as presented in Figure 3, notwithstanding the large applicative potential.
2.4 Tandem/Paired Electrocatalytic Reactions
Particularly in the field of biobased processes, there is a large potential for tandem/paired electrocatalytic reactions beyond the examples discussed above. Among the advantages are i) process intensification and develop low-carbon processes, ii) the use of renewable energy, and iii) in-situ generation of the redox reactants (hydrogen equivalents, active oxygen species), thus avoiding the production costs for reducing agents (typically H2) or oxidants. Notwithstanding being known for years, there are still no commercial examples, being necessary to overcome many difficulties, from identifying suitable electrocatalysts combining high Faradaic selectivity at high current densities, high stability (fouling of the electrode or leaching are typical issues), pairing match the requirements of anodic and cathodic reactions (e. g., balance in terms of reaction rates, electron and H+/OH− flow, etc.).
While research often focuses on a single electrode side, the cathodic one, exploiting both sides properly, is an unavoidable necessity.17b, 67 When the focus is on the cathodic part (in CO2RR or HER, for example), OER is the typical anodic reaction. Still, it has slugging kinetics and requires relatively high overpotentials, being a 4e− oxidation. Thus, alternative oxidation reactions have often been explored, including wastewater oxidation, which is mentioned in the introduction. There is recent interest in exploring also other energetically easy and kinetically fast reactions to speed up the process. For example, in the electrosynthesis of ammonia by nitrogen or nitrate reduction, hydrogen oxidation at the anode side has been proposed to enhance the performance.68 While reducing the energy requirements of the cell (but not the overall energy valance, by considering the energy request to produce H2), this is not the right approach to enhance the techno-economic viability and sustainability, which is the critical issue. Similarly, using nitrate as the feed for ammonia reduction rather than N2,69 improves the kinetics and performances but has a limited industrial relevance. Ammonia is mostly used to produce nitrate (as fertilizers). Thus, the interest is in converting N2 to ammonia or, better yet, nitrate directly (as outlined in Figure 4) rather than in reducing nitrate to ammonia. Also, to eliminate nitrate from wastewater, there are better industrial alternatives. The message is thus to address relevant value chain and industrial cases rather than use academic shortcuts to prove higher performances.
Creating case examples for effective integration within a valuable value chain is important to push research and industrial interest in this area. One interesting example, which, however, is still not at the exploitability level for the many issues outlined above, is that at the core of the EU project, TERRA (cited before). Poly(ethylene 2,5-furanoate) (PEF) is a biobased polymer alternative to the fossil-based polyethene terephthalate.70 PEF is produced from two monomers: FDCA and ethylene glycol. They can be produced in a paired electrocatalytic process at the anode and cathode, respectively, starting from sugars. Thus, a single electrocatalytic unit can generate simultaneously both the two monomers necessary for producing PEF, with a significant potential cost reduction in the process.
The electrocatalytic synthesis of adipic acid is another potentially large-scale example of paired electrosynthesis. It was at the core of the PERFORM EU project (cited before). Glucose is oxidised to glucaric acid at the anode, which is fed to the cathode for hydrodeoxygenation to adipic acid. There are some advances for the single reactions71, but the fully integrated process has not been demonstrated electrocatalytically, but only catalytically (Rennovia process72).
Several examples of other possible electrochemical conversion of biobased molecules (polyols, carbohydrates, heterocyclic, and other chemicals) exist. Many were discussed by Lucas et al73. They remarked, however, the gap between the lab-scale results and possible industrialisation. Several challenges were identified for industrialisation. The main issues indicated (among others) were i) the low faradaic efficiency and presence of competitive HER/OER reactions (with thus downstream costs for separation and loss of energy efficiency), ii) the low product concentrations and complex product streams, as well as the need to recycle the electrolyte, which determines costly downstream processing, iii) the insufficient current densities (thus high fixed costs, i. e. CAPEX), iv) electrode stability due to fouling, v) membrane crossover of chemical species. However, studies have been limited in these directions up to now, and also case examples to overcome them, at least in part, are scarce.
The cost of downstream processing is a key issue, and thus, a good case example to promote electrocatalytic technologies would be to identify possibilities to reduce these costs drastically. The EU project DECADE (grant 862030) addressed this question in two ways: i) by forming the same product at both the cathodic and anodic side and ii) by producing a stream which, with minimal downstream operations, can be directly used. The project concept is a PV/EC device, where at the cathodic side, the CO2 is electrocatalytic reduced (on a gas diffusion cathodic electrode - GDE) to acetate, which is used to esterify the co-feed ethanol to ethyl acetate (EA). On the anodic side, the ethanol is instead oxidatively dimerised to EA. A stream of ethanol and EA with some ethyl formate (side product) is produced that can be directly used as a green solvent or gasoline fuel additive. The integrated photovoltaic module allows direct operations with solar energy. The device was developed up to the bench-scale level.
This is a scientific area with very limited available results. There are several studies on CO2 electrocatalytic reduction to acetate and/or formate74, but all these studies refer to the use of aqueous electrolytes. When ethanol is used as an electrolyte (plus some additives to increase conductivity), the electrocatalysts selective in aqueous electrolytes are completely inactive, even when some water is present together with ethanol. For the anodic part, there are few studies75 of difficult reproducibility and/or applicability. There is, thus, novel electrocatalytic chemistry largely unexplored and, in general, still insufficient scientific bases to design tailored electrocatalysts for operations under less conventional conditions and electrolytes.
An analogous cell can be used to produce methyl formate (MF) from methanol by valorising the CO2 emissions in methanol plants to a higher-added-value chemical. MF is commercially produced by methanol carbonylation, but a new green route could be the production of methanol from CO2 and green H2. Then, the electrocatalytic conversion of methanol to MF using the possibility to produce on both sides of the cell, by methanol oxidative dimerisation at the anode and by CO2 reduction to formate, used to esterify methanol to MF.
There are other possible examples of the possibility of producing the same product at both anodic and cathodic sides. The electrocatalytic production of glycolic acid (GC) is an interesting example. GC is produced by oxalic acid reduction at the cathode, while at the anode, glycerol is electro-oxidised to glycolic acid (GC). Glycerol is a byproduct of biodiesel production, while oxalic acid could be produced by biowaste fermentation. GC is a monomer for green poly-glycolic acid production, a high-value polymer. This is another case example of the value chain and reuse of waste to realise carbon circularity through advanced electrocatalytic processes. However, the results of the literature on developing such a type of device and identifying the electrocatalysts are very limited or made under non-relevant reaction conditions.
These examples demonstrate how electrocatalysis can be a crucial technology for future chemical production and energy. Still, a creative effort is required to investigate electrocatalysts, electrodes, and cell design outside of the traditional approaches and conditions. It should be guided by a more in-depth and open-minded analysis of value chains where these technologies could bring innovation to current production technologies.
2.5 Electrocatalytic Anodic Selective Oxidation
Previous sections have already evidenced the need to broaden the studies on electrocatalytic anodic selective oxidation. This is an area much less investigated than cathodic reaction, except for total oxidation. However, raising this as a general methodology to produce valuable large-volume chemicals is a crucial element in the vision for future sustainable chemical production.
An example is phenol synthesis, an important chemical and monomer currently produced industrially through a multi-step, energy-intensive process with an overall yield of around 7 %. Direct electrocatalytic oxidation of benzene to phenol using the activated oxygen species generated on the surface of an anodic catalyst is an interesting option to explore, although with limited studies. Lee et al.76 reported a selectivity up to ~95 % (with a current efficiency of 76 %) in the direct hydroxylation of benzene to phenol over a VOx anode at 50 °C. With alternating current and using a VxOy-Sn0.9In0.1P2O7 electrode, they also show the possibility of producing phenol both at the anodic and cathodic sides77. The alternative approach is to generate insitu H2O2 and use it for the hydroxylation of benzene. For example, over nickel single-atom catalyst (on defective carbon nanofibres) acting as bifunctional electrocatalysts, able to make the two-electron ORR to H2O2 and H2O2-assisted benzene oxidation to phenol.78 Productivity is generally low, but these approaches seem preferable over the alternative of direct photocatalytic oxidation of benzene to phenol,79 which shares, however, several mechanistic analogies.
Direct epoxidation of olefins is another challenging and potentially large-volume reaction.80 Recently, it was shown that ethylene and propylene can be epoxidised electrochemically to the corresponding epoxides (ethylene and propylene oxide, respectively) with selectivities around 70 % at industrially-relevant current densities.81 However, chlorine is used to mediate the process, with related problems of safety corrosion, etc. At the anode, the olefin and chlorine are converted to chlorohydrin [RCH(OH)CH2Cl, where R is H for ethylene or CH3 for the propylene] and HCl, while hydroxide and H2 are produced at the cathode. Downstream mixing produces the epoxide, Cl− and water. When integrated with a CO2-to-ethylene step, an integrated CO2-to–ethylene oxide process is possible.
Another example is the electrocatalytic production of ethylene and acetate from CO2 and their (electro)catalytic coupling to produce vinyl acetate. These examples, together with the above case of phenol production (benzene could also be produced from CO2), demonstrate the potential feasibility of producing large-volume monomers from CO2, offering a full novel possibility for carbon circularity and reuse of CO2. Electro-carbonylation of olefins and diolefins (ethylene and butadiene82) is another route for making CO2-based monomers and chemicals. Butadiene, and not only olefins, could also be produced electrocatalytically from CO2.83
The electrocatalytic oxidation (ECO) of organic compounds can proceed through either direct oxidation at the electrode surface or (ii) indirectly, using redox mediators, as discussed later. During electrolysis in an acidic or alkaline solution, H2O (or OH–) electrode transfer at the anode generates adsorbed hydroxyl radicals, which may either react with an organic molecule or oxidise a transition metal ion of the electrocatalyst, subsequently reacting with the organic molecule. While generally, these mechanisms lead to full oxidation of the organic molecule, the challenge is to control the process to realise selective oxidation. Few studies have explored ECO possibilities84. Data for the predictive identification of suitable electrocatalysts are largely missing. Similarly, most of the studies on ECO are limited to small molecules, while those on more relevant chemicals are scarce.
Lignin and its derivative conversion by ECO is an interesting opportunity.53 Lignin can be electrocatalytically depolymerised to oligomers.85 These (and phenol derivatives) can also be electrocatalytically further upgraded to added-value chemicals. 64d, 86 Catechol can be selectively converted to 1,2-benzoquinone, resorcinol to 2,4-benzoquinone, p-cresol to 4-hydroxybenzyl alcohol and p-hydroxyl benzoic acid, phenol to hydroquinone and p-benzoquinone, as examples.53 PbO2 and Ni-based anodes are the most used, but deactivation is a common problem observed in many of these studies. There is a facile generation of radical species at the electrode surface, which starts the formation of polymeric species, such as humins, occurs, that deactivate the electrocatalyst by fouling.
An industrial-relevant example developed by BASF is the anodic conversion of p-methoxytoluene into p-anisaldehyde (Figure 5).87 Toluene derivatives undergo anodic acetalisation in methanol to form dimethylacetal intermediates, which can be hydrolysed to the corresponding aldehyde. This electrochemistry can be adapted to a variety of other syntheses of valuable chemicals. p-Anisaldehyde is also produced in paired electrolysis, where the cathodic hydrogenation of dimethyl phthalate is associated with the anodic methoxylation of the p-toluene88. BASF produces about 4000 t/y using this method, and other companies also use the method to convert other substituted toluene chemicals.11a
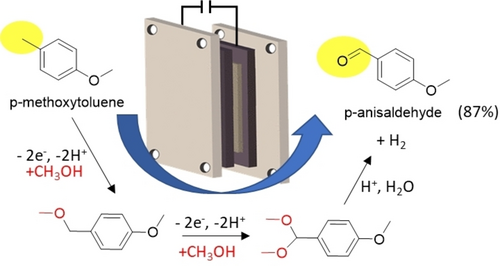
The BASF electrosynthesis of p-anisaldehyde from p-methoxytoluene. Conditions: bipolar graphite rings, 4–6 V, 3–5 A dm−2, 40–50 °C, in methanol.
The ECO of glucose and furanic compounds is another interesting area, as mentioned above. Various anodes have been explored, including, for example, Ni2P/Ni/NF,89 Ni3S2/NF,90 and Ni2P nanoparticles on nickel foam.91
Another relevant area is the Kolbe reaction, where one-electron oxidation at the anode results in the decarboxylation of carboxylates to give the corresponding alkyl radical, which may dimerise (due to the high concentration of radicals at the electrode surface) to produce R–R homo-dimer. On porous carbon graphite electrodes, two-electron oxidation is possible, resulting in the oxidation of the alkyl radical to the corresponding carbocation, R+ (Hofer–Moest reaction).92 When this reaction is combined with a cathodic production of carboxylates (from CO2),93 a very interesting synthetic chemistry is possible. Intramolecular biradical recombination of dicarboxylic acids to unsaturated compounds is alternatively a valuable option to produce alkenes, alkynes or cycloalkanes.94 As several carboxylic acids are derived from biomass, the above electrocatalytic chemistry offers many valuable options for a biorefinery. The process can also be scaled up to continuous flow conditions, for example, to convert valeric acid to n-octane.95
There is thus a variety of valuable electrocatalytic anodic reactions to convert platform biobased chemicals. Still, systematic studies are lacking, both in terms of identifying electrocatalyst characteristics (design) for the reactions of concern and clear identification of needs from an industrial perspective.
2.6 Electrocatalytic Mediated Synthesis
Electrocatalytic-mediated synthesis9a, 11, 96 (indirect electrolysis) is an approach well-used in organic electrosynthesis but less so in other electrocatalytic applications. The reason is that processing cost, including for separation when complex mixtures are present and the redox mediator should be recirculated, is a less critical aspect compared to the synthesis of commodities and large-volume chemicals. Nevertheless, it represents an opportunity that should be considered and evaluated.
In indirect oxidation, a redox mediator acts as the transfer agent to reduce the substrate (Figure 6), which may otherwise be more difficult to be converted directly. Figure 6 also shows some of the typical redox mediators used for anodic oxidation. Transition metal complexes can be used for cathodic reactions.96c Among the advantages of indirect conversion are the elimination of the kinetic inhibition of the heterogeneous electron transfer between electrode and substrate, e. g. the overpotentials can be reduced, ii) electron transfer mediators can exhibit higher or totally different selectivity, iii) deactivation of the electrode can be reduced or eliminated, due to lower potentials, iv) some side reactions occurring at high potentials are avoided. On the other side, additional costs, particularly in downstream separation and redox mediator recovery, may be crucial for large-volume productions, different from organic syntheses of high-added-value chemicals.
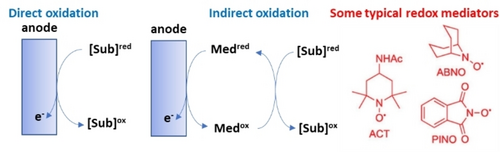
Difference between direct and indirect anodic oxidation and some typical redox mediators.
3 Enabling an E-Chemistry Future
The above examples, although not systematic and exhaustive of the landscape of electrocatalysis for chemical production and energy (further aspects and examples were discussed earlier3c, 5a), show that it is possible to consider a future scenario for the chemical production where the traditional thermal (catalytic) processes are substituted by novel technologies using directly renewable energy sources. This new future scenario we can indicate concisely as e-chemistry.
The e-chemistry concept addresses some important societal challenges: i) develop a resilient model of development which minimises dependence and constraints from resource externalities, ii) develop a CO2-neutral or even carbon-negative production system, which also minimises other impacts on the environment, and iii) realise a carbon circular economy beyond fossil fuels.3a Electrocatalysis is a key technology that can help us realise these challenges. The question is to identify how to prioritize and integrate diverse electrocatalytic reactions beyond the currently limited focus to effectively transition from traditional thermal catalytic processes to a comprehensive and sustainable e-chemistry and biorefinery interface. Some specific elements were discussed in the previous section, while here we attempt to outline the elements of a roadmap and blueprint vision to proceed in this direction.
We may distinguish two main types of possibilities: i) (photo) electrocatalytic routes to produce the raw materials for chemistry, e. g. light olefins, aromatic and syngas) and ii) routes to produce directly value chemicals/monomers, reducing the current multistep, energy-intensive processes or instead based on fossil resources. We discussed here, particularly the second category, why the first category was discussed earlier.3c, 5a
It is emerging from the above discussion that in front of the potentially crucial role of electrocatalysis in a transformative revolution of chemical production and energy, which requires a systematic analysis of all the possible solutions in the complex scientific landscape, the current studies are focused on too limited reactions (CO2 conversion, N2 fixation, water electrolysis or H2O2 production, conversion of few biobased platform molecules). Instead, it is necessary to explore a larger range of possibilities to build an e-chemistry and interface it with biotechnological paths (coupling biorefinery and e-chemistry).97 We have provided here some clues of reflection for new aspects and directions to investigate.
Biobased electrocatalytic processes hold significant potential, but their development was often disjoined from a more extended vision of their effective potential and integration with the possibilities offered by tandem/paired electrocatalytic reactions and the ability to generate active species by electrocatalytic conversion of small molecules. This concept is explained further below in discussing e-chemistry vision related to Figure 7. While there are scientific and technological challenges in developing improved and more stable performances, in part discussed in the previous section, we believe that this is not the conditioning factor to accelerate the transition from laboratory research to industrial application. It is rather a lack of vision about exploring new possibilities and an academic focus on not crucial aspects such as the identification of mechanistic aspects, which often are strongly limited by the insufficient understanding between electro and thermal catalysis. This aspect is commented on below in more detail.
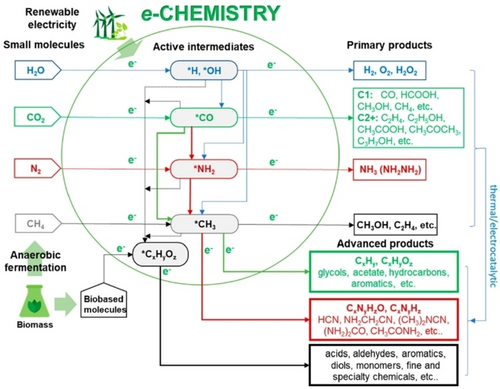
A new framework of electrocatalytically based reactions to develop a new e-chemistry alternative to that based on fossil fuels (petrochemistry). Reproduced with permission from ref.3c Copyright ACS, 2022.
Often, it is argued that these technologies may appear far from being applicable based on conventional TEA estimations. However, the deep transition also requires different assessment methods to be developed to evaluate the effective future impact properly.98
It is also necessary to rethink whether the very large activity in the design and development of electrocatalysts addresses the proper aspects that determine the reactivity. A parallel question is whether the current approach (essentially translating to electrocatalysis the methodologies developed for thermal catalysis, including theoretical ones) is appropriate. To focus on this personal account, we do not discuss here these aspects, which were already discussed elsewhere.
Centi and Perathoner31 analysed the pitfalls and scientific gaps in CO2RR, evidencing the limits of design and modelling studies in accounting for the complexity of the phenomena present in electrocatalysis. Under industrially relevant reaction conditions and types of testing cells, many parameters characterising the performances (such as Faradaic efficiency, carbon selectivity and potential onset, besides the current density) are strongly influenced and typically dominated by the effective population of adspecies on the electrode surface.49, 99 The latter aspect is related to mass control and transport resistances, local pH changes, multiphase boundaries, wettability and other aspects rather than (only) the nature of the electrocatalyst, the focus of most electrocatalyst design criteria. For example, in producing C2+ products in CO2RR,100 an inactive catalyst under normal operations can be turned into a selective catalyst when the electrode/cell design is changed to enhance the surface concentration of carbon dioxide.22e, 99b Thus, the intrinsic nature of the catalyst's active side plays a secondary role. Thus, even the preliminary screening of the catalysts could be incorrect and not operate under representative conditions, e. g., without properly choosing the electrode and reactor.
The mechanistic and design strategies used have an intrinsic methodological issue.101 We proposed that rather than conventional DFT (density functional theory) and derived computational quantum mechanical modelling method, where the energy profile of different paths/active sites is calculated, the key issue in electrocatalysis is determining how to realise a selective transferring energy for the activation barriers. This concept emphasises the role of localised phonons, polarons and interaction with excitons in determining the electrocatalytic conversion pathways.2a, 3b, 31 It is thus specific to electrocatalysis and differentiates electro- from thermal catalysis.
Creating the bases for e-chemistry and exploiting the effective full potential of electrocatalysis requires thus turning current approaches and giving more emphasis to a series of crucial aspects:
-
Improve the design and use of advanced electrocatalytic reactors, overcoming the use of too simple reactors (like H-cells)49, 99a and study novel solutions, from multiphasic electrocatalytic reactors102 to operations using non-conventional electrolyte, organic feed (as alcohols), electrolyte-less conditions, etc. Electrocatalytic behaviour depends on an intricate interplay between surface structure (both on the nano- and mesoscale), electrolyte effects (pH, buffer strength, ion effects) and mass transport conditions.103 Often, what is associated with a change in the nature of the active sites is rather associated with other aspects (wettability, for example), which determine a change in the effective surface population of species at the surface of the electrocatalyst and interface with the electrolyte (or gas-phase). The dynamics of the polarised interface also determine the selectivity and rate37, 104. Upon application of a potential, a significant dynamic reconstruction of the active surface may occur during electrocatalytic operations.105
-
On the application side, there is the need to broaden the approach in exploring novel electrocatalytic routes, valorise the potential of electrocatalytic processes in intensifying the process, reduce the steps, avoid costly reactants, and use renewable energy sources directly. It is necessary to i) investigate more systematically how to develop electro-synthetic paths for bulk chemicals, starting from biobased molecules, and in producing the raw materials for chemical production beyond fossil fuels; ii) develop new reactor/separation technologies that may bring organic electrochemistry from the synthesis of small-volume (high added-value) chemicals to commodities and large-volume chemicals. There are many electrocatalytic classes of reactions that can be the basis for creating novel e-chemistry, but they are still required to identify how to realise effective industrial processes. Examples include106 i) i) C−N coupling to form a range of N-containing chemicals (urea, amides, amines, and nitrile, etc.), ii) selective hydrogenation or hydrogenolysis, with the in-situ generation of H2 or hydrogen equivalents (H+/e−), and iii) selective oxidation or oxidative dehydrogenation, also without the addition of external oxidation agents, including O2. Coupling electro, photo, and bio-catalysis offers many novel possibilities, but this area of hybrid electrocatalytic/biocatalytic systems has been more systematically explored.107
On top of these aspects, there is a need for a more creative effort in electrocatalysis. Reactions to explore include i) direct coupling of in-situ generated intermediates, ii) tandem reactions using in-situ generated chemicals, and iii) the eventual coupling between electro- and other types of catalysis. In the electrocatalytic conversion of small molecules, the current targets are the final products. Instead, there is a large range of novel possibilities for using the in-situ generated intermediates108 produced in the electro-transformation of these small molecules (CO2, N2, H2O, CH4, the latter derived from biogas). The concept is presented in Figure 7, which highlights the possibilities of combining electrocatalytic intermediates produced in the conversion of small molecules to convert and valorise (electrocatalytically) biobased chemicals.
This novel area can produce a large range of products that will form the skeleton of new low-carbon chemical production, together with the other electrocatalytic paths discussed above.
For conciseness, many other possibilities for electrocatalytic technologies have not been discussed, including interesting examples for i) new routes to produce monomers for high-performance polymers via CO2 conversion to oxalic acid33, 109 and its electrocatalytic reduction to glycolic acid110 (for new polyesters) or ethylene glycol, another large-volume monomer (this was the core of the cited EU project OCEAN which scale-up part of the technology to a pilot unit), and ii) production of aminoacids (glycine is a base aminoacids that can be electrocatalytic produced from oxalic acid and nitrate111).
The coupling between electrocatalysis and hetero-/homogeneous catalysis is another area of interest to remark on, although largely unexplored. This development could combine with the possibilities mentioned in mediated electrocatalysis and the many possible variations explored in organic electrocatalysis.112
4 Conclusions and Outlooks
Accelerating the development of electrocatalytic technologies is a necessary objective to enable the transition to a new sustainable chemical and energy production model and a new model of distributed production for net-zero communities.
However, it is necessary to accelerate the development of these technologies. Notwithstanding the intense and growing research in the area, the potentialities of the field are largely unexplored, with a limited range of reactions and approach studies. There are also some limitations in the methodological approaches, which, however, were only marginally discussed here. This personal account aims to provide clues for reflection and indicate some emerging possibilities for the topics and reactions that may transformative change chemical production but are still limited investigated.
We believe that expanding the current approaches is the main barrier to accelerating a larger use of electrocatalytic processes rather than improving specific technical aspects. Accordingly, the motivation for this perspective is to highlight these aspects and outline emerging possibilities together with a blueprint vision rather than review a state-of-the-art one. Accelerating the progress towards commercialisation requires not (only) overcoming technical barriers but also rethinking the current approach to electrochemistry. This is the main message of this perspective, which is summarised in a bullet points message in the cover figure. Those indicated are the key tasks that should be considered to make electrocatalysis work on a commercial scale and play the expected crucial role in clean energy and chemical production.
While overcoming the gaps from the lab- to industrial scale is a necessary step for the commercial introduction of electrocatalytic technologies, we would emphasise that the limiting factor in accelerating the transformation of chemical production is the insufficient vision of the many, often disruptive possibilities of electrocatalysis. There is an urgent need to explore them rather than focus only on mechanistic aspects, which are often not capable of identifying the critical aspects determining the behaviour and possible implementation. A critical element regarding this question is the difference between electro- and thermal-catalysis and electrochemical versus electrocatalytic mechanisms.
Here, we discussed a series of case examples and areas of electrocatalysis deserving more research attention. Still, the aim is not to conduct a comprehensive survey nor discuss state-of-the-art and specific scientific or technological issues. Instead, the idea is to provide the stimulus to understand better the potential of electrocatalysis technologies and how they can transform the current panorama of chemical production and energy towards a resilient, sustainable, and carbon-circularity model.
This deep transformation also implies that new assessment models must be developed. At the same time, current TEA and LCA methodologies do not offer sufficiently solid and reliable bases to properly evaluate the impact of electrocatalytic technologies in a completely changing scenario.
There is certainly no consensus on many of the indications provided in this personal account, but we believe that a discussion is necessary and thus also discording ideas. On the other hand, analysing future scenarios and predicting needs, gaps, and limits is a necessary exercise to accelerate the transformation and overcome the limits commented on by the actual assessments. Without this vision and stimulation of a creative effort in the area, the only scientific advances are insufficient. They are one of the reasons why effective implementation progress in the area is still limited, notwithstanding the very extensive research activities, but focused on a too narrow vision and often on not crucial developments.
Acknowledgments
This work was done in the frame of the ERC Synergy SCOPE (project 810182), EU project DECADE (nr. 862030) and PRIN 2022 project MATISSE nr. 2022 K5SX27 002, which are gratefully acknowledged. Open Access publishing facilitated by Università degli Studi di Messina, as part of the Wiley - CRUI-CARE agreement.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Biographical Information
Gabriele Centi is a Full Professor of Industrial Chemistry at the University of Messina (Italy) and President of the European Research Institute of Catalysis (ERIC aisbl). He was the Coordinator of the Network of Excellence on Catalysis IDECAT and of several EU projects. He was the Chair of the Editorial Board of ChemSusChem and is Co-Editor-in-Chief of the Journal of Energy Chemistry. He was the Chairperson of various international meetings on catalysis on catalysis and has authored over 650 scientific publications. His current h-index (Google Scholar) is 101, with about 41500 citations.
Biographical Information
Siglinda Perathoner obtained her PhD in Chemical Science in 1988, working on the photophysics and photochemistry of supramolecular systems with V. Balzani and Nobel Laureate J. M. Lehn. In 2001, she joined the University of Messina and is now a Full Professor of Industrial Chemistry. She has coordinated many EU projects. She was the Co-Chair of Europacat 2017 in Florence, Italy. Her research interests include nanostructured oxides and nanocarbons for catalytic applications, particularly in the photo- and electrocatalytic sectors, using solar energy to convert CO2, H2O and N2. Her current h-index is 81, with about 30000 citations (Google Scholar).






