Life in the Fast Lane: Feeding and Growth of Juvenile Steelhead and Chinook Salmon in Main-Stem Habitats of the Columbia River Estuary
Abstract
Very little is known about the ecology of juvenile Pacific salmon Oncorhynchus spp. that rapidly traverse estuaries. For these species and life history types, main-stem habitats are thought to largely function as migratory corridors rather than as productive habitats that support feeding or growth, although little research has focused on this issue. This is especially true in the highly modified Columbia River estuary, where it is unclear whether fast-migrating salmon benefit from extensive tidal marsh restoration. To address this deficit, we sampled migratory juvenile steelhead O. mykiss and Chinook Salmon O. tshawytscha at locations spread across nearly 200 km of the Columbia River estuary. Our results demonstrated that these juvenile salmon were actively feeding and growing as they moved downstream; dominant prey included chironomids, other insects, and corophiid and gammarid amphipods. We also observed variation in diet composition and quantity between years, which was likely associated with the highly contrasting river conditions. Insulin-like growth factor 1 hormone levels and the size of juvenile salmon increased as the fish moved downstream, suggesting that prey quality and quantity were sufficient to fuel rapid growth, which may increase survival in marine waters. Our results have direct management implications for habitat restoration and suggest the potential for competition between hatchery and wild salmon. Overall, our results support a fundamental shift in the view of main-channel estuarine habitats from serving primarily as a migration corridor to serving as productive habitat where rapidly moving salmon actively feed and grow.
Pacific salmon Oncorhynchus spp. and steelhead O. mykiss (hereafter, collectively referred to as “salmon”) are renowned for their life history diversity across all stages of the life cycle (Groot and Margolis 1991; Quinn 2005; Beamish 2018). One expression of this variability is the duration and habitat use during estuarine residency as juvenile salmon transition from freshwater to marine waters. From the Yukon–Kuskokwim Delta, Alaska, to the Central Valley, California, use of estuaries by juvenile salmon is spatially and temporally variable, with abundances, diets, residence times, and growth rates varying by habitat location and type, as well as the species and size of salmon (Chapman et al. 2013; Teel et al. 2014; Carr-Harris et al. 2015; Goertler et al. 2016; Roegner et al. 2016; Miller et al. 2020; Chalifour et al. 2021). In general, Chum Salmon O. keta and subyearling Chinook Salmon O. tshawytscha (predominantly fall run; note that salmon run timing refers to the season [e.g., spring, summer, or fall] in which adult salmon return to freshwater to eventually spawn) are the most estuarine-dependent salmon, spending weeks to months feeding and growing in shallow-water estuarine habitats (e.g., tidal wetlands, edges of the main channel) before entering marine waters (Simenstad et al. 1982; Levings 2016; McNatt et al. 2016; Sather et al. 2016; Chalifour et al. 2021). By contrast, Sockeye Salmon O. nerka, yearling Chinook Salmon, Coho Salmon O. kisutch, and steelhead are thought to largely use estuaries as migration corridors. These fish are generally restricted to deep waters of the main channel, where they spend from a few hours to a few days in transit (Groot and Margolis 1991; Quinn 2005; Levings 2016).
In the Columbia River basin, yearling smolts from interior stocks of steelhead and Chinook Salmon (those originating above Bonneville Dam, located at river kilometer [rkm] 234; rkm 0 = Columbia River mouth) rapidly move through the Columbia River estuary (CRE; defined as tidal waters below Bonneville Dam) in deep waters of the main channel and are rarely encountered close to shore (Teel et al. 2014; Roegner et al. 2016; Bottom et al. 2021). Natural-origin fish from these stocks are of particular management concern, as they are listed under the U.S. Endangered Species Act (ESA) and poor survival through the estuary may limit their recovery (Kareiva et al. 2000). These salmon have transit rates exceeding 50 km/d (McMichael et al. 2010; Harnish et al. 2012; Dietrich et al. 2016), comparable to migration rates through other large estuaries (Melnychuk et al. 2010; Sandstrom et al. 2013; Stevenson et al. 2019). Migration rates vary with fish size and river discharge (Morrice et al. 2020). For example, median migration rates through the upper 160 km of the CRE exceeded 100 km/d for both steelhead (116 km/d) and yearling Chinook Salmon (103 km/d) in 2017, when river flow was exceptionally high (>14,000 m3/s; USGS 2016), whereas the median rates were somewhat slower (92 and 82 km/d for steelhead and yearling Chinook Salmon, respectively) in 2016 under more typical flow conditions (∼6,000 m3/s; Morris et al. 2017, 2018). Given the rapid migration rates through the CRE, it only requires 3–5 d during normal flow conditions for juvenile salmon to transit the 234 km from Bonneville Dam to the river mouth.
Far less is known about the value of estuaries for supporting the feeding and growth of these fast-moving salmon. In the CRE, thousands of acres of former tidal marsh habitat have been restored specifically to benefit juvenile salmon (Thom et al. 2011; Littles et al., in press). An underlying premise of this restoration is that prey such as chironomid insects (nonbiting midges) produced in restored tidal wetlands would be exported to the main channel, where they would be available to migrating salmon. Studies that were conducted in the CRE 40 years ago suggested limited feeding opportunities for juvenile salmon in the main channel (Dawley et al. 1986), but the extent to which this is true today is unknown (Johnson et al. 2018).
Our study had three objectives: (1) to characterize juvenile salmon with respect to their size, origins (geographic; hatchery versus wild), diets, and growth in the main channel during their downstream migration through the 234-km-long CRE; (2) to document the diets of these salmon as they moved through the estuary; and (3) to determine whether juvenile salmon were growing as they rapidly transited the estuary. We focused on interior stocks of steelhead and yearling Chinook Salmon because of their regional importance, their status as protected species under the ESA, and the limited knowledge of their ecology as they transit the CRE. We accomplished these objectives by collecting juvenile salmon in the main channel at four sites that spanned 195 km of the CRE during the spring out-migration in two highly contrasting years. We expected that salmon would feed as they moved downstream, and we expected higher gut fullness and more wetland-produced prey (e.g., chironomids) in diets of fish sampled lower in the CRE, where both extant and restored wetlands are more abundant (Marcoe and Pilson 2017; Littles et al., in press). We were uncertain whether we could document growth given the extremely short time (<1 week) it takes salmon to traverse the CRE. Our investigation is one of only a few studies to focus on the ecology of juvenile salmon migrating in the main channel of any large estuary, and we demonstrate that these fast-migrating salmon were feeding and growing as they transited the CRE.
METHODS
Study Area
The Columbia River is the largest river on the Pacific coast of North America and drains an area of 670,000 km2. We define the CRE as the portion of the basin influenced by tides, which stretches from the base of Bonneville Dam (rkm 234) to the river mouth (rkm 0). Like most West Coast estuaries, the CRE has been highly modified by anthropogenic activities over nearly 150 years and has lost 70% of its historic tidal wetlands, primarily to diking (Marcoe and Pilson 2017). A deep shipping channel (hereafter, referred to as the “main channel”) runs the length of the estuary, which is maintained at a minimum depth of 13 m (43 ft). Spring freshets in the basin are truncated by a series of hydroelectric dams on the main stem and its tributaries (Jay et al. 2016). Because of these dams, the water column is vertically well mixed upstream of rkm 40, the upper extent of salinity intrusion (Jay et al. 2016).
Collection of Fish
Our objective was to characterize juvenile steelhead and yearling Chinook Salmon, including their feeding and growth, as they transited the CRE. To accomplish this, juvenile salmon were collected at four sites that spanned the CRE and represented four ecohydrologic zones (Jay et al. 2016): Rooster Rock (RR [rkm 210], Upper Tidal River Zone), Willow Grove (WG [rkm 92], Lower Tidal River Zone), Steamboat (SB [rkm 61], Upper Estuary Zone), and estuary purse seine (EPS [rkm 15], Lower Estuary Zone; Figure 1).
Juvenile salmon were collected during 2 years (2016, 2017) at all sites in April, May, and June, representing the beginning, middle, and end of the approximately 3-month-long spring out-migration (Dawley et al. 1986; Weitkamp et al. 2012; Morris et al. 2017, 2018). For each month, we sampled the two upper stations (RR and WG) first and then sampled the lower stations (SB and EPS) in an attempt to capture the same groups of fish moving through the estuary. It took 1–3 d to complete sampling at each site; sampling dates (excluding weekends) were April 11–22, May 9–20, and June 9–20 in 2016 and April 18–28, May 16–25, and June 13–21 in 2017. Due to strong tidal currents and afternoon winds, sampling at the EPS site was restricted to days with early morning low tides (Weitkamp et al. 2012) and therefore determined the schedule of all sampling.
Juvenile salmon were collected using either a purse seine or a tow net. Juvenile salmon at the EPS site were collected with a fine-mesh purse seine, which was described in detail by Weitkamp et al. (2012) and is summarized here. The purse seine (10.6 m [35 ft] deep × 155 m [510 ft] long; stretched body mesh = 1.7 cm; stretched knotless bunt mesh = 1.5 cm) was repeatedly set and retrieved (and the catch processed) starting at low tide and continuing throughout the incoming tide. The net was fished at two locations within the EPS site (both in 10–15-m water depth): North Channel (46°14.2′N, 123°54.2′W) on the north side of the river and Trestle Bay (46°12.9′N, 123°57.7′W) on the south side. The density of salmon from quantitative round hauls (area = 1,912 m2) were averaged between the two stations to estimate CPUE, expressed as catch per round haul. Catch from nonquantitative sets (when the net was towed upstream before pursing to increase catch) were not included in CPUE estimates.
We used a two-boat tow net at all other stations to catch fish because using the purse seine was not practical. The tow net was deployed in 10-min tows in an upstream direction at the three upper sites (RR: 45°33.4′N, 122°13.7′W; WG: 46°10.2′N, 123°5.6′W; SB: 46°13.3′N, 123°25.9′W). The net was fished using two boats spaced 100 m apart, each pulling a 70-m-long tow rope attached to either side of the net; a flowmeter was deployed overboard to record the distance traveled during each tow. Two tow nets (small and large) were used due to concerns about the effectiveness of the smaller net for catching larger juvenile salmon and to increase the number of fish caught. Both nets had the same mesh size (3.18 cm [1.25 in] in the forward section, decreasing to 1.3 cm [0.5 in] in the cod end). The smaller net (mouth opening = 6.1 m [20 ft] wide, 2.9 m [9.5 ft] deep; 12.8 m [42 ft] long) was used during all of 2016 and in April 2017, while the large net (mouth opening = 7.6 m [25 ft] wide, 3.7 m [12 ft] deep; 16 m [52.5 ft] long) was used in May and June 2017. Volumes swept by the small and large nets during a 10-min tow were estimated as 19,940 and 25,600 m3, respectively. As will be discussed later, we believe that potential differential selectivity of these nets had a minor influence on our results and conclusions. We did not compare fish densities between years because we learned where high fish densities were located within sites in 2016, which likely increased catches in 2017.
Regardless of the net used, all captured juvenile salmon were field processed in the same manner. Juvenile Chinook Salmon and steelhead to be retained were identified to species, given a lethal dose of tricaine methanesulfonate (MS-222), checked for adipose fin clips (indicating hatchery origin) and coded wire tags (CWTs), measured for FL (nearest 1 mm), and immediately bled; the whole fish was then labeled and bagged. The blood was immediately placed on ice and was centrifuged within 4 h, and the plasma was extracted and frozen. Once on shore, retained fish were stored in a −80°C freezer. All remaining fish were lightly anesthetized with MS-222, identified to species, checked for tags and clips, enumerated, measured (up to 30 individuals of each species), allowed to fully recover, and released back into the river.
Our goal was to retain 50 juveniles of each salmon species at each station during each month and year. These fish were bled in the field and used for laboratory analyses, including diets, genetic stock identification (GSI), and retrieval of CWTs (if present). This represented a compromise between having enough fish for robust statistical analysis and permit limitations for species that are protected under the ESA.
Environmental Conditions
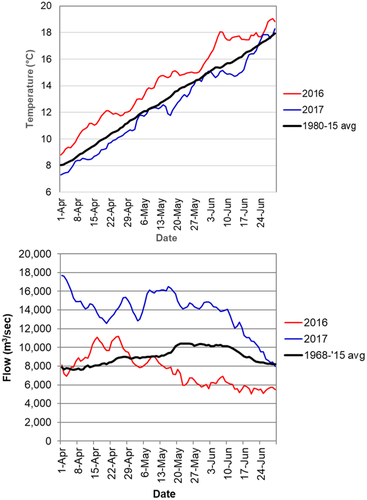
To help assess potential differences in fish characteristics between years due to environmental conditions, we report river flow and temperatures. We used flow data from the U.S. Geological Survey gauging station at rkm 76 (station 14,246,900, Port Westward; USGS 2016). This station captures flow from the Columbia River basin above Bonneville Dam but also includes large lower-river tributaries (e.g., Willamette, Cowlitz, and Lewis rivers) that provide substantial runoff during rapid melting of low-elevation snow in the spring. For water temperatures, we used measurements at Bonneville Dam (USACE 2016). Water temperatures at this station were strongly correlated with measurements from the CRE at fixed stations (CCMOP 2016) and our own field measurements (r > 0.98, P < 0.05; L. A. Weitkamp, unpublished data), but they provided historical context that could not be attained with our field measurements.
This study occurred during two highly contrasting years: 2016 was characterized by elevated main-stem temperatures and below-average flows, whereas 2017 was characterized by elevated flows and below-average temperatures (Figure 2). During the period from April 1 to June 30, spring daily temperatures averaged 1.82°C warmer and flows averaged 6,264 m3/s lower in 2016 than in 2017. Neither year was exceptional compared to conditions observed over the past 20 years (USGS 2016). Compared to discharge in the undammed historic Columbia River, however, peak modern spring freshets average 5,200 m3/s lower than historical spring freshets, which often exceeded 25,000 m3/s (Sherwood et al. 1990).
Laboratory Analysis
In the laboratory, frozen fish were thawed, their species identification was reconfirmed, and they were measured (FL, nearest 1.0 mm; weight, nearest 0.1 g). All fish were rechecked for adipose fin clips, CWTs, or PIT tags; their snouts were removed (CWTs), and tags were read visually or electronically (PIT) as appropriate. Whole stomachs and fin tissue were collected from each fish for analyses of diets and genetics, respectively, and were stored as appropriate (frozen or placed in preservative). To explore variation in fish shape, we calculated Fulton's condition factor (CF) for each fish as CF = 100,000 × W/(FL3), where weight (W) is expressed in grams and FL is expressed in millimeters (Murphy and Willis 1996).
Fish origins
We wanted to know the geographic origins, life history type (run timing, smolt age), and production type (known hatchery or presumed wild) of the salmon we caught. Geographic origins and life history type were determined from GSI, parental-based tagging (PBT; for hatchery fish), and internal tags (CWTs and PIT tags). As implemented in the Columbia River basin, PBT consists of genotyping as many adults as possible from the hatchery broodstock and then using parentage assignments to identify parental pairs for each offspring (Steele et al. 2013).
Hatcheries in the Columbia River basin released 14.8 million steelhead and 36.0 million yearling Chinook Salmon annually in 2016 and 2017; of these, 13.5% of steelhead and 35% of yearling Chinook Salmon were tagged with CWTs and 89% of steelhead and 91% of yearling Chinook Salmon had their adipose fins removed (PSMFC 2016b). During these years, 1.7 million hatchery and wild salmon (primarily steelhead and yearling Chinook Salmon) were PIT-tagged annually (PSMFC 2016b). Both CWTs and PIT tags provide information about genetic and geographic origins, production type, and Chinook Salmon smolt age. This information was queried from the appropriate online database: the Regional Mark Information System for CWTs (PSMFC 2016b) or the PIT Tag Information System for PIT tags (PSMFC 2016a). This release information was assigned to a geographic stock by using the criteria provided by Fisher et al. (2014), and age for untagged Chinook Salmon was determined from length-based age criteria (Weitkamp et al. 2015). We distinguished “known” hatchery fish from “presumed” wild fish by using PBT and internal tags and the absence of adipose fins; in the Columbia River basin, some unknown portion of “wild” fish actually consisted of unclipped, untagged hatchery fish.
Genetic methods (GSI and PBT) used in this study were described in detail by Hess et al. (2013) and Van Doornik et al. (2019) and are summarized here. Steelhead and Chinook Salmon were genotyped for 180 and 185 single-nucleotide polymorphism loci, respectively, through PCR. The resulting PCR products were visualized using the Fluidigm EP1 genotyping platform. Estimates of the genetic stock of origin for each fish were generated using GSI and the algorithms employed by the software ONCOR (Kalinowski 2007). Steelhead were assigned to one of five genetic stock groups within the Columbia River; Chinook Salmon were assigned to one of 10 genetic stock groups. Assignments of the hatchery of origin for individual fish employed PBT by using SNPPIT version 1.0 (Anderson 2010). For some Chinook Salmon that assigned to the Snake River spring group by GSI (based on single-nucleotide polymorphisms), PBT or internal tags indicated that they originated from the mid- or upper Columbia River basin. This conflict was due to the genetic legacy of historic transfers of fish between Snake River and upper Columbia River basin hatcheries. To correct for this, Chinook Salmon that assigned to the Snake River spring group by GSI but did not have PBT or internal tags to confirm their origins were assigned to the “interior spring” group.
Based on these combined methods, we grouped steelhead and yearling Chinook Salmon into three groups each. The three steelhead stocks were (1) lower Columbia River (LCR; which includes the Willamette River), (2) mid- and upper Columbia River/lower Snake River (M&UCR/LSnk), and (3) upper Snake River (Salmon and Clearwater rivers). For yearling Chinook Salmon, the stocks consisted of (1) LCR spring (which includes Willamette River spring), (2) interior spring (mid- and upper Columbia River spring [M&UCR Sp] and Snake River spring/summer), and (3) interior fall (upper Columbia River summer/fall [UCR Su/Fall] and Snake River fall).
Diets
We weighed the stomach contents of most fish directly. However, some fish stomach contents were estimated from the direct measurement of the whole stomach (contents and lining) minus the estimated weight of the lining, which was calculated from species-specific ratios of stomach lining to whole-fish weight. Stomach fullness estimates from both methods (n = 715) were similar (r = 0.84, P < 0.05). Stomachs were considered empty if no material (regardless of the level of digestion) was observed in stomachs.
Given water temperatures in both years (Figure 2), stomach contents should reflect feeding over the previous 24–36 h (Benkwitt et al. 2009). Although we only sampled salmon during daylight hours (between 0700 and 1500 hours), studies with diel sampling have shown that most feeding by juvenile salmon in estuarine and coastal waters occurs during the day (Benkwitt et al. 2009; David et al. 2014). We found no relationship between collection time and gut fullness for either species (R2 < 0.02).
Diet composition was analyzed using standard methods—namely, visual identification and enumeration of identifiable prey based on appropriate taxonomic guides. Only a subset of fish was analyzed for diet composition because fish that are caught together typically have high diet overlap (Weitkamp and Sturdevant 2008; Weitkamp, unpublished data). We largely restricted our analysis to the focal groups (interior stocks of steelhead and yearling Chinook Salmon), and we randomly selected fish to be processed from each cell representing species/stock, month, site, and year; in total, 234 steelhead and 311 yearling Chinook Salmon were analyzed for diet composition. Over 200 prey taxa (including life stage) were identified in the stomachs, with many identified to the genus or family level. We also observed “nonfood” material in the stomachs of some fish, consisting of small pieces of bark, leaves, or plastic, but due to its small contribution to diets (<2% of prey consumed), we did not include this category in the analysis.
We employed the plasma concentration of insulin-like growth factor 1 (IGF-1) as a fast-responding physiological measure of growth. Insulin-like growth factor 1 is produced by the liver and stimulates somatic growth; it reflects growth during the previous 2 weeks (Beckman et al. 1998, 2004; Duguid et al. 2018). The IGF-1 concentrations of blood plasma for individual fish were measured using the time-resolved fluorescence immunoassay developed by Small and Peterson (2005) as modified by Ferriss et al. (2014). Chinook Salmon were also assayed for plasma 11-ketotestosterone (Cuisset et al. 1994) as an indicator of precocious maturation. The IGF-1 concentrations from fish identified as maturing were excluded from the data set because the relationship between growth and IGF-1 in precociously mature males is weak (Larsen et al. 2010).
Statistical Analysis
Size, stomach fullness, and growth
We used generalized linear models (GLMs) to evaluate whether metrics of steelhead and yearling Chinook Salmon changed as fish moved downstream from RR (rkm 210) to the EPS site (rkm 15). We fitted separate GLMs to five response variables: length, weight, CF, IGF-1, and stomach fullness. The explanatory variables in each model included year, month, station order (i.e., rkm), stock, and production type (hatchery versus wild). For both steelhead and Chinook Salmon, we fitted GLMs to data encompassing the three geographically defined stocks described above. If station order was a significant explanatory variable (i.e., P < 0.05), we then fitted separate models to the three individual stocks to determine whether downstream changes in response variables were stock dependent.
Prior to the analysis, we quantified inherent correlations among all potential explanatory variables. Temperature and river flow were highly correlated with year and month and with each other; therefore, we excluded temperature and flow from the analysis. Steelhead and Chinook Salmon length and weight measurements were natural log transformed, and estimates of the proportion of stomach fullness were arcsine–square root transformed. A Gaussian distribution with an identity link function was used in all GLMs except for the IGF-1 models, in which a log link function was used. The correlation analysis was performed using the R package corrplot (Wei and Simko 2021), and all models were run in R using the glmmTMB package (Brooks et al. 2017; R Development Core Team 2019). Model fits were evaluated based on model residuals and quantile–quantile plots, and outliers were assessed using the R package car (Fox and Weisberg 2019). No evidence of spatial or temporal autocorrelation was detected during this process.
Diet composition
Stomach composition data were analyzed using three multivariate techniques to explore patterns among stocks or species of fish, collection location, month, and year: (1) nonmetric multidimensional scaling (MDS), (2) analysis of similarities (ANOSIM; a multivariate analog for ANOVA), and (3) permutational multivariate ANOVA (PERMANOVA; a multi-way multivariate ANOVA). These multivariate analyses used PRIMER-E software (Clarke and Gorley 2006) and were based on pairwise Bray–Curtis similarity coefficients calculated between individual fish. In this application, similarity coefficients ranged from 0 (no taxonomic groups in common) to 1 (identical taxonomic groups).
Similarity coefficients were based on the numeric abundance of prey in 12 taxonomic groups, including seven groups of insects (chironomid Diptera; Coleoptera [beetles]; other [nonchironomid] Diptera [true flies]; Ephemeroptera [mayflies]; Hemiptera [true bugs]; Hymenoptera [wasps, bees, and ants]; and other insects); two groups of crustaceans (corophiid amphipods and other amphipods) and one group each for arachnids (spiders and mites), fish, and other invertebrates. Count data were standardized and fourth-root transformed prior to analysis. Analyses using more taxonomic groups and different transformations resulted in similar overall patterns.
The matrix of pairwise Bray–Curtis similarity coefficients was used to construct MDS plots to graphically explore diet data. The MDS ordination technique places all points in MDS space in relation to their similarity. Random starting locations were used for each of 25 iterations to find the best solution; minimum stress was attained in multiple iterations, which suggests a true minimum solution. Resulting stress values were less than 0.20, indicating that a spatial representation of the data by the MDS plots was consistent with the structure of the original data set (Clarke and Gorley 2006).
We quantitatively evaluated patterns observed in the MDS plots using ANOSIM, specifically examining how location (site), species (steelhead or yearling Chinook Salmon), time (month or year), and production type (hatchery versus wild) influenced diets. This analysis produces global R-values that indicate the degree of separation between groups generated by a particular factor (or pair of factors). These global R-values range from 0 (no separation) to 1 (complete separation); the program also generates statistical probabilities by permutation. We used similarity percentages (SIMPER) analysis to determine the prey types that were driving similarities within groups and differences among groups. Finally, we used PERMANOVA to evaluate the influence of the same variables on diet composition because it is structurally very different from ANOSIM and can assign overall variation attributable to different factors of interest.
RESULTS
Effort and Catch
We completed a total of 232 townet sets and 35 purse-seine sets in 2016 and 195 townet sets and 35 purse-seine sets in 2017 during April, May, and June of each year (Table 1). We caught a total of 1,590 juvenile salmon (subyearling and yearling Chinook, Coho, Sockeye, and Chum salmon) and steelhead in 2016 and 1,740 juvenile salmon and steelhead in 2017. These catches included 224 steelhead and 338 yearling Chinook Salmon in 2016 and 507 steelhead and 435 yearling Chinook Salmon in 2017 (Table 1).
| Station | Category | 2016 | 2017 | Grand total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apr | May | Jun | Apr | May | Jun | |||||||||||
| CPUE | n | CPUE | n | CPUE | n | Total n | CPUE | n | CPUE | n | CPUE | n | Total n | |||
| Rooster Rock (rkm 210) | Yr Chinook | 0.91 | 21 | 2.62 | 89 | 0.16 | 7 | 117 | 1.19 | 32 | 10.86 | 152 | 0.00 | 0 | 184 | 301 |
| Steelhead | 0.22 | 5 | 0.56 | 19 | 0.00 | 0 | 24 | 3.52 | 95 | 3.64 | 51 | 0.22 | 2 | 148 | 172 | |
| Effort | 23 | 34 | 43 | 100 | 27 | 14 | 9 | 50 | 150 | |||||||
| Willow Grove (rkm 92) | Yr Chinook | 0.11 | 1 | 4.00 | 60 | 0.14 | 3 | 64 | 0.22 | 7 | 1.61 | 53 | 0.09 | 1 | 61 | 125 |
| Steelhead | 0.11 | 1 | 1.27 | 19 | 0.24 | 5 | 25 | 2.03 | 65 | 2.45 | 81 | 0.18 | 2 | 148 | 173 | |
| Effort | 9 | 15 | 21 | 45 | 32 | 33 | 11 | 76 | 121 | |||||||
| Steamboat (rkm 61) | Yr Chinook | 0.78 | 21 | 1.28 | 50 | 0.10 | 2 | 73 | 0.81 | 21 | 2.21 | 73 | 0.20 | 2 | 96 | 169 |
| Steelhead | 1.33 | 36 | 0.85 | 33 | 0.00 | 0 | 69 | 3.38 | 88 | 1.42 | 47 | 0.20 | 2 | 137 | 206 | |
| Effort | 27 | 39 | 21 | 87 | 26 | 33 | 10 | 69 | 156 | |||||||
| Estuary purse seine (rkm 15) | Yr Chinook | 1.57 | 34 | 6.42 | 50 | 0.00 | 0 | 84 | 0.33 | 27 | 3.83 | 68 | 0.00 | 0 | 95 | 179 |
| Steelhead | 0.57 | 30 | 8.85 | 74 | 0.13 | 2 | 106 | 0.33 | 34 | 1.00 | 40 | 0.00 | 0 | 74 | 180 | |
| Efforta | 13 (7) | 9 (7) | 13 (8) | 35 | 13 (6) | 13 (6) | 9 (9) | 35 | 70 | |||||||
| Total | Yr Chinook | 77 | 249 | 12 | 338 | 87 | 345 | 3 | 435 | 773 | ||||||
| Steelhead | 72 | 145 | 7 | 224 | 282 | 219 | 6 | 507 | 731 | |||||||
- a Purse-seine effort provides total effort and quantitative hauls in parentheses; CPUE was only estimated from quantitative hauls.
Catches of juvenile salmon at all sites showed a similar seasonal pattern. Catches of steelhead and yearling Chinook Salmon were generally low in April, peaked in May, and declined by June; very few yearling steelhead (n = 13) or Chinook Salmon (n = 15) were caught in June. Overall, more steelhead were caught at townet sites in 2017 than in 2016, while abundances of yearling Chinook Salmon were comparable between years, with the exception of high catches at RR in May 2017 (Table 1).
Origins of Juvenile Salmon
Most of the steelhead and yearling Chinook Salmon caught at all stations belonged to interior groups, which originate above Bonneville Dam in the mid- and upper Columbia River and the Snake River (Table A.1). Interior groups of yearling Chinook Salmon included Snake River, M&UCR, and interior spring (a mixture of Snake River and M&UCR spring) individuals, with lesser numbers of UCR Su/Fall and Snake River fall Chinook Salmon. Willamette River spring Chinook Salmon made up <5% of all yearling Chinook Salmon caught, and their abundance was highest at the EPS site. Most steelhead (>77%) belonged to the M&UCR/LSnk and upper Snake River groups. The catch of lower Columbia and Willamette River (i.e., LCR) steelhead was lowest at the uppermost site (RR).
Based on the presence of “marks” indicating production type, we estimated that the vast majority of steelhead (78.3%) and yearling Chinook Salmon (96.0%) were of hatchery origin across the 2 years. Accounting for unclipped hatchery fish, we estimated that 80.6% of the steelhead and 96.3% of the yearling Chinook Salmon caught were of hatchery origin. The composition of stocks and the proportion of hatchery fish were quite consistent between years.
Diets
Our assessment of stomach fullness indicated that salmon throughout the CRE were actively feeding, with variation among species, stocks, years, and sites (Tables 2–5; Figures 3, 4). Overall, stomach fullness was lower in steelhead (0.64% BW; range = 0.00–7.16% BW; n = 564) than in yearling Chinook Salmon (1.05% BW; range = 0.00–5.20% BW; n = 556). The percentage of empty stomachs was extremely low overall, averaging just 1.7% for steelhead and 1.3% for yearling Chinook Salmon.
| Variable | Intercept | Year | Month (May) | Station | LCR | M&UCR/LSnk | Production type | Pseudo-R2 | N |
|---|---|---|---|---|---|---|---|---|---|
| Length | |||||||||
| Coefficient | 99.896 | −0.047 | −0.051 | 0.014 | −0.088 | −0.014 | −0.131 | 0.35 | 566 |
| SE | 20.552 | 0.010 | 0.010 | 0.004 | 0.012 | 0.011 | 0.011 | ||
| t-value | 4.861*** | −4.605*** | −5.120*** | 3.181** | −7.128 | −1.255 | −11.400*** | ||
| Weight | |||||||||
| Coefficient | 265.40441 | −0.129 | −0.21416 | 0.03192 | −0.2318 | 0.00563 | −0.38521 | 0.33 | 562 |
| SE | 64.66394 | 0.03205 | 0.03055 | 0.01387 | 0.03885 | 0.03458 | 0.03484 | ||
| t-value | 4.104*** | −4.040*** | −7.010*** | 2.302* | −5.97*** | 0.163 | −11.055*** | ||
| Condition factor | |||||||||
| Coefficient | 28.699 | 0.016 | −0.053 | −0.006 | 0.025 | 0.039 | −0.001 | 0.26 | 561 |
| SE | 10.594 | 0.005 | 0.005 | 0.002 | 0.006 | 0.006 | 0.006 | ||
| t-value | −2.709*** | 2.793** | −10.578** | −2.527* | 4.002*** | 6.842*** | −0.177 | ||
| IGF-1 | |||||||||
| Coefficient | −18.092 | 0.011 | −0.011 | 0.027 | 0.120 | 0.041 | 0.058 | 0.07 | 540 |
| SE | 46.064 | 0.023 | 0.021 | 0.010 | 0.026 | 0.025 | 0.025 | ||
| t-value | −0.393 | 0.475 | −0.494 | 2.770** | 4.540*** | 1.636 | −2.316* | ||
| Stomach fullness | |||||||||
| Coefficient | 15.817 | −0.008 | 0.007 | −0.005 | −0.007 | 0.001 | 0.025 | 0.18 | 564 |
| SE | 5.686 | 0.003 | 0.003 | 0.001 | 0.003 | 0.003 | 0.003 | ||
| t-value | 2.78** | −2.77** | 2.56* | −4.15*** | −1.93 | 0.204 | 8.29*** | ||
| Variable | Stock group | Slope | SE | t-value | Pseudo-R2 | N |
|---|---|---|---|---|---|---|
| Length | USnake | 0.013 | 0.006 | 2.002* | 0.27 | 239 |
| M&UCR/LSnk | 0.013 | 0.007 | 1.723 | 0.35 | 198 | |
| LCR | 0.010 | 0.010 | 0.942 | 0.35 | 129 | |
| Weight | USnake | 0.031 | 0.020 | 1.551 | 0.25 | 239 |
| M&UCR/LSnk | 0.028 | 0.024 | 1.170 | 0.35 | 195 | |
| LCR | 0.018 | 0.033 | 0.534 | 0.36 | 128 | |
| Condition factor | USnake | −0.006 | 0.003 | −1.907 | 0.35 | 239 |
| M&UCR/LSnk | −0.003 | 0.004 | −0.821 | 0.19 | 193 | |
| LCR | −0.006 | 0.006 | −0.993 | 0.10 | 129 | |
| IGF-1 | USnake | 0.018 | 0.015 | 1.174 | 0.05 | 230 |
| M&UCR/LSnk | 0.045 | 0.016 | 2.754** | 0.07 | 190 | |
| LCR | 0.010 | 0.023 | 0.411 | 0.03 | 120 | |
| Stomach fullness | USnake | −0.003 | 0.002 | −1.694 | 0.18 | 240 |
| M&UCR/LSnk | −0.007 | 0.002 | −3.508*** | 0.15 | 196 | |
| LCR | −0.003 | 0.003 | −1.030 | 0.18 | 128 |
| Variable | Intercept | Year | Month (May) | Month (Jun) | Station | Int Fall | LCR Sp | Production type | Pseudo-R2 | N |
|---|---|---|---|---|---|---|---|---|---|---|
| Length | ||||||||||
| Coefficient | −19.00 | 0.01 | −0.05 | −0.08 | 0.01 | 0.04 | 0.04 | −0.14 | 0.24 | 570 |
| SE | 13.54 | 0.01 | 0.01 | 0.02 | 0.00 | 0.01 | 0.02 | 0.02 | ||
| t-value | −1.40 | 1.77 | −6.27*** | −3.48*** | 2.19* | 4.82*** | 2.00* | −7.43*** | ||
| Weight | ||||||||||
| Coefficient | −144.10 | 0.07 | −0.21 | −0.27 | 0.02 | 0.15 | 0.08 | −0.40 | 0.26 | 564 |
| SE | 44.39 | 0.02 | 0.03 | 0.08 | 0.01 | 0.03 | 0.06 | 0.06 | ||
| t-value | −3.25** | 3.32*** | −8.03*** | 0.00*** | 1.75 | 5.60*** | 1.27 | −6.33*** | ||
| Condition factor | ||||||||||
| Coefficient | −90.08 | 0.05 | −0.05 | 0.03 | 0.00 | 0.02 | 0.02 | −0.02 | 0.21 | 564 |
| SE | 11.76 | 0.01 | 0.01 | 0.02 | 0.00 | 0.01 | 0.02 | 0.02 | ||
| t-value | −7.66*** | 7.74*** | −7.36*** | 1.38 | −1.45 | −2.82** | 1.37 | −1.13 | ||
| IGF-1 | ||||||||||
| Coefficient | −231.50 | 0.12 | 0.06 | – | 0.02 | −0.11 | 0.09 | 0.04 | 0.13 | 518 |
| SE | 40.22 | 0.02 | 0.02 | – | 0.01 | 0.03 | 0.05 | 0.06 | ||
| t-value | −5.76*** | 5.85*** | 2.38* | – | 2.35* | −4.08*** | 0.06 | 0.65 | ||
| Stomach fullness | ||||||||||
| Coefficient | −38.39 | 0.02 | 0.00 | −0.02 | −0.01 | 0.00 | 0.00 | 0.01 | 0.21 | 556 |
| SE | 4.07 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | ||
| t-value | −9.42*** | 9.45*** | 0.74 | −2.64** | −7.06*** | 1.12 | 0.13 | 1.23 | ||
| Variable | Stock group | Slope | SE | t-value | Pseudo-R2 | N |
|---|---|---|---|---|---|---|
| Length | Int Fall | 0.003 | 0.006 | 0.504 | 0.33 | 120 |
| Int Sp | 0.007 | 0.003 | 2.132* | 0.08 | 424 | |
| LCR Sp | 0.009 | 0.056 | 0.163 | 0.11 | 26 | |
| IGF-1 | Int Fall | 1.138 | 0.927 | 1.228 | 0.19 | 113 |
| Int Sp | 1.178 | 0.493 | 2.389* | 0.08 | 382 | |
| LCR Sp | 0.181 | 5.694 | 0.032 | 0.25 | 23 | |
| Stomach fullness | Int Fall | −0.003 | 0.002 | −1.585 | 0.19 | 121 |
| Int Spring | −0.007 | 0.001 | −7.44*** | 0.25 | 409 | |
| LCR Sp | −0.007 | 0.010 | −0.707 | 0.09 | 26 |
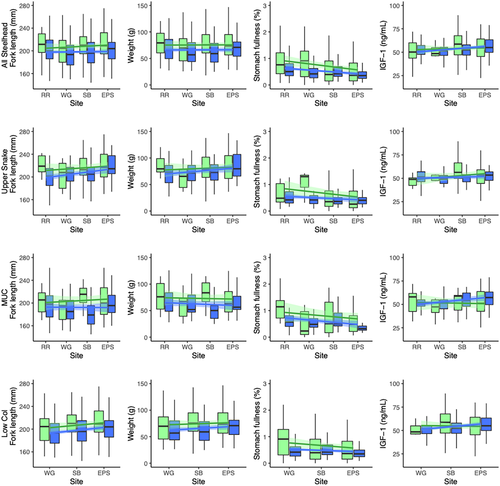
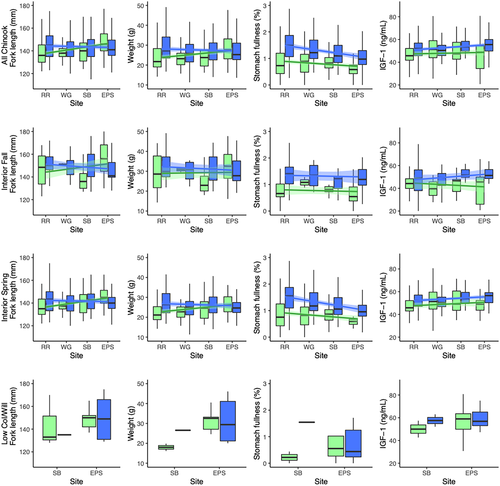
For both steelhead and yearling Chinook Salmon, multiple regression of stomach fullness showed significantly negative relationships between station order and stomach fullness: fullness was highest at RR and declined with distance downstream (Tables 2, 4; Figures 3, 4). Stomach fullness was also higher on average in 2017 (mean = 1.23% BW; n = 292) than in 2016 (0.86% BW; n = 271) for yearling Chinook Salmon. However, the reverse was true for steelhead: stomach fullness was higher in 2016 (mean = 0.79% BW; n = 216) than in 2017 (0.56% BW; n = 374). Stomach fullness was higher for presumed wild steelhead (mean = 1.01% BW; n = 150) than for hatchery steelhead (0.52% BW; n = 440), but no such difference was observed for yearling Chinook Salmon. Fullness did not vary on average among groups of steelhead or Chinook Salmon, although only M&UCR/LSnk steelhead and interior spring Chinook Salmon had statistically negative relationships between station order and stomach fullness (Tables 3, 5).
Analysis of diet composition for 311 yearling Chinook Salmon and 234 steelhead indicated that both species consumed many of the same prey taxa in similar quantities. The two most commonly consumed prey types—chironomid insects and corophiid amphipods—were consumed by 53% and 86% of steelhead, respectively, and by 66% and 83% of Chinook Salmon, respectively. Combined, these two prey taxa contributed 60% of all prey consumed by both species (Figure 5). Additional frequently consumed prey included other amphipods (primarily gammarids) and various insects (other dipterans, Ephemeroptera, Hemiptera, and Hymenoptera). Both juvenile salmon species also consumed fish, which were too digested to identify to species.
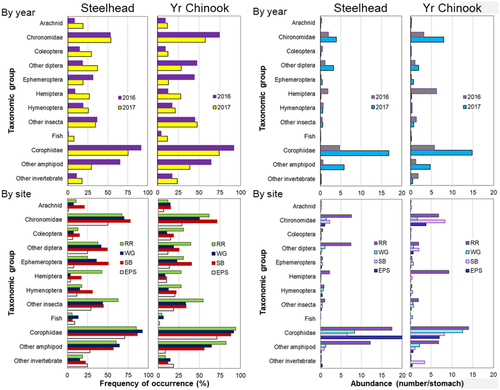
The frequency of occurrence of most prey taxa was quite similar between species, with minor variation between years and among sites (Figure 5). By contrast, the mean abundances of taxa consumed were less consistent, with variation due to both year and site. For both species, diets in 2016 (n = 78 steelhead; n = 151 Chinook Salmon) contained more hemipterans and arachnids, while those in 2017 (n = 156 steelhead; n = 160 Chinook Salmon) contained more chironomids, corophiids, and other amphipods (Figure 5). Steelhead (n = 68) and yearling Chinook Salmon (n = 101) diets at the uppermost site (RR) had higher contributions of corophiids, other amphipods, and insects overall, especially chironomids, hemipterans, and other dipterans.
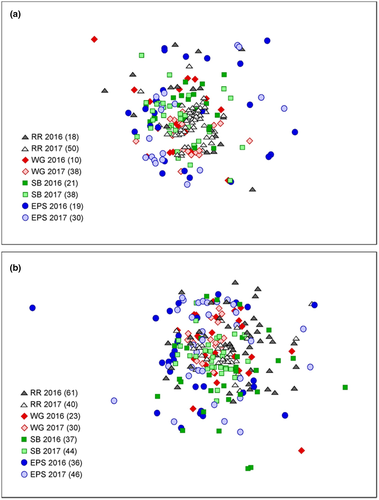
These similarities and differences in diets between species, years, and sites were evident in the statistical analyses of the diet composition data. However, none of the groups was particularly distinct, indicating generally high overlap in diets. For example, within-group similarities by station and year were relatively low (mean = 49.7; range = 0.35–0.61), no single factor formed particularly well-defined groups (global R < 0.40) or explained large amounts of variance (<13%), and there was nearly complete overlap among sites in MDS plots (Figure 6). This was also true of direct comparisons of diets between steelhead and yearling Chinook Salmon: the factor “species” produced poorly defined groups (r = 0.02, P = 0.027) in the ANOSIM and explained the lowest amount of variance (2.5%) of any factor examined in the PERMANOVA when analyzed together (Table 6).
| Analysis and factor | Steelhead | Chinook Salmon | Both species |
|---|---|---|---|
| ANOSIM | |||
| Year | 0.24 | 0.13 | 0.16 |
| Site | 0.13 | 0.07 | 0.09 |
| Production type | 0.00 ns | 0.08 | 0.00 ns |
| Stock | 0.00 ns | 0.05 | – |
| Species | – | – | 0.02 ns |
| Year, site | 0.36, 0.20 | 0.15, 0.13 | 0.21, 0.15 |
| PERMANOVA | |||
| Year | 13.8, 10.8% | 13.6, 6.3% | 22.0, 6.7% |
| Site | 7.2, 8.4% | 5.9, 4.8% | 11.0, 6.1% |
| Production type | 3.6, 2.5% | 3.5, 12.3% | 5.0, 3.0% |
| Stock | 1.5 ns, 0.6% | 4.5, 3.0% | – |
| Species | – | – | 7.9, 2.5% |
Whether steelhead and yearling Chinook Salmon were combined in a single analysis or evaluated separately, the factors “year” and “site” produced the best-defined groups (highest R) in both one-way and two-way ANOSIMs and explained considerable variance in the PERMANOVA (Table 6). The SIMPER analysis showed that these differences were caused by diets with higher amounts of corophiids and other amphipods in 2017 and higher contributions of chironomids in 2016 for both salmon species. Pairwise comparisons in both ANOSIM and PERMANOVA showed that diets at RR and EPS were the most distinct from each other, followed by EPS and SB and then RR and SB (all P < 0.05). These differences were driven by RR and SB diets that were higher in corophiids, other amphipods, and chironomids relative to diets at other sites.
For steelhead diets, neither production type (hatchery versus wild) nor stock group was an important source of variation (Table 6). By contrast, both factors explained variation in yearling Chinook Salmon diets, and production type explained the most variance of any factor considered (12.3%). Compared to hatchery yearling Chinook Salmon, wild fish had fewer corophiid amphipods and more dipterans, invertebrates, and chironomids in their diets. Both analysis types indicated that the UCR Su/Fall stock group had the most distinct diets due to more chironomids and other amphipods, while Snake River spring diets contained more corophiids.
Growth of Salmon
Our results indicated that juvenile salmon were growing (as indicated by increasing size and IGF-1 levels) as they migrated through the CRE, with differences among species and stocks (Tables 2–5; Figures 3, 4). The results of the steelhead multiple regression analysis demonstrated that there were significantly positive relationships between station order and length, weight, and IGF-1 and significantly negative relationships between station order and body condition (Table 2; Figure 3). We also found that on average, steelhead were larger in 2016 (218.7 mm FL; n = 341) than in 2017 (196.0 mm FL; n = 374) and hatchery fish were larger (mean = 214.1 mm FL; n = 565) and had lower IGF-1 levels (mean = 50.6 ng/mL; n = 528) than wild fish (179.5 mm FL, n = 150; 51.4 ng/mL, n = 140). By fitting separate models to the data for each geographic group, we found that the significant relationships between station order and steelhead metrics were mainly attributable to the upper Snake River and M&UCR/LSnk groups and less so for the LCR group (Table 3).
Like the steelhead results, the Chinook Salmon analysis showed significant positive relationships between station order and length, weight, and IGF-1 and the length (143.2 mm FL; n = 604) and weight (27.7 g; n = 600) of hatchery fish were greater on average than those of wild fish (127.9 mm FL, n = 23; 22.3 g, n = 22; Table 4; Figure 4). Unlike steelhead, however, Chinook Salmon had greater weight, CF, and IGF-1 levels on average in 2017 (28.5 g, n = 292; CF = 0.93, n = 291; 52.6 ng/mL, n = 288) than in 2016 (26.5 g, n = 330; CF = 0.88, n = 330; 47.0 ng/mL, n = 235). There were also geographic stock group, month, and year effects for many of the Chinook Salmon metrics (Table 5). In particular, the estimates of length, weight, and body condition of interior spring Chinook Salmon (140.7 mm FL, n = 476; 25.4 g, n = 472; CF = 0.89, n = 471) were lower on average than the estimates for interior fall Chinook Salmon (150.2 mm FL, n = 124; 32.8 g, n = 124; CF = 0.93, n = 124), and the estimates of IGF-1 were greater for the interior spring stock group (51.1 ng/mL; n = 385) than for the interior fall stock group (45.3 ng/mL; n = 113). Lastly, by fitting separate models to the three geographic stocks, we found that the significant relationships between station order and Chinook Salmon metrics were mainly attributable to the interior spring group (Table 5).
DISCUSSION
We sampled juvenile salmon across nearly 200 km of main-channel habitat in the CRE in 2 years to document whether they fed and grew while moving rapidly through the estuary. Our results demonstrated that juvenile salmon were actively feeding: we observed at least some material in the stomachs of nearly all juvenile salmon that were examined. Fish collected at the uppermost site had the highest stomach fullness and consumed more insects, whereas there was generally greater consumption of amphipods in 2017 than in 2016. We were able to show that juvenile salmon were growing by increases in size (length and weight) and IGF-1 levels as they moved downstream through the estuary. This detectable growth occurred over the approximately 3–5-d period in which salmon migrated from Bonneville Dam to the mouth of the Columbia River. Overall, our results indicate that main-channel habitat in the CRE serves a dual purpose, functioning as a migratory corridor but also as productive habitat that supports active feeding and growth of fast-moving migrants.
Diets
Diet composition
Main prey items found in the stomachs of both salmon species were insects (mainly chironomids) and corophiid amphipods (Figure 5). These two prey types typically dominate the diets of juvenile Chinook Salmon in West Coast estuaries from Alaska to California (Chittenden et al. 2010; David et al. 2014; Levings 2016; Miller et al. 2020), including the CRE (Dawley et al. 1986; Kidd et al. 2019). The dominance of chironomids in the diets of steelhead and yearling Chinook Salmon also indicates that an expected indirect benefit of tidal wetland restoration in the CRE—providing prey for fast-migrating interior juvenile salmon—is being realized. Chironomids are produced in both restored and extant tidal wetland habitats (Kidd et al. 2019; PNNL and NMFS 2020) and were also the single largest taxonomic group exported from tidal wetlands to the main channel in the CRE, with thousands exported on a single tide (PNNL and NMFS 2020). Chironomids are also relatively energy dense compared to other typical salmon prey (Munguia 2019). Our finding that juvenile salmon are consuming chironomids, some of which were likely produced within restored tidal wetlands, provides critical evidence that wetland restoration does indeed benefit fast-moving juvenile salmon.
Although the dominant prey types were similar, we detected spatial and temporal variation in salmon diets. Diets at the uppermost site, RR, were the most distinct and contained more terrestrial insects than diets at the other sites. This difference likely reflects the limited wetland area in that river reach, especially compared to lower-river sites, where both restored and existing wetlands are much more extensive. Limited sampling with a neuston zooplankton net at all sites also indicated more terrestrial insects in surface waters at the uppermost site (PNNL and NMFS 2020).
Greater similarity in diet composition for salmon at the lower three sites was expected given the similarity in habitat mosaics in the lower estuary (Marcoe and Pilson 2017) and given the relatively short travel time (∼1 d) in which the fish migrate through the lower estuary (Harnish et al. 2012; Morrice et al. 2020). Other studies that have examined salmon diets across large estuaries have also documented variation in diet composition with distance downstream (Arbeider et al. 2019; Miller et al. 2020).
We also observed greater contributions of corophiids and other amphipods to diets in 2017 for both species across all sites. Corophiid amphipods were also elevated in the diets during 2017 for subyearling Chinook Salmon occupying wetland (Kidd et al. 2019) and main-channel habitats (PNNL and NMFS 2020), suggesting widespread increased prey availability. Corophiids are benthic-dwelling, burrow-building crustaceans that seasonally move upstream or downstream but generally remain on or near the river bottom (Wilson 1983). We speculate that increased consumption of corophiids in 2017 was due to extremely high flows (17,800 m3/s [630,000 ft3/s]) in late March 2017 that scoured benthic burrows, forcing the corophiids into the water column, where they were vulnerable to predation by juvenile salmon. This idea is further supported by high corophiid abundances in surface neuston samples during April 2017 (PNNL and NMFS 2020).
Stomach fullness
We report stomach fullness as an imprecise index of food consumption for smolts in the CRE. Our main goal was to assess whether rapidly migrating smolts in the CRE were feeding (i.e., yes or no), not to generate quantitative estimates of how much they ate. A simple inference can be generated by comparing our data to those reported from other studies; however, use of qualitative measures by other studies to report stomach fullness limits our ability to make widespread comparisons. Limited data that are directly comparable indicate that our estimates (0.64% BW for steelhead; 1.05% BW for Chinook Salmon) are similar in magnitude to those from other estuaries (e.g., 0.4–1.9% BW; Miller and Simenstad 1997; Levings 2016; Miller et al. 2020) but somewhat below those reported for marine waters (0.9–2.0% BW; Weitkamp and Sturdevant 2008; Daly et al. 2011, 2014; Beamish 2018). Our stomach fullness estimates are also considerably higher than those reported for earlier CRE studies of the same species. For example, stomach fullness was 0.09% BW for steelhead and 0.16% BW for yearling Chinook Salmon during 1979–1983 (Dawley et al. 1986) and 0.02% BW for steelhead and 0.5% BW for yearling Chinook Salmon caught at the EPS site during 2007–2012 (Weitkamp, unpublished data). These comparisons support the hypothesis that rapidly migrating smolts in the main channel of the CRE can find and consume significant amounts of prey.
Stomach fullness reflects a combination of factors, including local prey availability, time spent forging, and stomach clearance rates. Without fine-scale observations of the time spent foraging and prey availability, it is difficult for us to discern why stomach fullness may vary. However, we did observe some interesting patterns within our study.
We expected that stomach fullness would increase as fish moved downstream, since more wetland area (and resulting prey production) existed adjacent to the lower sites (Marcoe and Pilson 2017). Instead, the reverse was true: fullness was highest at RR, the uppermost site, and decreased with distance downstream (Figures 3, 4), a pattern that was also observed four decades earlier in the CRE (Dawley et al. 1986). Higher stomach fullness at the uppermost site might result from local abundances of prey items or differences in foraging behavior as smolts left the Columbia River gorge and entered the more normative river below Bonneville Dam. Foraging conditions in reservoirs upstream of Bonneville Dam largely consist of seasonally abundant but energy-poor zooplankton (Haskell et al. 2017). Increased foraging behavior at the uppermost site may also result from anthropogenic factors that limit feeding opportunities (spending up to 12 h in dam bypass systems or 2–3 d in barges during transport downstream).
Differences in temperature could relate to differences in stomach fullness because temperature regulates how quickly ectothermic salmon can digest their prey. Although water temperatures in 2016 were warmer than those in 2017, this daily difference was only 1.8°C on average, which would have relatively little influence on digestion rates (Benkwitt et al. 2009; David et al. 2014). A larger source of temperature variation was month; observed water temperatures between sampling trips in April and June increased by 6.0°C and 5.8°C in 2016 and 2017, respectively. However, most salmon were caught in April and May, when water temperatures differed by an average of 3.1°C. Gut fullness did not vary between months for yearling Chinook Salmon (Table 4) but was lower in April than in May for steelhead (Table 2)—the opposite of what one might expect if cooler April temperatures slow digestion and therefore increase apparent gut fullness. Unfortunately, we could not separate the influence of temperature and the influence of prey availability on patterns of stomach fullness.
Prey availability and feeding location
Knowing what prey were available to salmon and where juvenile salmon were feeding would increase our understanding of many of the diet patterns we observed. Unfortunately, effectively documenting the prey field in a large, rapidly flowing river for fish swimming over 50–100 km/d was beyond the scope of this study. This is one of the challenges of working in the open waters of large, highly dynamic systems like the CRE. The fact that both juvenile salmon species—regardless of where or when they were caught—consumed large quantities of chironomids, other aquatic insects, and corophiid amphipods indicates that those species were abundant and available. Stomach contents should represent the prior 24–36 h of feeding given observed water temperatures; therefore, most prey were consumed within the estuary. We do not know where and how migrating yearlings forage—that is, whether it is a constant behavior as fish move downstream in the main channel or whether fish alter their swimming behavior and position in the channel during short foraging bouts. Direct observations of fish swimming and foraging behavior would allow us to develop a better mechanistic understanding of feeding in these animals and when, where, and how migrating smolts benefit from wetland production of prey.
Growth of Salmon
Many studies have measured the growth of juvenile salmon (primarily subyearlings) in estuaries, with reported rates ranging between 0.2 and 2.0 mm/d (Miller and Simenstad 1997; Goertler et al. 2016; Levings 2016; Moore et al. 2016; Chalifour et al. 2021). We estimate that upper Snake River steelhead and interior spring Chinook Salmon may have increased by as much as 12 and 7 mm, respectively, as they moved through the CRE, based on simple linear regression of length versus location across the 195 km of our study area. If this distance took 5 d to transit, then maximum growth rates for these steelhead and yearling Chinook Salmon populations were 2.4 and 1.5 mm/d (1.1% and 1.0% of body length per day), respectively. This growth rate for Chinook Salmon is only slightly higher than the mean growth rate (1.4 mm/d) estimated by Norrie et al. (2022) during the 7 d prior to ocean entry for a subset of the Chinook Salmon used here; their estimate was based on back-calculated size using otoliths.
Comparable IGF-1-based growth rates for salmon in other estuaries do not currently exist. However, the mean IGF-1 levels that we recorded (∼45–60 ng/mL) generally overlapped with IGF-1 levels reported for juvenile Chinook Salmon and steelhead in marine waters (Figure 7), suggesting that growth in the CRE is comparable to growth in some marine areas. However, the highest rates reported for marine waters (>70 ng/mL) were much higher than our mean values from the CRE. Interestingly, our IGF-1 levels for CRE steelhead overlapped those measured in marine waters off the Washington–Oregon coasts during 2006–2011 and were substantially higher than levels measured in a limited number of juvenile steelhead sampled in the CRE (at the EPS site) during 2008 (Figure 7; Daly et al. 2014). Taken together, these comparisons indicate that the salmon we sampled in the CRE were growing rapidly.
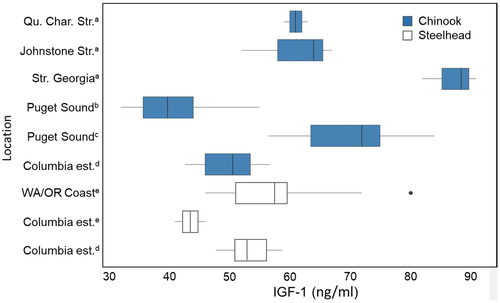
Our results demonstrate that juvenile steelhead and yearling Chinook Salmon were growing as they migrated through the CRE. This finding contradicts the long-held view that the main channel of the CRE serves as a “pipe” for fast-migrating juvenile salmon and that they neither feed nor grow as they move downstream (Lichatowich et al. 2006). By contrast, available evidence suggests that the Sacramento–San Joaquin River estuary, California, does function as a pipe for steelhead and Chinook Salmon, with little use of shallow-water bays and little feeding as fish swim toward the ocean (MacFarlane 2010; Chapman et al. 2013). Several recent studies have demonstrated that the ocean survival of Columbia River steelhead and Chinook Salmon is positively related to juvenile length or growth rates at ocean entry (Claiborne et al. 2011; Wilson et al. 2021; Gosselin et al. 2022; Norrie et al. 2022). These studies suggest that estuarine growth can be an important “carryover” effect of estuary rearing (Gosselin et al. 2021) that relates to subsequent ocean survival. Together, these findings reinforce the importance of understanding the link between wetland restoration, chironomid production and export from wetland areas, and consumption of chironomids by migrating salmon smolts.
Differences between Hatchery and Wild Salmon
The vast majority of the juvenile salmon we collected throughout the CRE were produced by numerous hatcheries spread across the Columbia River basin (Table A.1). We estimated that only 3.7% of the yearling Chinook Salmon and 19.4% of the steelhead we caught were wild. It is worth emphasizing this finding; the vast majority of yearling salmon smolts found in the largest river on the west coast of North America were produced in hatcheries. Presumed wild individuals were smaller than their hatchery counterparts, a well-documented difference (Quinn 2005; Weitkamp et al. 2015; Bottom et al. 2021). Diets of hatchery and wild steelhead were not statistically distinguishable, whereas yearling Chinook Salmon showed subtle differences in the amounts (but not types) of prey consumed by wild and hatchery fish.
Previous studies have warned of potential competition between larger and numerically dominant hatchery salmon and smaller wild salmon in the CRE based on high temporal and spatial overlap in shallow and main-stem habitats (Bottom et al. 2011, 2021; Teel et al. 2014; Weitkamp et al. 2015). Here, we observed high physical and temporal overlap over nearly 200 km of the CRE, as hatchery and wild individuals were typically caught together, including fish originating from different parts of the Columbia River basin. More importantly, our diet analyses demonstrated that hatchery and wild salmon had high diet overlap, potentially resulting in direct competition if resources were limited. A lack of good prey field data hinders our ability to determine whether prey are indeed limiting—a necessary requirement for demonstrating competition. Our observations of relatively high levels of stomach fullness and positive growth as fish moved downstream through the estuary suggest that overall prey abundances were likely not limiting at the time of this study. It is plausible, however, that future environmental conditions or increases in other native (and nonnative) competitors could greatly reduce prey availability in the CRE, resulting in direct competition. Although considerable efforts have been undertaken to isolate hatchery and wild salmon in tributary habitats (Araki et al. 2007), competition between hatchery and wild fish in the CRE is likely to be detrimental to smaller, less-abundant wild fish. This is an area of research that warrants additional attention.
Study Assumptions
Our study relies on two fundamental assumptions: (1) that the fish we sampled were indeed traveling in “the fast lane” and moving rapidly through the estuary and (2) that the two net types used did not unduly influence the size of captured fish. We do not believe that violations of these assumptions unduly affected our results or conclusions.
For the first assumption (that salmon were rapidly migrating), we caught 232 yearling Chinook Salmon and 69 steelhead with CWTs, which were estimated to have migrated at rates as high as 40 km/d from release at hatcheries to recovery in our study (Table A.1). These rates likely include time spent milling before the fish started to actively move downstream (Weitkamp et al. 2015), suggesting that these fish were moving even faster through the CRE. We also caught 7 yearling Chinook Salmon and 10 steelhead with PIT tags that were detected at main-stem dams prior to capture in our study. We estimated migration rates (from detection to recovery) of 70–80 km/d for these PIT-tagged salmon, typical of rates for juvenile salmon tagged with acoustic, radio, and PIT tags in other studies (McMichael et al. 2010; Harnish et al. 2012; Dietrich et al. 2016; Morris et al. 2017, 2018). Furthermore, the urge to swim downstream is initiated during smoltification; all of the fish we examined displayed shiny silver coloration typical of smolting fish (Wedemeyer et al. 1980), and many had swum over 500 km before we caught them (Table A.1). Therefore, it seems reasonable to conclude that the smolting salmon we captured were “in the fast lane” to the ocean.
Our second assumption was that any potential size selectivity due to the two nets used to capture fish (tow net and purse seine) did not unduly influence our results and conclusions. Most fish capture methods have size-selective biases (Murphy and Willis 1996). We know that the purse seine can catch large fish (i.e., adult salmon; Weitkamp et al. 2012), but whether the tow net is also capable of catching large fish is less clear. We do not know how much bias these different collection methods may have had on the characteristics of the salmon we collected or what proportion of the population our samples truly represented, but we generally believe that the bias was minor. For example, we observed increases in IGF-1 levels with distance downstream, which clearly demonstrated that salmon were growing, even if the increase in physical size with downstream distance included bias from the size selectivity of the nets. Such size bias would most likely influence the catch of the largest species, steelhead. However, the length of the largest steelhead caught with the tow net (320 mm FL) exceeded the length of the largest steelhead caught with the purse seine (307 mm FL), suggesting that the tow net is capable of capturing at least some large individuals.
We also observed many of the same patterns repeated in both species of salmon, such as seasonal patterns in abundance and differences in diet composition, during the 2 years. This suggests that even if we were not equally sampling all fish by using the two net types, the bias did not exceed the signals we were trying to measure. We had planned to conduct trials to estimate net size bias in 2017, but poor weather prevented us from doing the work. This is an important task for future studies.
Summary
We sampled yearling Chinook Salmon and steelhead during their rapid downstream journey in main-stem habitats across nearly 200 km of the CRE in two highly contrasting years. We found that most juvenile salmon were actively feeding, with diets dominated by chironomid insects and corophiid amphipods. We determined that juvenile salmon were growing during their 3–5-d migration through the estuary based on increases in size and based on a physiological indicator of growth. Our results demonstrate that the CRE serves not only as a migratory corridor for these fast-moving fish, but also as productive habitat where tidal wetland-produced prey fuel rapid growth; this estuarine growth may be important for marine survival. From a management perspective, the consumption of chironomids provides critical evidence that extensive tidal wetland restoration is having intended benefits (i.e., prey production) to interior stocks of juvenile salmon. Our results document high spatial, temporal, and diet overlap between hatchery and wild fish, which may result in direct competition if prey resources become scarce. Although this study greatly expanded what is known about juvenile salmon use of main-channel habitats in the CRE, some important details were beyond the scope of this study. In particular, future studies should examine fine-scale foraging behavior by migratory salmon and should characterize the extremely dynamic prey field in the main channel across the CRE.
ACKNOWLEDGMENTS
The research was conducted under National Oceanic and Atmospheric Administration (NOAA) Fisheries Determination of Take for Research Purposes permit numbers 23-16-NWFSC-108 and 06-17-NWFSC-108, Washington Department of Fish and Wildlife research permit numbers 16-086 and 17-011, and Oregon Department of Fish and Wildlife permit numbers 20246 and 21122. Field and laboratory work benefited greatly from the considerable efforts of Jake Biron and Wayne Haimes. The original concept for this work was inspired by Bob Emmett and designed by Gary Johnson (retired; Pacific Northwest National Laboratory) and Kurt Fresh (retired; NOAA, Northwest Fisheries Science Center [NWFSC]). The manuscript was improved by constructive comments provided by M. Rowse, R. McNatt, and three anonymous reviewers. The study was funded by the U.S. Army Corps of Engineers (USACE) Northwestern Division's Anadromous Fish Evaluation Program (Study EST-P-15-01) and NOAA Fisheries–NWFSC, with input and assistance from USACE staff Mike Turaski, Ida Royer, and Cynthia Studebaker. There is no conflict of interest declared in this article.
APPENDIX
RELEASE AND RECOVERY DATA FOR JUVENILE SALMON
| Hatchery region, run | Hatcheries | n | Release date | Days at large | Distance traveled (km) | Migration rate (km/d) |
|---|---|---|---|---|---|---|
| Steelhead | ||||||
| Snake River | Big Canyon Trap, Clearwater, Dworshak, Hagerman, Irrigon, Little Sheep Creek, Lyons Ferry, Magic Valley, Pahsimeroi, Wallowa | 46 | Apr 11 (Mar 15–May 1) | 26.3 (10–57) | 1,051 (448–1,425) | 44.0 (17.1–74.3) |
| MCR | East Fork Hood River | 1 | May 1 | 45 | 29 | 6.5 |
| UCR | Chiwawa, Wells, Winthrop | 22 | Apr 25 (Apr 15–May 8) | 22.1 (8–33) | 811 (520–952) | 39.2 (25.0–65.0) |
| Yearling Chinook Salmon | ||||||
| LCR Sp | Sandy | 4 | Mar 15 (Mar 8–Apr 6) | 44.3 (41–48) | 182 (182) | 4.1 (3.8–4.4) |
| Willamette River Sp | Dexter Ponds, Minto Ponds | 3 | Mar 22 (Feb 6–Apr 16) | 32.0 (7–81) | 384 (293–429) | 39.5 (3.6–61.3) |
| MCR Fall | Bonneville | 12 | Mar 4 (Feb 28–Mar 20) | 45.2 (29–51) | 359 (255–452) | 8.3 (5.0–15.6) |
| MCR Sp | Carson, Klickitat, Little White Salmon, Round Butte, Umatilla, Warm Springs | 25 | Apr 4 (Mar 21–Apr 13) | 16.8 (3–43) | 336 (41–467) | 24.4 (5.8–52.6) |
| UCR Sp | Chewuch, Chief Joseph, Chiwawa, Cle Elum, Leavenworth, Methow, Nason Creek, Twisp, Winthrop | 46 | Apr 12 (Mar 14–Apr 20) | 35.4 (19–71) | 767 (585–910) | 23.0 (10.1–30.8) |
| UCR Su | Carlton, Chelan Falls, Chief Joseph, Dryden Pond, Entiat, Similkameen, Wells | 55 | Apr 13 (Apr 5–17) | 33.7 (10–64) | 711 (570–952) | 23.1 (9.1–81.6) |
| Snake River Fall | Lyons Ferry | 28 | Apr 2 (Mar 30–Apr 6) | 16.8 (11–28) | 575 (407–807) | 35.5 (23.9–54.8) |
| Snake River Sp | Clearwater, Grande Ronde, Imnaha, Irrigon, Kooskia, Lookingglass, Nez Pierce Tribal, Rapid River, Sawtooth | 35 | Mar 27 (Mar 12–Apr 12) | 44.7 (15–67) | 807 (532–1,232) | 19.6 (11.8–36.2) |
| Snake River Su | Clearwater, Little Sheep Creek, Lyons Ferry, McCall, Pahsimeroi | 24 | Apr 7 (Mar 12–Apr 21) | 37.7 (17–67) | 1,055 (448–1,312) | 30.6 (9.9–58.7) |




