Multi-vital on-skin optoelectronic biosensor for assessing regional tissue hemodynamics
Ming Xin, Tong Yu, and Yongchang Jiang contributed equally to this study.
Abstract
Evaluation of the oxygen-mediated effects of clinical and daily activities demands an on-skin device that can track multi-vital regional tissue hemodynamics simultaneously. For example, peripheral arterial disease (PAD) is the third most prevalent cardiovascular disease, but the means of diagnosing and monitoring this disease are limited because the affected area is usually in the non-pulsatile area away from the heart. Herein, we report on an ultrathin and ultralight multi-vital near-infrared optoelectronic biosensor for the diagnosis and rehabilitation monitoring of regional tissue hemodynamics, which is suitable for mounting on the skin for long-term measurement. The device can simultaneously detect tissue oxygen saturation, heart rate, arterial blood oxygen, and tissue perfusion and shows potential for various hypoxia monitoring applications. Moreover, the tissue hemodynamics detected by this device showed a highly accordance with the ankle-brachial index and CT angiography obtained by traditional clinical methods. Therefore, our design was able to accurately diagnose and effectively evaluate PAD patients before and after surgery. The on-skin optoelectronic biosensor shows potential in biological oxygen-mediated behavior evaluation, injury-state monitoring, PAD clinical diagnosis optimization, and after surgery care.
1 INTRODUCTION
Developing an on-skin diagnosing device that is capable of precisely detecting specific physical parameters of human being, has great clinical importance in evaluation, diagnosing, monitoring, and after surgery care for specific diseases. For example, peripheral arterial disease (PAD) is the third-most common cardiovascular disease and is usually associated with other cardiovascular and cerebrovascular diseases and has a high disability and fatality rate.1, 2 More than 220 million patients worldwide experience PAD resulting in intermittent claudication,3, 4 resting pain, extremity ulcers, and limb gangrene, and this number is rapidly increasing due to the aging of the population. It is worth noting that the progress of the disease is hidden, while PAD monitoring is not included in general physical examinations.5 Often, the disease is not noticed until the late stage, when the clinical symptoms are serious; the consequences include amputation, rupture of abdominal aortic aneurysms, renal failure, and so forth.6, 7 At present, the most common methods used to diagnose and monitor PAD are the ankle-brachial index (ABI),8 oxygen partial pressure (pO2),9 CT angiography (CTA),10 and so on;11-13 however, these methods are costly, complex and require highly trained clinicians, especially the pO2, which needs the electrode to be inserted into the tissue to measure the local oxygen content.14-17 In addition, none of these methods addresses the need to monitor the hemodynamics of patients' distal tissue after discharge. Monitoring regional tissue hemodynamics to evaluate blood perfusion and tissue oxygenation levels is critical for preventing potentially severe PAD for both immediate diagnosis and after-surgery patient discharge, which calls for the development of a rapid diagnosis and monitoring device.
Tissue oxygen saturation (rSO2), one parameter that is widely used in various physiological and pathological processes, reflects the oxygenation capacity of skin tissue including the blood transportation and oxygenation level of the mixture of micro-veins in dermal tissue. rSO2 can be measured by near-infrared spectroscopy,18-22 but wearable devices have not yet used this method to evaluate PAD.23, 24 With the rapid development of epidermal electronics,25, 26 wearable optoelectronic devices have shown potential for the in situ monitoring of jaundice, arterial blood flow, and cerebral hemodynamics.27-29 Thus, it may be possible to develop a flexible on-skin device that is capable of comprehensively detecting the oxygenation level of skin tissue, including the rSO2, for the prevention, diagnosis, and postoperative care of PAD.
Herein, we present the design and implementation of an on-skin optoelectronic biosensor for the multi-vital monitoring of mixed arteriovenous blood flow that can be applied to analyze various clinical symptoms caused by PAD, such as lower limb ischemia, surgical vascular suture, and skin ulceration. The device featured an ultrathin, ultralight, and gentle interface that allows the device to be easily mounted on the skin for long-term monitoring. A newly designed circuit pattern is adopted to meet the low impedance requirements of the device as well as 30% biaxial stretchability to match the deformation of the skin in daily use. The device is able to simultaneously measure multiple vital parameters, such as the rSO2, heart rate (HR), arterial blood oxygen (SpO2), and tissue perfusion (PI), as proven by continuous real-time hypoxia and muscle oxygen consumption experiments. Clinical data obtained by the on-skin device from patients with lower extremity atherosclerotic occlusion disease (LEAOD) of a typical PAD, show a high correlation with the ABI and the CTA measured by traditional clinical methods, indicating that the device can be utilized for preoperative diagnosis, surgical guidance, and postoperative rehabilitation for PAD. Moreover, for arteriosclerosis and low lesion plane patients, the on-skin device showed the capability to correct the false positive result of ABI,30 which is in high accordance with the results of CTA. The on-skin device has wide application potentials in biological oxygen-mediated behavior evaluation, injury-state monitoring, PAD clinical diagnosis optimization, and after surgery care.
2 MATERIALS AND METHODS
2.1 Fabrication of the ultrathin substrate and patterned interconnects
A quartz glass was cleaned with acetone, ethanol, and deionized water (DI water). Then, poly(methyl methacrylate) (PMMA, 15 mg/ml) was spin-coated onto the cleaned glass chip at 2000 r/min for 30 s, which was then heated on the hotplate at 200°C for 30 min. Polyamic acid solution (12.0 wt% ± 0.5 wt%) was spin-coated onto the PMMA layer at 3000 r/min for 30 s, and heated on the hotplate at 250°C for 30 min to form ultrathin polyimide (PI, with thickness of 2 μm) substrate. Cr/Au (10 nm/140 nm) was deposited on PI film by electron beam evaporation. Then the glass was ready for the photolithography. In the photolithography steps, a positive photoresist (PR, AZ 5214; AZ Electronic Materials) was first spin-coated on the glass chip at 2500 r/min for 30 s and baked at 110°C for 120 s. Then the glass was covered with a patterned mask and exposed to ultraviolet light for 7.5 s. After that, it was soaked in the developing solution for 50 s. The Au was etched with potassium iodide, then washed with DI water and baked at 115°C for 5 min. The unwanted PI area was removed with selectively dry etching (Oxford Plasma-Therm. 790 RIE system, 200 W) for 10 min. Next, the sample was immersed in acetone for 12 h to dissolve the sacrificial PMMA layer. When the PMMA layer was wholly dissolved, the water-soluble tape was applied to pick up the interconnects transfer printing to the stretchable W1624 thin film.
2.2 Assembly of the biosensor
After the fabrication process, the contact between the interconnection circuit and LED and photodetector (PD) is bonded with the ACF tape (3M, 3M9703). The other electrode is interconnected with the conductor using silver paint (SPI; #5001-AB) and then encapsulated with biological dressing (W1624; Tegaderm, 3M).
2.3 Data analytics
All analyses used MATLAB (R2019b) for technical computing. The following hemodynamic parameters were used during the analysis: = 0.4, = 0.18, = 0.13, = 0.24.
2.4 Clinical studies
Clinical studies were performed under the permission of the Institutional Review Board (IRB No. 2022-131-01) at Nanjing Drum Tower Hospital of Nanjing. Patients were recruited from the inpatient department of vascular surgery of Nanjing Drum Tower Hospital. A consent was read to each patient before the study. After the staff answered all questions raised by the patient or his/her family, consent was then signed by the patient or his/her family, and the staff obtained consent. We used a peripheral vascular diagnostic system (Viasonix) to record the ABI. Information of all subjects were collected before and after the surgery; ABI was collected in the collection room, and the on-skin optoelectronic biosensor was used to collect information in the ward. The subjects lay flat on the hospital bed, and the biosensor was attached to the instep of the affected leg of the subjects. The collection time of each subject was 5 min.
3 RESULTS
3.1 Design of a multi-vital on-skin optoelectronic biosensor for accurate tissue hemodynamics monitoring
Figure 1A shows a conceptual schematic of the device and its application scenario. The biosensor consists of small optoelectronics, serpentine-honeycomb interconnects, a colorless PI substrate, and a biocompatible package. It is integrated with a wireless measuring module to produce an on-skin system. Three inorganic light-emitting diodes (LEDs) are placed on one side of the device, and two PDs are placed 10 and 20 mm to the side of the LEDs to create a multichannel sensing structure with sufficient spatial resolution for detecting the absorbed light intensity at different locations on the skin (Figure 1B). The small optical semiconductors hybrid-integrated here, include near-infrared light (750, 850 nm GaAs based) for obtaining the largest difference in the absorption coefficient in the near-infrared band, as well as red light (660 nm, GaAs based), and photodetector (400–1100 nm, silicon-based). A 150 nm-thick metal sheet (Au/Cr) is patterned in a serpentine-honeycomb shape to serve as the electrical interconnects for power and signal transmission, as shown in Figure 1C. This shape has low resistance and prominent biaxial stretchability. The metal interconnect is supported by an ultrathin (~2 µm), transparent, and colorless PI substrate and packaged by biological excipients (W1624; Tegaderm, 3M), which are widely utilized to cover and protect wounds and catheters and show excellent conformality to the skin based on van der Waals interactions alone (Figure 1D,E). Due to the ultrathin structure and low modulus mechanics, the device does not place severe constraints on the natural motions of the skin when pressed or stretched (Figure 1F,G, and Supporting Information: Figure S1).

The on-skin device is connected to an integrated wireless measuring module by a flexible conductor to perform signal processing and wireless transmission. Supporting Information: Figure S2 shows the overall view of the device. The multichannel spatial resolution sensing structure integrates three LEDs and a pair of PDs in different locations to achieve light-emitting and receiving sequences (Supporting Information: Figures S3 and S4). The electroluminescence (EL) spectra of the LEDs and the spectral responsibility of the PDs are shown in Figure 2A. Each LED turns on alternately at a frequency of 500 Hz and its driving voltage, and the PDs (AD conversion frequency of 1 kHz) detect the light reflected through the dermal tissue, which contains the most abundant arteries and veins in the tissue after the LEDs light up (Figure 2B). The spatial resolution structure can produce light signals of three wavebands carrying physiological information simultaneously. In this way, the value of tissue hemodynamics can be analyzed through the six groups of data. Here, we utilize a standard commercial chip to perform LED driving, amplification, filtering, and analog-to-digital conversion and wirelessly communicate to an external device via Bluetooth protocols (the circuit design is shown in Supporting Information: Figure S5). This strategy can not only allow signal processing within the chip, greatly reducing the signal attenuation and power consumption, but also minimize the number of components used and the design of the device. Powered by an easily replaceable button battery, the device can work continuously for more than 30 days, allowing long-term monitoring of PAD patients in surgical care and at-home measurements for people at risk of PAD.
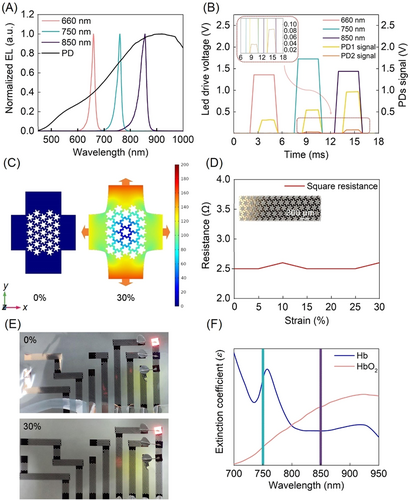
Daily activities require the device to possess a certain degree of stretchability; the maximum skin stretchability is 30%.31, 32 Generally, the shape of the interconnects determines the stretchability of the electronic skin device. The most widely used shape is the serpentine or serpentine-based pattern;33 however, the high ductility of the serpentine wire is achieved at the expense of the actual length of the wire. The interconnects for tissue hemodynamic monitoring transmit high-frequency signals and parasitic capacitance. Herein, we propose a novel serpentine-honeycomb composite structure to guarantee both stretchability and low impedance. Finite element analysis revealed that the serpentine-honeycomb structure follows the deformation law of honeycomb structures in the early stage of the deformation process (Figure 2C). When the tension reaches the limit of honeycomb structure, the serpentine wire can relax the stress (note the center of honeycomb). Additionally, the serpentine structure significantly enhances the tensile limit beyond the maximum reached by the honeycomb structure (Supporting Information: Figure S6). The square serpentine-honeycomb wires (thickness 150 nm) were designed with aside length of 12 mm, and tested for biaxial tension. The results of the real-time biaxial tensile test under 30% strain (the largest practical value for skin tissue) show that the square impedance of the serpentine-honeycomb structure remained stable at 2.6 Ω (Figure 2D,E).34 The above analysis demonstrates that the serpentine-honeycomb structure greatly improves the electronic and mechanical properties of the device.
3.2 Measurement of multi-vital parameters
Accurate monitoring of the hemodynamics of lower limb skin tissue depends on the maximized difference molar extinction coefficient (ɛ) of oxyhemoglobin (HbO2) and hemoglobin (Hb) in the visible and near-infrared spectra (Figure 2F).35 It is worth noting that the largest difference in HbO2 and Hb's ɛ lies in the 750 nm and 850 nm wavelengths. Human tissue is almost “transparent” in the 700–900 nm waveband,36 which allows for the transdermal design of our optical device. The key feature of the device's accurate ability to monitor regional tissue oxygenation dynamics is its accurate dual-band infrared light, the mechanism is essentially different from that of ABI in PAD diagnosis. The device is sensitive to tissue ischemia and is the main index to judge tissue ischemia, while the ABI is mainly related to blood pressure, so it is inaccurate to judge ischemia.
The procedures for extracting the rSO2, SpO2, HR, and PI from the measured data are outlined in Figure 3A. With the spatial resolution structure, the 750 and 850 nm wavelength signals captured by f-PD and the rear PD (r-PD), at source-detector distances of 10 and 20 mm, respectively, reflect the hemodynamics of deep skin tissue and can be used to define rSO2; the 660 and 850 nm wavelength signals captured by the front PD (f-PD) at a source-detector distance of 10 mm reflect the hemodynamics of the shallow peripheral tissues and can be used to define SpO2; finally, both PDs contribute to the detection and optimization of HR and PI.
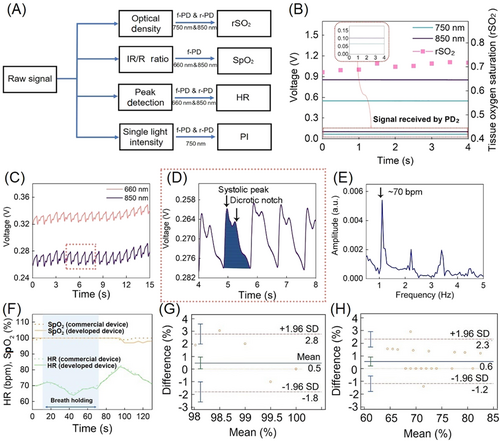
The measured photoplethysmography (PPG) signals corresponding to 17 cardiac beat cycles are shown in Figure 3C and include direct current (DC) and alternative current (AC). Figure 3D shows the AC value, including the systolic peak (the maximum pressure generated by the systolic ejection) and the dicrotic notch, which contributes to the accurate calculation of SpO2. PI is defined as the intensity of 750 nm light absorbed by Hb in the tissue, which is the band with the strongest absorption capacity in the near infrared range. HR is calculated via a Fourier analysis of the PPG signal, showing the different components of the signal (Figure 3E), where the fundamental frequency corresponds to the heart rate (in this case, 70 beats per min).
3.3 Evaluation of rSO2 in the hypoxia experiment
The device can quickly assess the severity of PAD by evaluating the level of tissue ischemia and hypoxia directly via continuous long-term monitoring. Different from the current mainstream indirect diagnosis of PAD,38 the PI quantifies the amount of Hb in the tissue to evaluate tissue perfusion and the microenvironment, and rSO2 reflects the self-regulatory response of the tissue vascular system to the change in oxygen concentration over time. In addition to the regional hemodynamics of the tissue, as part of current care standards, systemic hemodynamic parameters such as HR and SpO2 provide a better understanding of the overall health of the subject.39
The accuracy and sensitivity of the prepared sensor and a commercial sensor were verified by a breath holding experiment as shown in Figure 3F. Bland–Altman analysis of SpO2 and HR (Figure 3G,H) showed a mean difference of 0.5 (±1.96 SD, n = 2) and 0.6 (±1.96 SD, n = 2), respectively, indicating that the sensor has almost the same sensitivity and accuracy as the commercial sensor.
Human forearm venous occlusion was used to simulate limb insufficiency; the standard procedure is to place an inflatable cuff around the biceps muscle to block venous (but not arterial) blood flow, and the biosensor placed inside the forearm as shown in Figure 4A. The pressure of the cuff manometer was set to 100 mm/Hg; the pressurization process was, by default, completed instantly, and the blocking time was 3 min. During this process, the arm began to show a purplish red color due to the increase in blood volume and then gradually faded to dark red, while the rSO2 gradually decreased (Figure 4B). After the blockage was removed, the device detected a rapid increase in the rSO2 with overshoot due to the recovery of blood circulation, followed by gradual stabilization to the value before the compression. We note that body location can affect the overall signal levels but not the ratios and, therefore, the oxygenation results.
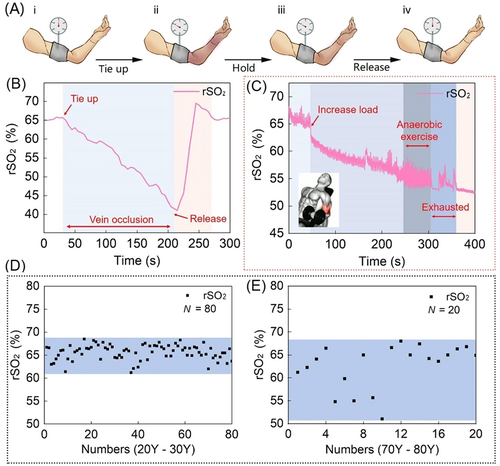
Monitoring the oxygen consumption process of human muscle tissue is key to starting the clinical PAD monitoring and diagnosis experiment.40 The device can accurately judge the time at which the muscle lactate threshold is reached, which could be of great significance to the field of sports medicine and health rehabilitation. As shown in Figure 4C, during the experiment, the device accurately revealed that the muscle rSO2 decreased linearly from start to 250 s after increasing the load. Between approximately 250 and 300 s, the rSO2 of the muscle did not decrease; that is, the muscle cells did not absorb oxygen, indicating that it was performing anaerobic respiration. The on-skin device determined muscularity and the lactate threshold by monitoring the dynamic oxygenation of the muscle. This technology could be used to perform long-term monitoring for people suffering from lower limb muscle atrophy caused by PAD.
The rSO2 of human skin is not directly related to age. Different age groups’ forearm rSO2 values were collected (20–30 years old and 70–80 years old). It was found that the skin oxygenation level of most elderly people was close to that of young people, but the range of oxygenation level was significantly larger than that of young people (Figure 4D,E). The device experiments revealed that the large difference in individual health levels among elderly individuals leads to the increase in the range of oxygenation levels in this group; however, there is no evidence that the skin oxygenation level decreases with age.
3.4 Evaluation of clinical PAD of the lower limbs by the rSO2
With the unique ability to monitor rSO2 and integrate systemic hemodynamic parameters, the device exhibits great potential in the clinical diagnosis, surgery monitoring, and postoperative nursing of PAD patients. To date, the most commonly used parameter for PAD diagnosis and monitoring is the ABI. Clinically, CTA and pO2 are used for auxiliary diagnoses, which are accurate, but allow only a single measurement, costly, complex and require highly trained clinicians. Although the ABI can be used to make a rapid diagnosis, different situations can lead to misjudgment and inconvenience for the patient, such as (1) abnormal arterial blood supply of the upper limb; (2) arteriosclerosis; (3) serious lower limb artery calcification; and (4) the inability of the patient to remain stationary during the examination. Compared with the CTA and ABI, the on-skin optoelectronic biosensor is easy to assemble, economical, and portable, friendly to limited resources areas; the ABI is mainly related to blood pressure while the rSO2 obtained by the on-skin optoelectronic biosensor can directly reflect the degree of hypoxia and/or ischemia of the lower limb tissue, which is suitable for the diagnosis and monitoring of PAD. The device is suitable for long-term wearing due to its imperceptibility and communication by Bluetooth with a smart device for big data analysis, which will be helpful for reducing the risk of PAD for elderly individuals, optimizing clinical diagnosis, and conducting after-surgery care. During surgery, the doctors can obtain the blood supply of the patients’ lower limbs in real time to evaluate the operation status. After surgery, the device can allow continuous remote monitoring to address the high recurrence rate of PAD. Notably, balloon dilatation and stent implantation are the first two choices for treating PAD, but the incidence of in-stent restenosis is as high as 40%–60%.41, 42 The on-skin device performs daily monitoring and early warning for discharged patients, thus increasing the possibility of medical intervention in advance and greatly reducing the recurrence of PAD.
The clinical PAD verification experiments were performed on-site at Nanjing Drum Tower Hospital. 10 subjects with severe LEAOD (the most typical PAD), characterized by intermittent claudication, pain, ulcers, and low lower limb temperatures, were randomly selected. The diseased region and corresponding procedure methods for the subjects represent typical patients with LEAOD and their treatment (see Table 1 for details). The first eight subjects are typical cases, as shown in Figure 5A–C. Taking the first subject as an example, the device shows that the rSO2 of the lower limb changes from 9% before surgery to 65% after surgery, while the ABI changes from 0.01 before surgery (ABI instrument displays 0.01 instead of 0 for points that cannot be measured) to 0.83 after surgery, indicating that the rSO2 is correlated with the ABI. The PI of the lower limb changes from 1.08 before surgery to 0.85 after surgery, verifying its negative correlation with the ABI. The rSO2 test showed that the oxygen supply to the patient's lower limb was improved after vascular dredging surgery. The data and clinical manifestations of other subjects also proved the same.
| Subject number | Diseased region | Procedure |
|---|---|---|
| 1 | SFA1+PA2 thrombosis | CDT3 |
| 2 | PA+BTK4 | POBA5 |
| 3 | Left iliac artery arteriosclerosis | PTA6 with stenting |
| 4 | SFA+BTK | PTA stenting (SFA)+BTK(POBA) |
| 5 | SFA | PTA with DCB7 |
| 6 | BTK | POBA |
| 7 | PA+BTK | POBA |
| 8 | PA+BTK | PTA with stenting |
| 9 | Right iliac artery arteriosclerosis | PTA with stenting |
| 10 | SFA | PTA with stenting+ CDT |
- Note: 1. Superficial femoral artery. 2. Popliteal artery. 3. Catheter direct thrombolysis. 4. Below the knee artery arteriosclerosis. 5. Percutaneous old balloon angioplasty. 6. Percutaneous transluminal angioplasty. 7. Drug-coated balloon.
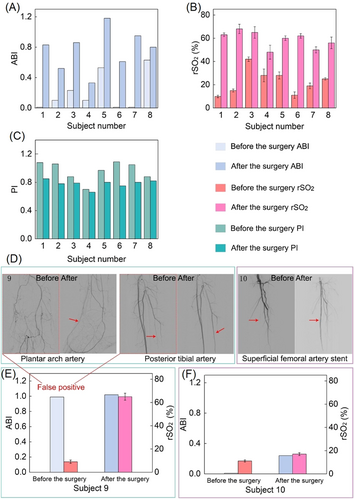
The on-skin device showed capability to correct the false positive result of ABI and was in high accordance with the results of CTA. The subject 9 with toe ulcer was diabetic foot and complicated with severe vascular calcification and low lesion plane (below ankle); ABI was positive (0.99), but rSO2 measured by the biosensor was low (9%). Arteriography showed the occlusion of plantar arch artery and posterior tibial artery (Figure 5D), reminding false positive ABI. After treatment of the lesions, the rSO2 recovered to normal and ulcer headed after 2 weeks. The data of biosensor are consistent with the conclusion of CTA in the therapy process proving that the biosensor can correct the clinical diagnosis of ABI (Figure 5E). The subject 10 was superficial femoral artery (SFA) stent restenosis with distal small vessels occlusion, complicated with foot pain. After reconstruction of SFA, ABI and rSO2 still remained at a low level and symptoms relief was mild. It is suggested that although the large arteries were opened, there was still severe tissue ischemia for the microcirculation was insufficient, calling doctors to seek other treatments (Figure 5F).
Other subjects had different diseased regions and underwent different procedures; the conclusions of postoperative ABI and CTA were highly consistent with rSO2, indicating that the on-skin biosensor can reflect LEAOD and postoperative recovery conveniently in real time. The device shows unparalleled advantages in the comparison of existing mainstream clinical diagnosis methods. The comfortability and flexibility of the on-skin device provide a wider space for its application, is easy to assemble and cheap; the portable alternative device is of great significance for areas with limited resources, reducing the risk of infection caused by repeated operation of the device. With the development of telemedicine technology, the device is expected to aid in continuous remote monitoring to address the high recurrence rate of LEAOD.
4 CONCLUSION
This paper introduces an ultrathin and ultralight multi-vital near-infrared optoelectronic biosensor for the continuous monitoring of mixed arteriovenous blood flow and can be applied to analyze various clinical symptoms caused by PAD. The biosensor exhibited a strong capability to simultaneously detect multiple vital parameters through real-time hypoxia and muscle oxygen consumption experiments. The device can accurately and directly respond to tissue hemodynamics, which are highly correlated with the results of ABI and CTA analysis and allowed a convenient and accurate differential diagnosis of PAD. Especially for arteriosclerosis and low lesion plane patients, the on-skin device showed the capability to correct the false positive result of ABI, which will make up for the existing clinical diagnostic methods. The on-skin optoelectronic biosensor shows great potential in organ oxygen-mediated, sports medicine, and artificial intelligence monitoring of PAD.
ACKNOWLEDGMENT
This research was supported by the National Key Research and Development Program of China (No. 2021YFA1401103), China National Funds for Distinguished Young Scientists (No. 61825403), The National Natural Science Foundation of China (Nos. 61921005 and 61674078), and Nanjing Scientific & Technological Talents Program.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Clinical studies were performed under the permission of the Institutional Ethics Review Board (IRB No. 2022-131-01) at Nanjing Drum Tower Hospital of Nanjing. Patients were recruited from the inpatient department of vascular surgery of Nanjing Drum Tower Hospital.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.




