Hierarchically Tailored Porous Carbon via Precursor Engineering for Dual-Redox Electrochemical Capacitors with Record-High Energy Density
Abstract
In energy storage systems utilizing redox reactions in the electrolyte (redox-enhanced electrochemical capacitors; redox ECs), electrode materials play a critical role: pore size distribution, free volume, and internal surface area directly impact the adsorption and diffusion of redox-active species at the electrode/electrolyte interface, thereby influencing overall energy storage capacity and efficiency. Importantly, achieving optimal full-cell performance requires tailored hierarchical pore architectures capable of accommodating structurally distinct redox-active species (catholytes and anolytes). Here, a streamlined precursor engineering strategy is presented to fabricate hierarchical, multi-scale porous carbon structures, providing a simplified alternative to conventional acid etching or resource-intensive pre-treatments. The porous carbon is engineered through thermal oxidation of precursor composites at moderate temperatures, combined with precise modulation of K2CO3 during activation. This approach yields a carbon material with a well-balanced pore structure, featuring a micropore volume of 0.74 cm3 g−1 and a mesopore volume of 1.64 cm3 g−1, and a specific surface area of 3,309 m2 g−1. When applied in a pentyl viologen/bromide dual redox EC, this system achieves a record-high energy density of 125 Wh kg−1. These findings highlight the significant relationship between pore structure and redox EC performance, offering valuable insights for advanced carbon materials in energy storage systems.
1 Introduction
Escalating environmental pollution and the depletion of fossil fuel reserves underscore the urgent need for advanced energy storage devices to efficiently harness renewable energy sources.[1, 2] In response, redox-enhanced electrochemical capacitors (redox ECs) have emerged as a promising energy storage solution.[3, 4] These systems utilize a hybrid energy storage mechanism, combining Faradaic charging from redox reactions with electrochemical double-layer charging, to achieve higher energy densities compared to conventional electrochemical double-layer capacitors (EDLCs), while also retaining key advantages such as high power output and long-term cycle stability.[5-9] Among the various redox EC configurations, aqueous-based redox ECs are particularly attractive due to the favorable properties of aqueous electrolytes, including safety, non-flammability, high ionic conductivity, and environmental sustainability.[10, 11] However, aqueous redox ECs still face critical challenges, such as limited operating cell voltages, low energy densities, and rapid self-discharge.[12-16] Increasing the concentration of redox-active materials in the electrolyte can significantly enhance energy density; however, this strategy may exacerbate the cross-diffusion of redox-active species that promote self-discharge.[11] While ion exchange membranes can effectively reduce self-discharge, their high cost remains a significant limitation.[17-21] Consequently, further research is essential to improve energy performance while minimizing self-discharge.
Evanko et al. proposed a dual redox system utilizing 1,1′-dipentyl-4,4′-bipyridinium dibromide (pentyl viologen, PV) as the anolyte and bromide (Br) as the catholyte.[22] This system alleviates self-discharge by enabling reversible, counter-ion-induced solid complexation between PV cations and bromide anions during charge-discharge cycles. The pentyl substituent effectively facilitates this precipitation process due to its intrinsic hydrophobicity; however, the resultant PV diameter, reaching up to 2 nm, imposes significant constraints on mass transport.[23] As a result, PV's inability to penetrate pores smaller than its size confines redox reactions on the carbon surface, leading to suboptimal energy storage performance.
The pore structure of porous carbon electrodes critically impacts the electrochemical performance of EDLCs, influencing key parameters such as capacitance, rate capability, and self-discharge rate.[24-28] Extensive research has elucidated the pivotal roles of micropores (< 2 nm) and mesopores (2–50 nm) in these systems. Micropores provide a high surface area for ion adsorption and storage, while mesopores facilitate efficient electrolyte diffusion into the electrode.[29, 30] However, this understanding primarily applies to systems involving small ions (< 1 nm) and does not directly extend to redox ECs, where complex electrochemical redox reactions, along with adsorption and diffusion, occur. Additionally, few studies have explored the interface between redox-active species and porous carbon electrodes.
For example, Zhao et al. investigated the effect of the pore size distribution on energy density, rate performance, and self-discharge rate in potassium iodide (KI) redox ECs.[31] Their results indicate that pores ranging from 1.1 to 3 nm enhance high-rate capability, while pores smaller than 0.8 nm suppress self-discharge, as governed by the sizes of iodide ions (I−, 0.22 nm) and polyiodide ions (I2, ≈0.5 nm; I3−, ≈0.9 nm). Furthermore, the void volume contributes to the overall redox capacity. Similarly, Calcagno et al. utilized ordered mesoporous carbon (CMK-8) in PV/Br redox ECs and found that PV species primarily interacted with 4.3 nm mesopores, with adsorption occurring on the carbon particle surfaces.[23] These findings emphasize the crucial role of mesopores as adsorption sites for large redox-active species, such as PV. However, because mesopores do not provide as high a specific surface area as micropores, their use generally results in limited surface utilization and lower energy storage performance. Therefore, the nano-engineering of porous carbon electrodes with a hierarchical pore structure, incorporating both micropores and mesopores to achieve a multi-scale pore distribution, is essential.
Extensive efforts have been devoted to developing mesopores in carbon materials while preserving their intrinsic microporous structure, employing strategies such as soft and hard templating, chemical activation, and self-activation.[32-36] However, these approaches frequently encounter critical challenges, including the collapse or excessive enlargement of pre-existing micropores during mesopore formation.[37, 38] Such issues become particularly pronounced under severe conditions, for instance, high temperatures or the excessive use of activating agents, which often lead to the degradation of the microporous framework and the unintended formation of mesopores and macropores.[39] To mitigate this trade-off, researchers have explored alternative methodologies, including the incorporation of silica-based hard templates, the activation of melamine-formaldehyde resin or graphene, and precursor modification via semi-carbonization.[40-45] Nevertheless, these strategies suffer from several inherent limitations, such as the high cost of precursors (e.g., graphene), the complexity of multistep processes involving template removal and acid-based post-treatments, the use of hazardous solvents or chemicals, and the stringent requirements for semi-carbonization, which typically necessitates temperatures above 450 °C under an inert atmosphere.
In this study, we introduce a practical precursor composite engineering strategy for synthesizing porous carbon, successfully addressing the trade-off between micropore loss and mesopore formation. This approach enables the pre-formation of mesopores within the precursor composite through a thermal oxidation (TO) step conducted at a moderate temperature of 250 °C in air. The mesopores generated during the TO step serve as channels, allowing the activation agent to access both the surface and interior of the precursor composite. Consequently, the increased number of activation sites facilitates carbon etching by the activation agent, leading to the formation of activated carbon with enhanced total porosity while effectively minimizing the associated structural trade-off. By optimizing the quantity of the activation agent, we precisely controlled the balance between micropore and mesopore volumes, resulting in a nano-engineered carbon material with significantly enhanced mesopore development while preserving a substantial proportion of micropore volume, which accounted for 31% of the total pore volume. This porous carbon material exhibited an exceptionally high surface area of 3309 m2 g−1 and a total pore volume of 2.38 cm3 g−1. Notably, it demonstrated a threefold increase in mesopore volume within the 2–10 nm range compared to the carbon derived from the precursor composite without the TO step. When the resulting carbon material was utilized as an electrode in PV/Br dual redox ECs, this well-balanced hierarchical pore structure enabled maximal utilization of PV's redox activity, achieving a record-high energy density of 125 Wh kg−1 using 1.5 m PV in a 3 m NaBr aqueous electrolyte solution. These findings underscore the critical importance of tailoring carbon pore sizes to match the molecular dimensions of redox-active species and suggest a promising framework that could be broadly extended to other redox-active species.
2 Results and Discussion
Figure 1 illustrates the synthesis process of highly porous carbon, encompassing a hydrothermal reaction, a TO step, and K2CO3-assisted activation. Initially, the tannic acid-melamine composite (TMC) was synthesized via a hydrothermal reaction using tannic acid and melamine as the carbon precursor and structure-directing agent, respectively, with zinc acetate serving as a linker to establish a solid framework (Figure 1a).[46, 47] The TMC was subsequently oxidized by heating at 250 °C in air for 5 h, promoting the formation of mesopores and macropores within the TMC particles and resulting in thermally oxidized TMC (TMC_TO). Following this, TMC_TO was uniformly mixed with K2CO3 using a ball mill and then carbonized under an inert atmosphere (Ar). The resulting black powder was washed with water at 80 °C to remove K2CO3 residues and subsequently dried in a convection oven. The carbonized and activated products derived from TMC_TO were designated as KX_TO, where X represents the mass ratio of TMC_TO to K2CO3 (Figure 1b). For comparison, the KX series was synthesized using TMC, adhering to the same procedure as KX_TO but excluding the TO step (Figure 1c).
The mesopores introduced by the TO step increased the contact area between K2CO3 and TMC_TO, enhancing mesopore formation in KX_TO through K2CO3-assisted activation. The resulting K1.5_TO, synthesized with an optimized quantity of K2CO3, exhibited enriched micro- and mesoporous structures, with a total pore volume of 2.38 cm3 g−1 and a micropore volume fraction of 31%. In contrast, K1.5, produced by activating non-porous TMC with the same amount of K2CO3, demonstrated that micropores contributed 68% to its total pore volume of 1.55 cm3 g−1. The multi-scale pore size distribution of the KX_TO series provides abundant channels and spaces to facilitate the efficient adsorption and diffusion of PV and bromide species, thereby substantially enhancing the energy density of redox ECs (Figure 1d,e).
The effects of the TO step and the quantity of K2CO3 on the pore structure of porous carbon materials were systematically investigated using N2 adsorption and desorption experiments. The results demonstrate that the total pore volume of the carbon materials increases proportionally with the amount of K2CO3 used, up to 1.5 times the weight of the precursor composite, thereby confirming its role as an activation agent (Figures S1 and S2, and Table S1, Supporting Information). In the TMC, a higher quantity of K2CO3 facilitated the formation of additional pores smaller than 3 nm. Conversely, for TMC_TO, pore development predominantly occurred within the 2–10 nm range. Moreover, the KX_TO series exhibited twice the pore volume in the 10–30 nm range compared to the KX series, with only minor variations depending on the K2CO3 quantity. Based on these observations, four porous carbon materials with distinct pore size distributions (K0.5, K0.5_TO, K1.5, and K1.5_TO) were selected to evaluate the impact of carbon pore structure on the energy storage performance of redox ECs. In terms of yield, K0.5 and K1.5 derived from TMC exhibited values of 27.3% and 16.8%, respectively, whereas the use of TMC_TO resulted in lower yields of 14.5% for K0.5_TO and 7.75% for K1.5_TO. This trend further underscores the influence of K2CO3 content, as the product yield exhibited a consistent decline with increasing amounts of the activation agent (Table S2, Supporting Information).
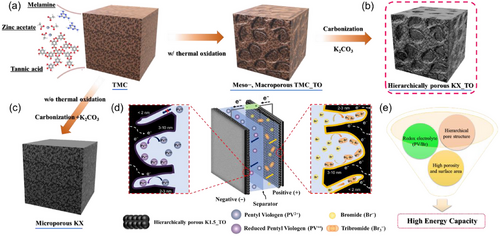
N2 adsorption and desorption isotherm curves for TMC-derived K0.5 and K1.5 feature the shape of the IUPAC Type I classification, indicative of a predominantly microporous structure (Figure 2a).[48] In contrast, the isotherm curves for TMC_TO-derived K0.5_TO and K1.5_TO follow the IUPAC Type IV classification, suggesting a combination of micro- and mesoporous structures. The pronounced increase in the p/p0 < 0.1 region of the isotherm curves across all carbon materials is attributed to abundant micropores. Additionally, the hysteresis with a positive slope in the p/p0 = 0.5–0.9 region confirmed the presence of mesopores in K0.5_TO and K1.5_TO.
A quantitative comparison of pore volumes between K1.5 and K0.5 reveals that increasing the quantity of K2CO3 results in a 41% increase in the volume of pores smaller than 3 nm (Figure 2b; Figures S1 and S2, Supporting Information). In contrast, K1.5_TO exhibits a 16% increase in total pore volume alongside a 5% reduction in the volume of pores smaller than 3 nm compared to K0.5_TO (Table 1). This indicates that higher amounts of K2CO3 lead to the destruction of the micropore walls, facilitating the formation of mesopores within the 2–10 nm range. Notably, K0.5_TO demonstrates an 81% higher total pore volume than K0.5, which is mainly attributed to an increase in pores within the 2–10 nm range. In K1.5_TO, the pore volume within the 2–10 nm range increased to 1.52 cm3 g−1, which is 1.5 times higher than that of K0.5_TO (1.03 cm3 g−1) and ≈3.5 times greater than that of K1.5 (0.44 cm3 g−1). Despite the development of these mesopores, the micropore volume of K1.5_TO (0.74 cm3 g−1) remains only 30% lower than that of K1.5 (1.06 cm3 g−1), suggesting that the removal of micropores is constrained during activation. Comparing K0.5_TO, K1.5_TO, and K1.5, these findings reveal that the TO step not only enables the formation of a substantially greater mesopore volume with the same quantity of K2CO3 but also facilitates the synthesis of carbon materials with superior mesoporosity while requiring only one-third of the K2CO3. Additionally, the amount of K2CO3 primarily impacts micropores and mesopores smaller than 3 nm in the KX series, while influencing mesopores below 10 nm and mitigating micropore collapse in the KX_TO series.
| Sample | SBET [m2 g−1] | Salpha − s [m2 g−1] | Vp [cm3 g−1] | Vs [cm3 g−1] |
|---|---|---|---|---|
| K0.5 | 2302 | 1941 | 1.13 | 11.92 |
| K1.5 | 3137 | 2582 | 1.55 | 17.43 |
| K0.5_TO | 3405 | 2699 | 2.05 | 10.33 |
| K1.5_TO | 3309 | 2623 | 2.38 | 18.12 |
Furthermore, the void space volume (Vs) of K1.5 and K1.5_TO was measured as 17.43 cm3 g−1 and 18.12 cm3 g−1, respectively, both exceeding those of K0.5 (11.92 cm3 g−1) and K0.5_TO (10.33 cm3 g−1). These results suggest that void volume is more affected by the quantity of K2CO3 than by the TO step (Table 1; Table S3, Supporting Information). It can be attributed to a combination of factors, including the formation of KOCN and KCN, the evolution of CO or CO2 gases during activation, the thermal decomposition of the precursor composites, the salt template role of K2CO3, KCN, and KOCN, and its reactions with the precursor composites at elevated temperatures.[49, 50]
Raman spectroscopy and X-ray diffraction (XRD) analyses were conducted to elucidate the microstructural characteristics of the carbon materials. The Raman spectra for K0.5, K0.5_TO, K1.5, and K1.5_TO exhibited no significant variations in the intensity ratio of the D band (≈1340 cm−1) to the G band (≈1590 cm−1) (Figure 2c), indicating that the addition of excess K2CO3 and the TO step had negligible effects on the microstructure.[51] The XRD patterns predominantly displayed peaks corresponding to amorphous carbon (002) and (101), without discernible peaks attributable to Zn particles derived from the zinc acetate linker used during synthesis (Figure 2d).[52] Consistently, X-ray photoelectron spectroscopy (XPS) analysis confirmed the absence of Zn in the synthesized carbon materials (Tables S4 and S5, Supporting Information). This absence of Zn alleviates concerns regarding the potential interference from Zn species in evaluating the energy storage performance of the activated carbon materials, suggesting that the carbon pore structure plays a dominant role in determining the energy density of redox ECs.
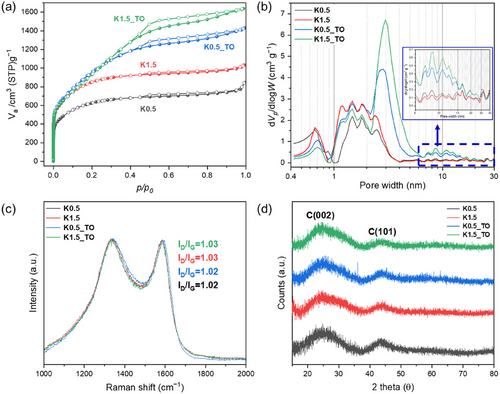
Transmission electron microscopy (TEM) and scanning electron microscope (SEM) images revealed that both precursor composites and porous carbon materials primarily consisted of aggregated particles ranging from 50 to 300 nm in diameter (Figure 3; Figures S3 and S4, Supporting Information). While there was a minimal difference in particle size and distribution between the TMC and its thermally oxidized counterpart, TMC_TO exhibits the formation of new mesopores and macropores smaller than 100 nm (Figure 3a,d). The formation of meso- and macropores results from the structural collapse of micropore walls during the TO step. This structural transformation is confirmed by pore size distribution analyses, which reveal that TMC_TO exhibits a 6.4% increase in mesopore volume and a corresponding 7.2% reduction in micropore volume compared to TMC (Figure S5 and Table S6, Supporting Information). These mesopores contributed to the presence of 10–30 nm pores in the KX_TO series, in contrast to the TMC-derived KX series, which lacks mesopores of this dimension (Figure 3b,c,e,f; Figure S6, Supporting Information). However, the enhanced development of 2–10 nm pores in the KX_TO series compared to the KX series remains unclear. We hypothesized that the pore structure of TMC_TO significantly influences the carbon activation process; specifically, the mesopores in TMC_TO extend the interface with K2CO3, thereby intensifying the carbon etching by K2CO3.
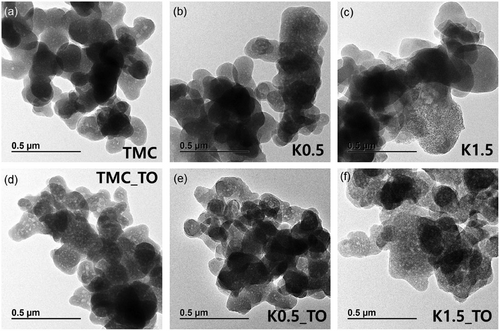
Fourier-transform infrared (FT–IR) spectroscopy was employed to examine the structural characteristics of TMC and to assess modifications induced by the TO step. In the spectrum of TMC, distinct peaks corresponding to tannic acid and melamine confirmed the successful formation of the composite through a hydrothermal reaction (Figure 4a; Figure S7, Supporting Information). The spectrum of TMC_TO closely resembled that of TMC, however, peaks corresponding to ─OH (1000–1300 cm−1) and ─NH (1400–1600 cm−1) functional groups were significantly diminished.[53] The removal of unstable functional groups during the TO step was accompanied by the formation of mesopores in TMC_TO.
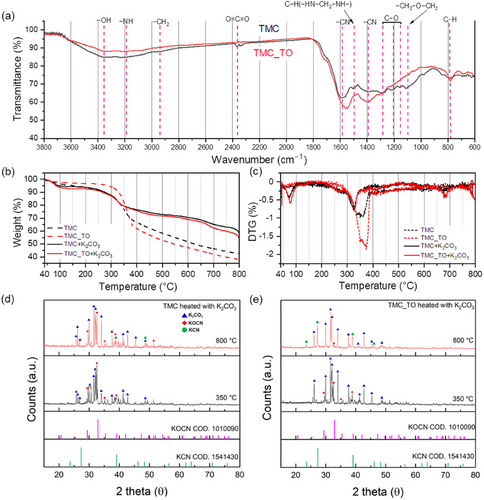
Thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG) curves were used to investigate how the structural properties of precursor composites influence K2CO3-assisted carbon activation. The weight of TMC_TO remained stable up to 300 °C, as unstable species were removed during the TO step at 250 °C (Figure 4b,c). However, the reduced functional groups suppressed polymerization above 250 °C, resulting in decreased thermal stability.[54] Therefore, in the 350–370 °C range, TMC_TO exhibits a more pronounced weight change than TMC. This behavior is attributed not only to the polycondensation of residual melamine and tannic acid derivatives but also to an increased extent of thermal decomposition.[55, 56]
For mixtures of TMC or TMC_TO with K2CO3 at a weight ratio of 1.5 (designated TMC+K2CO3 or TMC_TO+K2CO3, respectively), weight loss due to water evaporation occurred below 100 °C. The overall weight changes for TMC+K2CO3 and TMC_TO+K2CO3 were less severe than for samples without K2CO3, owing to K2CO3 residues in the TGA containers. Notably, the weight loss peak for TMC and TMC_TO in the 350–400 °C range shifted to ≈330 °C upon K2CO3 addition, possibly due to the dehydrogenation of carbon precursors at a lower temperature.[57, 58] Weight loss differences between TMC+K2CO3 and TMC_TO+K2CO3 remained comparable up to 650 °C. However, TMC_TO+K2CO3 exhibited a significantly greater weight loss approximately twice that of TMC+K2CO3 at 680 °C, the onset temperature for chemical agent-assisted activation (Figure S8a, Supporting Information). In both TMC+K2CO3 and TMC_TO+K2CO3, weight losses associated with additional carbon activation by K2CO3 were observed at 770–800 °C without noticeable variations between the TO steps (Figure S8b, Supporting Information).
To further elucidate the underlying mechanism of multi-scale pore structure development in TMC_TO+K2CO3, we investigated the interactions between the precursor composite and K2CO3 using XRD patterns. XRD measurements were conducted at 350 and 800 °C to track the structural evolution of the precursor and K2CO3 composites at intermediate and post-activation stages. Both TMC and TMC_TO exhibit a broad diffraction peak around 22°, corresponding to tannic acid within the composite (Figures S9 and S10a, Supporting Information). Additionally, a prominent diffraction peak around 28°, along with weaker peaks ≈22° and 33°, indicative of hydrothermally treated melamine (Figure S10b, Supporting Information).[59] After the removal of K2CO3, no significant differences were observed between TMC and TMC_TO following heat treatment at 350 and 800 °C without featuring Zn acetate and K2CO3 (Figures S9 and S10c,d, Supporting Information).
During the thermal treatment of nitrogen-containing compounds such as melamine with K2CO3, an intermediate species (KOCN) is initially formed through the reaction of K2CO3 with edge nitrogen sites (─CN). At temperatures exceeding 500 °C, KOCN facilitates carbon oxidation, thereby promoting mesopore development and subsequently transforming into KCN (see Note S1, Supporting Information for details).[49] Thermal treatment of TMC+K2CO3 and TMC_TO+K2CO3 at 350 °C led to the formation of KOCN, as indicated by characteristic XRD peaks alongside residual K2CO3 (Figure 4d,e; Figure S10d, Supporting Information).[60] At this stage, TMC_TO exhibited an 11.4% higher mesopore volume than TMC (Figure S11 and Table S6, Supporting Information), a difference attributed to the prior step. The presence of pre-formed mesopores contributed to enhanced interfacial contact with K2CO3, facilitating more effective precursor–activator interactions during subsequent high-temperature treatment.
Upon further heating to 800 °C, a pronounced conversion of KOCN to KCN was observed in the TMC_TO+K2CO3 sample, whereas KOCN signals largely persisted in TMC+K2CO3 (Figure 4d,e). This distinction underscores the critical role of TO in improving the accessibility of reactive carbon sites. In TMC_TO, the enhanced interfacial connectivity between KOCN and the carbon matrix enables more efficient carbon etching and mesopore formation. In contrast, the limited transformation observed in TMC implies reduced activation due to insufficiently developed porosity. These results demonstrate that the enhanced interfacial contact in TMC_TO facilitates more vigorous carbon activation by K2CO3, contributing to the development of 2–10 nm mesopores. This precursor engineering strategy ultimately enables the KX_TO series to achieve higher mesopore and total pore volumes while preserving microporosity.
For the electrochemical characterization of the synthesized carbon in redox EC systems, PV was synthesized (Figures S12 and S13, Supporting Information) and employed as a redox-active electrolyte. Pore volume contributions by size for the synthesized carbon materials are presented in Figure 5a for comparison. Asymmetric cells were constructed using 0.5 m PV in 3 m NaBr aqueous solution and carbon materials with tailored pore architectures. In this configuration, the weight ratios of the carbon electrodes were meticulously adjusted to ensure that additional redox reactions occurred exclusively at one electrode, while the opposing electrode was restricted to capacitive charging. This strategic design enables the precise observation of the electrochemical Faradaic charging behavior of a single redox species in an electrolyte containing dual redox species.[22]
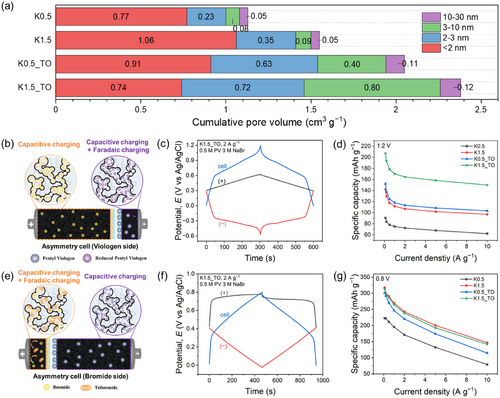
To examine the effect of engineered pore structures in carbon electrodes on the PV redox reaction as the anolyte, asymmetric three-electrode cells were constructed with a 12:1 weight ratio between the cathode and anode (Figure 5b). As shown in Figure 5c, the cathode (black line, (+)) exhibited only capacitive charging, with no bromide redox activity detected. In contrast, the anode (red line, (−)) displayed both capacitive and distinct Faradaic charging associated with PV. This experimental setup allows a fair capacity comparison driven by PV, as the observed capacity closely correlates with the quantity of PV adsorbed, which is determined by the specific pore structure of each carbon electrode.
The galvanostatic charge-discharge (GCD) profiles of asymmetric two-electrode cells for K0.5, K1.5, K0.5_TO, and K1.5_TO show capacities of 72, 107, 113, and 164 mAh g−1 at 1.2 V and 2 A g−1, respectively (Figure S14a, Supporting Information). Capacity values for these cells were also evaluated across a range of current densities (Figure 5d), revealing a pronounced dependence of PV capacity performance on the pore size and volume of the carbon electrode. The highest capacity, achieved with the K1.5_TO electrode, which has the largest mesopore volume, aligns with the expectation that mesopores markedly contribute to increased PV adsorption and efficient diffusion due to the size of PV being up to 2 nm. Moreover, while K1.5 and K1.5_TO have similar specific surface areas, K1.5 shows a 35% lower capacity than K1.5_TO, highlighting the critical influence of mesopores on PV redox activity compared to micropores. However, the mesopore effect is notably diminished when comparing K0.5_TO and K1.5. Despite K0.5_TO possessing 2.3 times the mesopore volume of K1.5, it shows only a 6% capacity increase. This might be a combined outcome stemming from void space functioning as an electrolyte reservoir and mesopores serving as channels for PV.[31] Since void space volume is governed by the quantity of K2CO3 used, K1.5 exhibits a 69% higher void space volume than K0.5_TO (Table 1).
The correlation between the carbon pore structure and the redox activity of bromide as the catholyte was evaluated in an asymmetric cell with a cathode-to-anode weight ratio of 1:12 (Figure 5e). As shown in Figure 5f, only capacitive charging occurred at the anode, with no indication of PV redox reactions, while both capacitive and Faradaic charging of bromide were observed at the cathode. GCD profiles of the asymmetric two-electrode cells for each carbon material present capacities of 195, 269, 246, and 264 mAh g−1 at 0.8 V and 2 A g−1, respectively (Figure S14b, Supporting Information). Notably, excluding K0.5, which exhibited relatively low micro- and mesopore volumes, the capacities of redox ECs using the other three carbon materials were similar. Given the size of ≈0.19 nm, bromide ions can easily access micropores (< 1 nm) and adsorb readily onto the electrode surface, indicating that differences in mesopore volume have little impact on bromide redox activity. Specifically, although K1.5_TO has a 30% lower micropore volume than K1.5, its sufficient micropore volume and superior mesopore volume provided ample surface area for bromide adsorption, ensuring that bromide redox reactions were not restricted.
However, in contrast to PV, the capacity of bromide across all carbon electrodes decreased significantly by ≈66% when the current density was increased to 10 A g−1, compared to the initial current density of 0.1 A g−1 (Figure 5g). The reduction can be attributed to the substantial size mismatch between bromide ions and the pore size. As pore size increases relative to ion size, the effective diffusion distance to the electrode surface becomes longer.[31, 61] At higher current densities, the time available for bromide ions to diffuse and adsorb onto the carbon electrode surface is markedly limited, leading to reduced bromide adsorption. This diminished adsorption ultimately results in lower capacity values. These results demonstrate that the tailored pore architecture of K1.5_TO primarily enhances the adsorption and diffusion of PV rather than bromide due to the relationship between ion diameter and pore size.
Symmetric two-electrode cells containing 1 m PV and 3 m NaBr were assembled to investigate the correlation between carbon pore structures and the electrochemical behavior of redox species under conditions where Faradaic charge storage occurs at both the anode and cathode. Cyclic voltammetry (CV) was conducted to evaluate redox behavior and electrolyte reversibility, revealing a pair of reversible redox peaks at 1.1 V and a broadened CV area compared to systems without redox-active materials (Figure 6a; Figure S15a, Supporting Information). This behavior arose from the combined contributions of viologen and bromide redox reactions, coupled with capacitive charging due to the electrostatic adsorption of bromide and PV ions at the electrode-electrolyte interface during charge and discharge. The CV area increased across the carbon materials in the order of K0.5, K1.5, K0.5_TO, and K1.5_TO, a trend that was also reflected in GCD profiles. The GCD results revealed energy densities of 35.0, 54.2, 65.6, and 88.6 Wh kg−1 at 1.4 V and 2 A g−1, respectively (Figure 6b). Specific energies were measured across a range of current densities from 0.1 to 10 A g−1 (Figure 6c), with the energy order consistently observed as K1.5_TO > K0.5_TO > K1.5 > K0.5. This trend aligns with the PV redox activity observed in asymmetric cells using these carbon materials, suggesting that enhanced PV redox reactions play a crucial role in improving the overall performance of PV/Br redox ECs. Although pore architecture clearly plays a dominant role, other factors such as nitrogen doping in carbon were also considered to assess their potential contribution to charge storage. While nitrogen doping can, in principle, influence electrochemical charge storage by introducing redox-active surface functionalities, its contribution appears limited in the present system. Given the near-neutral pH of the PV in 3 m NaBr electrolyte (≈6.5), the conditions are not favorable for activating nitrogen-induced pseudocapacitive reactions, which typically manifest under acidic or alkaline environments.[62-64] As detailed in Figure S15 and Table S5 (Supporting Information), K0.5_TO, which exhibited the highest nitrogen content (4.64 at%), showed lower capacitance performance than K1.5_TO, despite the latter containing only 2.09 at% nitrogen. These results further support that, in the present system, hierarchical pore architecture, particularly mesopore development, plays the primary role in governing charge storage. Consequently, engineering carbon pore structures, particularly mesopores, emerges as an effective strategy to enhance PV redox activity, thereby significantly advancing the energy density potential of redox ECs.
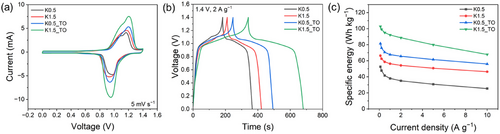
Based on a comprehensive analysis of the results, K1.5_TO exhibited optimal compatibility for PV/Br redox EC, demonstrating superior capacity and energy performance. This can be attributed to its well-engineered pore structure, which facilitates enhanced PV redox activity and more efficient ion transport. To further enhance energy storage performance, the PV concentration was increased to 1.5 m, and the K1.5_TO electrode was employed to maximize PV adsorption at the carbon electrode interface. Figure 7a presents the GCD profiles of the symmetric two-electrode cell across a range of charging rates, from 0.1 to 10 A g−1. The cell achieved specific energies of 125, 121, 114, 104, 85, 64, and 46 Wh kg−1 at current densities of 0.1, 0.2, 0.5, 1, 2, 5, and 10 A g−1, respectively. Increasing the PV concentration to 1.5 m successfully enhanced the energy density, improving 22.3 Wh kg−1 at 0.1 A g−1 compared to the 1 m solution.
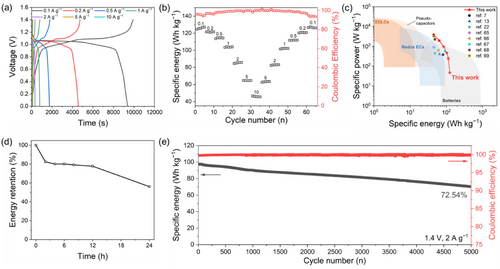
To assess rate capability, a GCD cycling test was conducted by progressively increasing the current density from 0.1 to 10 A g−1 and subsequently returning it to 0.1 A g−1 (Figure 7b). Upon reducing the current density back to 0.1 A g−1, the cell demonstrated stable rate capability, consistently reproducing the initial average specific energy while retaining high Coulombic efficiency. The Ragone plot highlights the superior energy performance of the PV/Br redox EC employing the tailored K1.5_TO carbon electrode, outperforming peer state-of-the-art redox ECs (Figure 7c).[7, 13, 22, 65-69] This remarkable performance stems from the synergistic effect between the increased solubility of the electrolyte and the optimized carbon structure of K1.5_TO, which significantly increases the adsorption capacity and enhances overall redox activity within the carbon pores.
In the self-discharge test, the cell was charged to 1.4 V at 1 A g−1 and then held at open-circuit voltage for various durations (2, 4, 6, 8, 12, and 24 h). After 24 h, the cell retained 56.07% of its energy, using low-cost filter paper as a separator (Figure 7d). This impressive performance is attributed to the effective suppression of cross-diffusion between redox species, enabled by the reversible solid complexation between bromide and PV during charge and discharge cycles.[29] Additionally, in a long-term cycle stability test, the cell retained 72.54% of its energy over 5000 cycles at 2 A g−1 (Figure 7e). In summary, these redox ECs demonstrate exceptional energy density, self-discharge resistance, and cycle stability, achieved by tailoring the porous carbon architecture with the PV/Br redox electrolyte. This performance surpasses that of other reported redox ECs (Table S7, Supporting Information).
3 Conclusion
This study presents a tailored porous carbon architecture designed to enhance energy storage performance in a dual redox system utilizing PV and bromide redox species. The carbon electrode material was synthesized through a simple and effective precursor engineering strategy that integrates a TO step with conventional chemical activation. The resulting carbon features a unique multi-scale pore structure with a substantial mesopore volume while achieving a specific surface area exceeding 3000 m2 g−1 typically associated with micropore-dominant carbons. Comprehensive analyses, including microscopy imaging, pore structure characterization, and thermogravimetric analysis, reveal that meso- and macropore development during the TO step significantly accelerates carbon activation. These hierarchical pores offer accessible pathways for K2CO3 and KOCN to interact extensively with the carbon matrix, generating abundant activation sites that facilitate the formation of 2–10 nm mesopores. This strategy effectively mitigates the conventional trade-off between mesopore development and micropore collapse. Notably, the optimized K1.5_TO carbon material exhibits more than a threefold increase in mesopore volume relative to K1.5, with only a 30% reduction in micropore volume.
By controlling the pore structure through optimized synthesis parameters, the K1.5_TO electrode harnessed the redox capacity of PV without compromising bromide reactivity, as demonstrated in asymmetric cell experiments. In symmetric cells, the dual redox system achieved an energy density 63% higher with K1.5_TO than with K1.5 using 1 m PV and 3 m NaBr. Under conditions with 1.5 m PV and 3 m NaBr, where the effect of PV reactivity was further amplified, the K1.5_TO electrode reached a record-high energy density of 125 Wh kg−1 at 1.4 V and 0.1 A g−1, maintaining stable energy retention of 72.54% over 5000 cycles at 2 A g−1. These findings underscore the critical importance of designing carbon pore structures to optimize the utilization of redox-active species. Furthermore, this resource-saving pore-engineering approach establishes a valuable framework for advancing next-generation, high-performance energy storage systems using porous carbon electrodes.
Acknowledgements
J.G.K. and Y.H.C. contributed equally to this work. This research was supported by the “National Research Foundation of Korea (NRF)” grant funded by the Korean government (MSIT) (NRF-2022M3J1A1085379) and the “National Research Foundation of Korea (NRF)” grant funded by the Korean government (MSIT) (RS-2024-00347887).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




