Fast Near-Infrared Photodetectors Based on Nontoxic and Solution-Processable AgBiS2
Abstract
Solution-processable near-infrared (NIR) photodetectors are urgently needed for a wide range of next-generation electronics, including sensors, optical communications and bioimaging. However, it is rare to find photodetectors with >300 kHz cut-off frequencies, especially in the NIR region, and many of the emerging inorganic materials explored are comprised of toxic elements, such as lead. Herein, solution-processed AgBiS2 photodetectors with high cut-off frequencies under both white light (>1 MHz) and NIR (approaching 500 kHz) illumination are developed. These high cut-off frequencies are due to the short transit distances of charge-carriers in the ultrathin photoactive layer of AgBiS2 photodetectors, which arise from the strong light absorption of this material, such that film thicknesses well below 120 nm are sufficient to absorb >65% of NIR to visible light. It is also revealed that ion migration plays a critical role in the photo-response speed of these devices, and its detrimental effects can be mitigated by finely tuning the thickness of the photoactive layer, which is important for achieving low dark current densities as well. These outstanding characteristics enable the realization of air-stable, real-time heartbeat sensors based on NIR AgBiS2 photodetectors, which strongly motivates their future integration in high-throughput systems.
1 Introduction
Near-infrared photodetectors (NIR PDs) have been gaining increasing attention over the past decade, owing to the increasing needs in automotive vehicles, smart phones, augmented reality, machine vision, biometric monitoring, and imaging.[1-4] Many high throughput applications, such as optical communication[5] and computed axial tomography,[6] also require NIR PDs with fast photo-response. The speed of the photo-response of PDs is usually characterized by the cut-off frequency (f–3 dB), which is highly associated with the rise and fall times of PDs under pulsed light excitation.
In the wavelength range between 850–1100 nm, which is crucial for LiDAR (light detection and ranging) technology[7] and biomedical imaging,[8, 9] silicon-based PDs dominate the current market due to their low cost and easy integration. However, silicon-based PDs are usually bulky and require sophisticated manufacturing processes, making them less attractive as portable or wearable devices. On the other hand, organic materials,[10-12] lead-halide perovskites,[7, 13] and chalcogenide quantum dots (QDs)[14-16] have demonstrated higher absorption coefficients in the NIR region than silicon, and their solution processability at low temperatures enables them to be compatible with polymer substrates, which are ideal for flexible and lightweight electronics. Nevertheless, NIR PDs based on these materials also face various challenges. For example, the performance of NIR organic PDs is limited either by the low charge-carrier mobility or high non-radiative recombination losses,[17] while perovskite PDs are not stable in air and encapsulation is usually needed. Additionally, heavy metal contamination to the environment (e.g., Pb or Cd pollution) remains a concern for PDs based on metal-halide perovskites (e.g., Pb-Sn perovskites) and chalcogenide QDs (e.g., PbS and CdTe QDs) used for NIR photodetection. Indeed, the usage of Pb and Cd in consumer electronics is regulated in many jurisdictions, such as by the EU Restriction of Hazardous Substances (RoHS) directive to up to 0.1 wt.% for Pb and 0.01 wt.% for Cd.[18] Apart from these challenges, another limiting factor is that very few of the above solution-processed PDs could achieve cut-off frequencies exceeding 300 kHz under visible light illumination, and it is even rarer in the NIR region (Table S1, Supporting Information). So far, to our knowledge, cut-off frequencies exceeding 300 kHz in the NIR region have only been reported in PDs based on toxic PbS QDs and a small handful of organic materials.[19, 20] The limited options for fast, solution-processable NIR PDs could be a potential hindrance in high-speed applications, such as optical communication and data transfer.
AgBiS2 is composed of RoHS-compliant elements with sufficient availability for commercial applications.[21] It has emerged as a promising absorber for photovoltaics, especially due to its high absorption coefficients exceeding those of conventional inorganic thin film materials.[22-24] As a result, efficient photovoltaics are achievable using only 30 nm thick absorber layers.[23] Surprisingly, although the bandgap of AgBiS2 (≈1.0–1.3 eV) is also suitable for NIR light detection, there are very few investigations into AgBiS2 NIR PDs. Some early studies have demonstrated that a high cut-off frequency in the visible region (630 nm wavelength) and a high specific detectivity D* in the NIR region could be achieved in PDs based on solution-processed bulk thin films of AgBiS2[25] and AgBiS2 nanocrystals (NCs),[26, 27] respectively (Table S1, Supporting Information). Nevertheless, AgBiS2 PDs that simultaneously exhibit outstanding photodetection along with a fast response in the NIR range have not yet been realized. It is important to note that fast responses in the UV or visible wavelength ranges cannot guarantee the same performance in the NIR range, as recently shown by Kang et al.[27]
With escalating demand for ultrafast NIR technology over the past decade, developing cost-effective, high-performance and solution-processable NIR PDs that are fully compliant with regulations for consumer electronics are urgently needed. In this work, we develop PDs that fully address these requirements based on colloidal AgBiS2 NCs. We hypothesized that the thickness of the photoactive layer is a critical parameter to achieve optimal performance. Thus, we finely tuned the NC film thickness through layer-by-layer (LBL) deposition from 20 to 115 nm and determined the effect on the external quantum efficiency, dark current density and noise current. Having established devices giving high responsivities and specific detectivities, we sought to understand the influence of thickness on the response speed of the PDs under white light or NIR (940 nm wavelength) illumination. We rationalized the results obtained by analyzing the drift lengths, transient responses, and transit times. From these measurements, we identified ion migration as a hidden factor affecting the transient response of AgBiS2 PDs. Furthermore, we quantified the activation energy barrier for ion migration, and how it influences PD performance as a function of AgBiS2 layer thickness. Finally, we demonstrated the practical applications of AgBiS2 PDs by testing them as real-time heartbeat sensors operating without encapsulation in ambient air.
2 Results and Discussion
2.1 Photodetector Performance of AgBiS2 PDs in the NIR Region
The AgBiS2 NCs used in this work were synthesized through a modified hot-injection process[22] (see the Experimental Section for details), and they had a mean size of 4 ±1 nm (Figure S1a, Supporting Information). These AgBiS2 NCs have a rocksalt crystal structure, with both Ag+ and Bi3+ randomly occupying the same crystallographic lattice site. Drop-cast AgBiS2 NCs could retain their phase and visual appearance in ambient air with relative humidity ranging from 60–70% for over a month (Figure S2, Supporting Information), suggesting their high air stability.
Herein, AgBiS2 PDs were fabricated using an established n-i-p photodiode architecture[22, 24] (Figure 1a), where ZnO and PTB7/MoOx were adopted as the electron (ETL) and hole transport layers (HTL), respectively. The energy levels of the ETL and HTL were acquired from Kelvin probe (KP) and photoelectron yield spectroscopy (PYS) measurements[28] (Figure S12, Supporting Information). As displayed in Figure S12c (Supporting Information), although photo-excited holes could be extracted effectively, a small barrier might be present between AgBiS2 and the ETL, which will be discussed in Section 2.4. Ligand exchange treatment with tetramethylammonium iodide (TMAI) in methanol solution enabled the use of the LBL method to fabricate PDs with controlled photoactive layer thickness (see details in the Experimental Section). A representative secondary electron microscopy (SEM) image of the cross-section of a complete PD is shown in Figure S3 (Supporting Information), where we can see the well-defined ETL, photoactive layer, and Ag electrode, verifying the uniformity of each layer over a large length scale. Our TMAI-treated AgBiS2 films showed a bandgap of 1.2 eV (Figure S4b, Supporting Information) along with high absorption coefficients from the UV to NIR region, which exceeded 104 cm−1 for wavelengths below 1200 nm, and exceeded 105 cm−1 for wavelengths below 775 nm (Figure S4, Supporting Information), making them suitable for NIR photodetection.
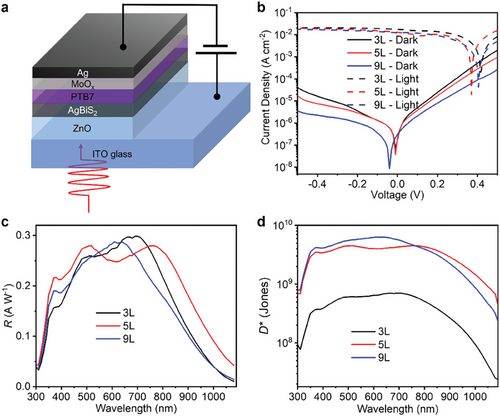
To achieve high-performing PDs, the number of photoactive layers (i.e., TMAI-treated AgBiS2 NC films) was tuned from 2 to 9 to reduce the dark current density JD. Herein, JD was only monitored at reverse biases smaller than 0.5 V (i.e., –VA ≤ 0.5 V). Although a larger reverse bias (−1–−10 V) is commonly used in commercial silicon PDs in order to extract charge-carriers out of their thick photoactive layers (hundreds of micrometers), it is not necessary for AgBiS2 PDs because ultrathin photoactive layers (<120 nm) are sufficient for absorbing >65% of visible and near-infrared light (wavelengths <800 nm). Being able to operate these devices under smaller applied biases is advantageous for applications in wireless sensors as part of the Internet of Things, where reducing external power requirements is critical for these autonomous devices. The JD–V curves of AgBiS2 PDs with 2–9 photoactive layers deposited (denoted as 2L–9L) are shown in Figure S5a (Supporting Information), where we can see that there is an overall decrease in JD with an increase in the number of the photoactive layers deposited. At the same time, AgBiS2 PDs with different photoactive layers exhibited distinct external quantum efficiency (EQE) spectra (Figure S5b, Supporting Information), possibly caused by different optical interference effects. To compare the capability of these AgBiS2 PDs in terms of NIR photodetection, the EQE values at 940 nm wavelength, and the JD values at −0.5 V, are simultaneously shown in Figure S5c (Supporting Information), where we can clearly see that the highest EQE and the lowest JD value, which are both desired for ideal NIR PDs, were not obtained from the same thickness of photoactive layer. Therefore, to investigate how the photoactive layer thickness affects PD performance, AgBiS2 PDs with 3, 5, and 9 photoactive layers (denoted as the 3L, 5L, and 9L AgBiS2 PD) were characterized in detail, as will be discussed below. We note that in order to make direct comparisons between these devices, the operational conditions used (i.e., light sources and applied biases) were all the same for these different measurements. Herein, 5L and 9L AgBiS2 PDs account for the devices showing the highest EQE values in the NIR region and the lowest JD, respectively, while 3L AgBiS2 PDs account for the thinnest devices among the three types as a reference.
The representative current density–voltage (J–V) curves of the AgBiS2 PDs in the dark and under 1-sun illumination are displayed in Figure 1b, where we can see a JD value of 4.1 × 10−5, 1.8 × 10−5, and 0.37 × 10−5 A cm−2 at −0.5 V for the 3L, 5L, and 9L PD, respectively. This decrease in JD with increasing AgBiS2 NC film thickness was possibly caused by a decrease in shunt pathways. We therefore estimated the shunt resistance Rshunt of the devices from the reciprocals of the first derivative of the JD–V curves at 0 V (Figure S6a, Supporting Information). Despite the large variation between devices, we could still observe an overall enhanced Rshunt in thicker PDs. As shown in Figure S6b (Supporting Information), the average Rshunt value of the 3L, 5L, and 9L PD is 0.22 ± 0.08, 0.61 ± 0.17, and 0.76 ± 0.17 MΩ cm2, respectively. While we cannot make a like-for-like comparison in the JD values with other reports due to the different measurement conditions used by different groups, the JD values achieved with AgBiS2 PDs at 0.5 V applied bias are within the range of strongly-performing NIR organic,[10, 29] lead-halide perovskite[30, 31] and PbS QD PDs[32, 33] (see Table S1, Supporting Information). Figure 1c shows the responsivity (R) spectra of these devices. Since R is proportional to the EQE value, it is not surprising that the 5L PD would display higher R values in the NIR region (750–1080 nm) with a peak value of 0.28 A W−1 at 770 nm.
In Equation 1, q is the elementary charge, iD the static dark current, k the Boltzmann constant, T the temperature, and Rshunt the shunt resistance. In practice, other sources of noise could contribute to in as well. Therefore, using Equation 1, or even only, which is also widely used in the literature,[32, 36] would overestimate D* values, as depicted in Figure S8 (Supporting Information). To avoid such an overestimation, we determined the in spectra of the devices by performing a Fast Fourier Transform (FFT) on the time evolution of the dark current (details in the Experimental Section). As shown in Figure S7a (Supporting Information), thicker devices tended to show lower in values, mainly due to their lower dark currents. The in spectra for all of the devices were invariant with frequency, indicating that flicker noise, which is usually ascribed to the random trapping and de-trapping processes,[37] does not play a significant role in these devices. The calculated in values based on Equation 1 for all devices are displayed as horizontal lines in Figure S7a (Supporting Information), and it can be seen that all of the measured in were at least an order of magnitude higher than the values calculated from Equation 1. This result again verifies the importance of direct noise current measurements for precise D* determination. The tendency of the wider field working on solution-processed PDs to assume that the noise current only comes from shot noise, or a combination of shot and thermal noise, rather than directly measuring the noise current makes a comparison of D* values between different reports difficult, with many previous reported values being over-estimated (see footnote to Table S1, Supporting Information).
The D* spectra of AgBiS2 PDs were determined by their R spectra and the directly-measured in values, as shown in Figure 1d. Herein, the in value was extracted from the high-frequency region (>1 kHz) of the in spectrum for each device biased at −0.5 V (Figure S7a, Supporting Information), which is estimated to be 8.9 × 10−11, 1.3 × 10−11, and 9.5 × 10−12A Hz−1/2 for the 3, 5, and 9L PD, respectively. The 9L PD showed the highest D*, with values over 109 Jones in the region below 780 nm wavelength due to its lowest in value, which is consistent with the lowest JD value occurring in the 9L PD. However, the higher R values in the NIR region of the 5L PD enabled it to exhibit the highest D* values (between 7 × 108 and 4 × 109 Jones) for wavelengths > 780 nm. Additionally, the peak D* values of each PD (at 710, 770, and 620 nm wavelength for the 3L, 5L, and 9L PD, respectively) calculated based on the in values at different frequencies are also displayed in Figure S7b (Supporting Information), where we can see that the D* values are almost frequency-independent. This frequency independence further verifies the validity of the D* values reported in our work.
The linear dynamic range (LDR) and film morphology were also characterized for our AgBiS2 PDs. Under 940 nm wavelength illumination, the 3L, 5L, and 9L PDs biased at −0.2 V could reach LDR values of 52, 68, and 65 dB, respectively (Figure S9a, Supporting Information). The slight decrease in the LDR value for the 3L device mainly resulted from the increased JD values, which determined the lower limit of LDR. On the other hand, the AgBiS2 PDs biased at −0.5 V showed a slightly sub-linear variation in photocurrent with light intensity (Figure S9b, Supporting Information), making the acquired LDR values less reliable. Such sub-linearity is possibly affected by ion migration taking place in AgBiS2, which will be discussed in Section 2.4.
In addition, the morphology of the TMAI-treated AgBiS2 films with different thicknesses deposited on top of the ZnO layer was characterized by atomic force microscopy (AFM). As displayed in Figure S10 (Supporting Information), all of the AgBiS2 films showed a compact surface with a small root-mean-square (RMS) roughness (3.7, 3.5, and 3.3 nm for the 3, 5, and 9L AgBiS2 films, respectively). Although smaller roughness values might be observed in thicker films, we emphasize that the present roughness values achieved in all of the samples are comparable to or even smaller than those reported in PbS QD (≈0.5–5 nm),[38, 39] organic (≈0.5–10 nm),[10, 40] and lead-halide perovskite films (≈10–50 nm),[41-43] suggesting that film morphology is not limiting the performance of these devices.
2.2 Fast Photo-Response of AgBiS2 PDs
In Equation 2, I0 is the photocurrent measured under continuous-wave (CW) light illumination. As the damping of the photocurrent drops to −3 dB, the associated frequency is f−3dB. Figure 2a displays the damping measurements for AgBiS2 PDs excited by a 940 nm LED driven by sinusoidal voltages at different frequencies, where we can see the high f−3dB values of 244, 496, and 399 kHz for the 3L, 5L, and 9L PDs, respectively. We note that when PDs are illuminated by visible or UV light, they usually display higher f−3dB values,[25, 30, 31] which might be due to higher diffusion currents as higher excess charge-carrier densities are created near the surface or increased filling of traps due to the higher power densities achieved in the shorter wavelength range. Indeed, this was also the case for the AgBiS2 PDs under white light (400–800 nm wavelength) illumination, where f−3dB values could reach 1.20, 1.44, and 1.35 MHz for the 3L, 5L, and 9L devices, respectively. These cut-off frequencies are not only the highest values for AgBiS2 PDs, but they also exceed most solution-processed NIR PDs, as can be seen in Table S1 (Supporting Information).

2.3 Rationalizing the Fast Response of AgBiS2 PDs
In Equation 3, V is the applied voltage across the two electrodes of the photodiode, d the thickness of the photoactive layer, and µe and µh the electron and hole mobilities, respectively. In most solution-processed NIR PDs, d is at least a few hundreds of nanometers in order to achieve sufficient light absorption,[7, 15, 36] and their f−3dB values would thus be limited by the long transit distances required. In addition, organic PDs suffer from low or unbalanced charge-carrier mobilities,[29, 44] which could further lower their f−3dB values, since charge-carrier transport will be limited by the smallest charge-carrier mobility. By contrast, the high absorption coefficients of AgBiS2 NCs enable the fabrication of ultrathin devices here. As determined from AFM measurements, the AgBiS2 film thicknesses in the 3L, 5L, and 9L PDs were only 19 ± 3, 38 ± 4, and 115 ± 9 nm, respectively. Furthermore, calculations also predicted the comparable effective masses of electrons and holes (the hole effective mass mh* is approximately only double the electron effective mass me*)[45, 46] in AgBiS2, which could also be beneficial for achieving higher f−3dB values by having relatively balanced electron and hole transport.
In Equation 4, vdrift is the drift velocity. From time-resolved microwave conductivity (TRMC) measurements,[47] the electron and hole diffusion lengths of TMAI-treated AgBiS2 NC films have been reported to be ≈60 and 150 nm, respectively.[47] Considering the AgBiS2 PDs with different photoactive layers biased at −0.5 V, and assuming no screening of this field within the device, a Ldrift value of at least 3516, 1758, and 611 nm (for holes) could be estimated for the 3L, 5L, and 9L devices, respectively. We can see that all of the Ldrift values obtained from Equation 4 are substantially larger than the corresponding photoactive layer thicknesses in each device. Therefore, most photo-generated charge-carriers would be quickly swept across the photoactive layers under bias even in the thickest 9L AgBiS2 PD. It has been reported in a PbS QD/metal Schottky diode, the response speed of the diode could be significantly improved when photo-generated charge-carriers were transported mainly by drift.[20] We note that a similar condition could be met in our AgBiS2 PDs, where the thin photoactive layers were all included in the depletion regions so that drift transport would dominate and lead to a fast response.
| Device | trise/tfall [µs] | f−3dB [Hz] | 1/RtC [Ω−1 F−1] | ttr [µs] |
|---|---|---|---|---|
| 3L | 5.2/8.3(940 nm);3.7/3.6(white light) | 244 k(940 nm);1.20 M(white light) | 8.50 × 104 | 2.3 (940 nm);0.5 (white light) |
| 5L | 1.9/1.9(940 nm);2.6/2.5(white light) | 496 k(940 nm);1.44 M(white light) | 2.71 × 104 | 1.1 (940 nm);0.4 (white light) |
| 9L | 2.1/2.5(940 nm);3.0/3.7(white light) | 399 k(940 nm);1.35 M(white light) | 2.05 × 104 | 1.4 (940 nm);0.4 (white light) |
In Equation 5, A0 is a constant typically equal to ≈3.5 and RtC refers to the effective resistance-capacitance product of the overall experimental system. The series resistances of the AgBiS2 PDs could be estimated from the reciprocals of the slopes of the photocurrent density–voltage curves (Jph–V curves) at open-circuit (dashed lines in Figure 1b), and the effective resistance of the experimental system was then estimated by the summation of the series resistance of each device and the connected resistance within the oscilloscope (50 Ω). The C values were directly measured from the high-frequency range of the capacitance-frequency plots (Figure S11, Supporting Information). The RtC values for different AgBiS2 PDs are listed in Table 1, from which we can calculate the transit time ttr.
As can be seen from Table 1, the calculated ttr values were close to the trise/tfall values under 940 nm light illumination, suggesting that the device response times were mainly determined by the charge-carrier transit times through the devices. On the other hand, under white light illumination, the trise/tfall values tended to be slightly longer than the calculated ttr values for each device. Indeed, smaller trise/tfall values were initially expected to be reached under white light illumination (larger f−3dB values) and also in thinner devices (larger drift velocities for charge-carriers), both of which were not the case, as can be seen from Table 1. These unusual phenomena along with the slightly sub-linear variation in photocurrent with illumination intensity when biased at −0.5 V (Figure S9b, Supporting Information) imply the influence of a hidden factor, ion migration, might occur in AgBiS2 devices. Here, we propose that ion migration is facilitated by a stronger electric field (e.g., in thinner PDs or PDs biased at larger reverse voltages), or higher light intensity from the white light source, which could potentially induce a large charge-carrier gradient and hence drive ions migration more easily.[50, 51] It is therefore important to understand the role of ion migration in AgBiS2, which is examined in the next section.
2.4 Ion Migration in AgBiS2
Ion migration has been observed in several perovskite and PbS QD-based devices,[53, 54] where mobile cations or halide ligands could easily move when driven by the external electric field. In order to verify whether ion migration could also occur in AgBiS2, temperature-dependent transient dark current measurements (see details in the Experimental Section) were performed here. At higher temperatures, more ions will be thermally activated[56] and accumulated near the electrodes, which would gradually screen the electric field between the two electrodes. As a result, the transient dark currents excited by voltage pulses would decay faster when the external field was screened more by the built-in field from the accumulated ions.
To simplify the analyses of ion migration in AgBiS2, electron-only devices (architecture: ITO/ZnO/AgBiS2/PCBM/Ag) with 3 photoactive layers (3L) were used here. An electron-only device was used because of the n-type nature of TMAI-treated AgBiS2 films, as verified from KP and PYS measurements (Figure S12c, Supporting Information). Figure 3a shows the dark current transients of the electron-only AgBiS2 device excited by high-voltage (4 V) pulses from 200 to 320 K, where we could indeed see the transients decaying faster at higher rates k1 (Table S2, Supporting Information) as temperature was increased. The corresponding Arrhenius plot (analysis details in Note S1, Supporting Information) of AgBiS2 is then displayed in Figure 3b, and an activation energy barrier (Ea) of ≈126 ± 17 meV was extracted. This activation energy barrier is comparable to or smaller than those reported in lead-halide perovskites (typically in the range of 100–300 meV, with some reports of up to 600 meV[53, 54, 56, 57]), where ion migration has been proven to strongly influence their device performance. We thus conclude here that ions could easily migrate in AgBiS2 PDs, and possibly impact the photo-responses more significantly under a stronger electric field (e.g., in the 3L device) or higher light intensity[59] (e.g., under white light illumination).
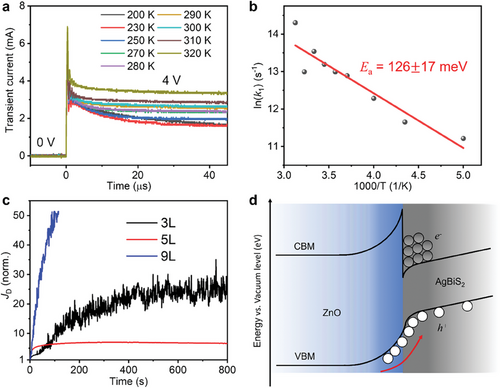
To examine the possible impact of ion migration on the dark current density JD of AgBiS2 PDs over a longer time scale, we tracked the dark current density JD of the 3L, 5L, and 9L AgBiS2 PDs under continuous bias for a few minutes. As shown in Figure 3c, we noticed that the initial JD value of the 3L and 5L devices were enhanced by a factor of 24 and 7, respectively, after 13 min, while the 9L device JD was increased by a factor of 51 after only 2 min. Such an enhancement in JD values suggests ion migration to occur in all the AgBiS2 PDs biased over a long timescale. The accumulated ions at the AgBiS2/transport layer interfaces could induce charge-injection from the electrodes, therefore leading to dark current drift. Such a JD enhancement over long-term biasing was also observed in tetrabutylammonium iodide (TBAI)-treated PbS QDs,[60] and iodide anions have been claimed to be the main source for ion migration owing to their weaker binding energy with the PbS. Considering the similar crystal structure and iodide ligand used for AgBiS2 films, iodide anions may also be the main source of migrating ions in AgBiS2 PDs as well.
In addition, KP and PYS measurements indicated that the ZnO films used in this work had a lower electron affinity than the TMAI-treated AgBiS2 film (Figure S12c, Supporting Information; i.e., negative conduction band offset). This could lead to a contact barrier (type-II heterojunction) at the ZnO/AgBiS2 interface in these devices. Although some electrons may still tunnel through this barrier via the tail of states extending from the ZnO band edges[60] (which are also shown to be present from the broadband photoluminescence emission of the ZnO films; Figure S13, Supporting Information), they are more likely to accumulate at this heterointerface, which could encourage hole injection from ZnO under reverse bias, and hence increase the dark current, as displayed in Figure 3d.
Given that ions are driven by the application of an external field, thinner devices were expected to exhibit more significant ion migration, with a larger increase in JD over time under continuous reverse bias. Therefore, it is not surprising to see a larger JD enhancement and slower photo-response in the 3L device compared to the 5L device (Figure 3). Interestingly, although the 9L device had the smallest JD at −0.5 V (Figure 1b), its JD enhancement is still larger than other devices, suggesting that the applied field is not the only factor facilitating ion migration. As a result, the smallest JD enhancement factor was actually seen from the 5L device. We proposed that such a small JD increase could have benefited from 1) a smaller electric field compared to the 3L device, and 2) a smaller number of defects introduced during the ligand-exchange process compared to the 9L device, which is further explained in Figure S14 (Supporting Information). The small JD enhancement in the 5L device indicates the minor effect of ion migration in this device, which also contributes to its fastest photo-response (Figure 2).
Herein, we show that tuning the thickness of the photoactive layer is an effective strategy to achieve high shunt resistance while minimizing ion migration. The combination of these two factors is crucial for realizing strongly performing and fast NIR PDs. Increasing the number of layers deposited in the LBL process could lead to a larger shunt resistance, and hence lower JD values. However, more defects are also introduced in thicker devices, which can be activated with the application of an electric field, facilitating ion migration. These mobile ions can accumulate via drift at the photoactive layer/transport layer interfaces, which could induce extra charge-carrier injection and result in dark current enhancement. As indicated above, the population of ions accumulated at these heterointerfaces is associated with both the strength of the electric field within the photoactive layer and the concentration of defects. Therefore, ion migration can be suppressed by fine-tuning the photoactive layer thickness in AgBiS2 PDs to simultaneously reach a field strength and defect concentration as low as can be, as demonstrated in Figure S15 (Supporting Information). We note that although charge-carrier extraction is still efficient in all of our devices because of the thin photoactive layers, future efforts should focus on minimizing ion migration in order to achieve faster photo-responses. Based on the insights gained from this work, we also believe that reducing defects by using passivation agents and less polar solvents or changing the band alignment at the AgBiS2/ZnO interface are important areas to target in future efforts.
2.5 Real-Time Heartbeat Monitoring Using AgBiS2 PDs
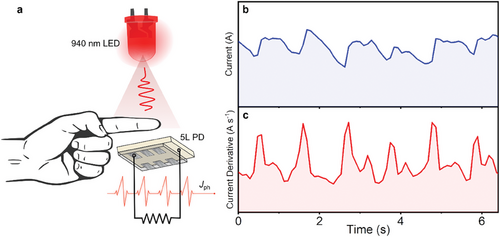
To demonstrate the practical application of AgBiS2 PDs, we performed real-time photoplethysmogram measurements using the 5L device. As shown in Figure 4a, the finger of a volunteer was placed between a 940 nm LED and a 5L AgBiS2 PD. The pulsating bloodstream through the capillaries changed the transmission of 940 nm NIR light from an LED. In the NIR region, not only could light penetrate living tissue efficiently but also the difference in absorbance between oxygenated and deoxygenated hemoglobin will be maximized. As a result, the heartbeat rate of the volunteer could be precisely recorded as electrical signals by the 5L PD, as shown in Figure 4b. A clearer figure could be made when we differentiated the current signals with time (Figure 4c), where we could easily estimate a heartbeat rate of ≈60 beats per minute. Finally, it is worth mentioning that unlike lead-halide perovskite PDs, where JD values might be enhanced by a factor of two after exposure to air for 10 days,[62] the JD value of the unencapsulated AgBiS2 PD was not increased when stored in an ambient environment (relative humidity: 60–70%) over 34 days (Figure S16, Supporting Information). The results here therefore strongly indicate the utility of AgBiS2 in practical applications in air, and this could inspire future studies on using these fast PDs in high-throughput applications.
3 Conclusion
In this work, fast and air-stable NIR PDs that are comprised of fully RoHS-compliant elements have been realized by using AgBiS2 NCs, and the electronic–ionic transport of the AgBiS2 NC films was comprehensively studied. We showed that the fast response of these PDs comes about from the balanced charge-carrier transport and ultrathin photoactive layer, which benefits from the high absorption coefficients of cation-disordered AgBiS2. Consequently, high f-3 dB values over 1 MHz under white light illumination, and approaching 500 kHz in the NIR region, were achieved, which are higher than the values reported in previous reports of AgBiS2 PDs as well as most solution-processed NIR PDs. At the same time, we found a small activation energy barrier of 126 meV in AgBiS2 NC films, showing there to be facile ion migration, which limits how fast the photo-response can be and leads to dark current drift. By carefully tuning the photoactive layer thickness, we could strike a balance between high shunt resistance, strength of the electric field in the photoactive layer, and ion migration, leading to outstanding PD performance. Finally, AgBiS2 PDs were demonstrated as heartbeat sensors under NIR illumination, which shows their great potential for practical applications, including for non-invasive diagnostics and optical communication, and this motivates future efforts on integrating AgBiS2 in high-throughput applications. Our work therefore shows the benefits of these ultrathin light harvesters in terms of achieving high cut-off frequencies at a low reverse bias, which could be advantageous for applications in portable consumer electronics by reducing the requirements of the power supply unit.
4 Experimental Section
Synthesis of AgBiS2 NCs
Bismuth acetate (99%, Alfa Aesar, 1mmol) and 0.8 mmol silver acetate (99.99%, Merck) were dissolved in 10 mL oleic acid (90%, Merck). The solution was degassed at 100 °C in a Schlenk line to remove oxygen and moisture for at least 2 h. The reaction atmosphere was then switched to an Ar line, and 1 mmol hexamethyldisilathiane (HMS) (Synthesis grade, Merck) dissolved in 5 mL 1-octadecene (ODE) (90%, Merck) was quickly injected into the degassed solution. The whole solution was heated at 100 °C under the Ar line for 20 min and then cooled down to room temperature in a water bath. The cooled-down solution was diluted by toluene (90%, Merck) and 1.5 times of acetone (90%, Merck) was added as the anti-solvent. The solution was then centrifuged at 5000 rpm for 3 min. The precipitated AgBiS2 NCs were re-dispersed in toluene and the same purification process was repeated for another two times to ensure non-reacted precursors were completely removed. The purified AgBiS2 NCs were dissolved in toluene and centrifuged at 3500 rpm for 3 min to precipitate larger NCs. The final NC solution was extracted after filtering the supernatant after the final centrifugation step by a 0.22 µm polytetrafluoroethylene (PTFE) filter.
Fabrication of AgBiS2 PDs
ZnO sol–gel solution was prepared by dissolving 1 g zinc acetate (Merck, dihydrate) in 10 mL 2-methoxyethanol (>99%, Merck) and 284 µL ethanolamine (>99%, Merck). The ZnO ETLs were deposited by spin-coating the ZnO sol–gel solution at 3000 rpm for 30 s and heated at 200 °C for 10 min. 40 µL of 20 mg mL−1 AgBiS2 NC solution with toluene as the solvent was spin-coated on ZnO at 2000 rpm for 35 s. 40 µL of 5 mg mL−1 TMAI (99%, Merck) solution with methanol as the solvent was dropped onto the AgBiS2 film and kept there for 40 s to allow solid-state ligand exchange treatment to take place. During this treatment, the long-chain oleate ligands attaching to the surface of the AgBiS2 NCs during synthesis were replaced by short-chain iodides, enabling more efficient charge-carrier transport between the NCs. Afterward, the TMAI-treated AgBiS2 film was washed once individually by methanol and toluene at 2000 rpm for 35 s to remove the residual ligands. The effectiveness of ligand exchange was verified from the Fourier-transform Infrared (FTIR) absorbance spectra of pristine and TMAI-treated films (Figure S17, Supporting Information), where it could see a significant decrease in the intensity of the characteristic carboxylic peaks from oleate ligands in the latter, suggesting that most oleate ligands have been replaced. Thicker AgBiS2 films could then be fabricated layer-by-layer by repeating the above processes (LBL process). Forty microliters of 5 mg mL−1 PTB7 (1-material) solution with 1,2-dichlorobenzene (99%, Merck) as the solvent was spin-coated at the AgBiS2 films at 2000 rpm for 30 s. Finally, 3 nm MoOx and 100 nm Ag were evaporated onto the PTB7 throughout a mask, which defined the device area of 4.5 mm2. AgBiS2 PDs were stored in air overnight before characterization.
Transmission Electron Microscopy (TEM) Measurements
TEM samples were prepared by putting a small drop of NC solution onto the carbon-coated copper grid. TEM images were recorded using FEI Tecnai F20 (200 kV).
Current Density–Voltage (J–V) Measurements
Noise Current (in) Measurements
The in was extracted from the dark current density recorded by a digital oscilloscope (Siglent SDS6054A) with a current amplifier, followed by a fast Fourier transform (FFT).
Responsivity (R) Characterization
R values were measured using an integrated system from Quantum Design PV300.
Transient Current Measurements
Transient current measurements were performed using a digital oscilloscope (Siglent SDS6054A). The PDs were illuminated by either 940 nm or white light LEDs driven by a function generator (ThorLabs DC2200). For determination of the rise and fall time, a series of 5 kHz square voltage pulses were applied to the LEDs using the function generator. For determination of the cut-off frequency, sinusoidal functions with varying frequencies between 100 Hz and 2 MHz were used to drive the LEDs.
Photoplethysmography Measurements
Photoplethysmography measurements were performed by directly connecting the PDs to a Keithley 4200 Source-Measure unit and recording the current as a function of time upon illumination with 940 nm LED driven by a function generator (ThorLabs DC2200).
Impedance Spectroscopy
To record the impedance signal, a STAT-I-400 potentiostat of Metrohm LTD was used. The signal was measured starting from 1 MHz down to 1 Hz over 50 frequency points, using a potential amplitude of 20 mV, under 100 mW cm−2 at open-circuit voltage.
Temperature-Dependent Transient Current Measurements
Square voltage pulses with a voltage amplitude of 4 V and a time width of 50 µs were generated by a function generator (Hewlett Packard 8116A) and applied to the electron-only device connected in series with a resistor (330 Ω). The transient voltage across the resistor could be probed by an oscilloscope (RS PRO RSDS1304CFL) and transferred into a transient current. The device was mounted in a Desert TTP4 Probe Station system, which could control the temperature range from 150 to 360 K via an integrated cryostat in a high vacuum (10−6 mBar). At each temperature, devices were measured after being placed in the Probe Station system for at least 15 min in order to reach thermal equilibrium.
Kelvin Probe (KP) and Photoelectron Yield Spectroscopy (PYS) Measurements
Secondary Electron Microscopy (SEM) Imaging
SEM sample cross sections were cleaved and fixed upright into the chamber. SEM images were recorded using a Gemini 1 Zeiss Sigma 300 with an accelerating voltage of 5 keV.
Atomic Force Microscopy (AFM) Imaging
AFM measurements were conducted using an Agilent Technologies 5500 AFM in tapping mode. Images were analyzed using Gwyddion software.
Acknowledgements
Y.-T.H. would like to thank the Ministry of Education, Taiwan and Downing College Cambridge for funding. N.G. and F.F. would like to acknowledge support from European Commission Research Executive Agency (Grant Agreement Number: 859752 HEL4CHIR-OLED H2020-MSCA-ITN-2019). L.D. thanks the Cambridge Trusts and the China Scholarship Council for funding. Y.-T.H. and R.L.Z.H. acknowledge support from the Silverman Research Fellowship, Downing College Cambridge. S.D.S. acknowledges the Royal Society and Tata Group (no. UF150033) for funding. The work received funding from the European Research Council under the European Union's Horizon 20202 research and innovation programme (HYPERION, no. 756962). R.L.Z.H. would like to thank EPSRC for financial support (grant no. EP/V014498/2), as well as the Royal Academy of Engineering through the Research Fellowships scheme (no. RF∖201718∖17101). M.H. would like to thank EPSRC (no. EP/T028513/1).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Y.-T.H. and D.N. contributed equally to this work. Y.-T.H., N.G., and R.L.Z.H. conceived of this work. Y.-T.H. developed and optimized the synthesis of AgBiS2 NCs, fabricated AgBiS2 devices, and performed XRD, FTIR, stability tests as well as temperature-dependent transient current measurements under the supervision of A.R. and R.L.Z.H. D.N. and F.F. performed PD characterization under the supervision of N.G. Y.Z. assisted the temperature-dependent transient current measurements under the supervision of H.S. M. Rusu and X.G. performed the KP and PYS measurements, under the supervision of T.U. and R.L.Z.H., respectively. L.D. took the TEM images under the supervision of S.D.S. Z.A.-G. performed the PDS measurements. D.D. helped with the cross-sectional SEM measurements. M. Rimmele helped with the AFM measurements, under the supervision of M.H. All authors discussed the results and wrote the paper together.
Open Research
Data Availability Statement
The data accompanying this paper and the supporting information is available open access from the Oxford Research Repository under the DOI: 10.5287/ora-010ynqeyr. https://doi.org/10.5287/ora-010ynqeyr.




