Sintering Metal–Organic Framework Gels for Application as Structural Adhesives
Abstract
Metal–organic frameworks (MOFs)/coordination polymers are promising materials for gas separation, fuel storage, catalysis, and biopharmaceuticals. However, most applied research on MOFs is limited to these functional materials thus far. This study focuses on the potential of MOFs as structural adhesives. A sintering technique is applied to a zeolitic imidazolate framework-67 (ZIF-67) gel that enables the joining of Cu substrates, resulting in a shear strength of over 30 MPa, which is comparable to that of conventional structural adhesives. Additionally, systematic experiments are performed to evaluate the effects of temperature and pressure on adhesion, indicating that the removal of excess 2-methylimidazole and the by-product (acetic acid) from the sintered material by vaporization results in a microstructure composed of large spherical ZIF-67 crystals that are densely aggregated, which is essential for achieving a high shear strength.
1 Introduction
Metal–organic frameworks (MOFs) are promising materials for gas separation,[1] fuel storage,[2] catalysis,[3] and biopharmaceuticals.[4] However, to date, most applied research has focused on the use of MOFs as functional materials for the above-mentioned applications.[5, 6] This is because the MOF bulk materials investigated thus far have significantly inferior mechanical properties, which is the most important factor for their application as structural materials, compared with metals, ceramics, and plastics.[7-9] Therefore, the methods of formation of bulk MOFs have been actively studied recently[10-13] and some studies have reported MOFs with mechanical properties comparable with those of structural plastics.[14-16] However, for the practical application of MOFs as structural materials, they should possess additional unique properties. Thus, nearly no studies have specifically reported the possibility of the practical use of MOFs as structural materials thus far.
This study focuses on the potential of MOFs as structural adhesives for metals. Typically, structural adhesives have several requirements in terms of strength and stability. First, it should adhere well to, and bond strongly with, the metal adherend to prevent interfacial failure. Many studies have reported the formation of dense films or sheets in which MOFs are well adhered to substrates using the layer-by-layer method,[17-19] in situ synthesis,[20-22] sputter/atomic layer deposition,[23] chemical solution deposition,[24, 25] and chemical vapor deposition.[26] These studies have been presented in detailed in literature.[27-30] Moreover, among the organic ligands comprising MOFs, some interact relatively strongly with metals. For example, imidazole and their derivatives, which constitute the zeolitic imidazolate framework (ZIF) series, are known to bond with specific metals,[31, 32] such as Cu that forms Cu-imidazole polymers or Cu coordination complex films, which are corrosion inhibitors in various environments.[33-35] Therefore, it is expected that relatively strong interactions will occur between MOFs containing these organic ligands and metal substrates.
Second, to ensure stability in temperature-fluctuating environments, the thermal expansion coefficient of the adhesive should be similar to that of the metal adherend, so that thermal stress should be suppressed. Conventional polymers used as adhesives have a thermal expansion coefficient that is one or two orders of magnitude larger than that of metals, which causes problems such as reduced strength in environments where temperature is repeatedly applied.[36-38] In contrast, as shown in Figure S1 in the Supporting Information, many MOFs and coordination polymers (CPs) have much smaller coefficients of thermal expansion than ordinary polymers, which are similar to those of metals. This is a significant advantage for their application as structural adhesives compared with conventional polymers.
Third, its bulk form should be rigid to prevent cohesive failure. However, the imposed strength requirements are extremely high. Zhao et al. reported that a colloidal solution containing 2D nanoplates of cyano-bridged CPs was used to fabricate structures with a high cohesive strength, which were suitable for use as general-purpose adhesives.[39] This is of great value because it was the first study to demonstrate the potential application of CP as a structural material; however, the adhesive strength of CP was significantly lower than that required for structural adhesives (≈0.5 MPa).[40] Based on the above considerations for the application of MOFs as structural adhesives for metals, the third requirement, seemingly the most difficult, should be satisfied, while maintaining the first and second requirements.
In this study, a novel adhesive method using ZIF-based MOF gels is proposed to demonstrate these ideas using Cu substrates. Cu joint specimens are fabricated using ZIF-67 gel as an adhesive. For adhesion, a sintering technique with the hot-press method under optimized temperature and pressure condition is used, considering the cohesiveness of the ZIF bulk. Furthermore, the fracture surface and sintering behavior of the ZIF gel are evaluated to elucidate the origin of the adhesive strength.
2 Results and Discussion
2.1 Adhesive Body and Its Mechanical Properties
Chen et al. reported that ZIF-67 films were fabricated using the spray method with ZIF gel at 150 °C without additional pressure and the microstructure was observed to be continuous and smooth by scanning electron microscopy (SEM).[25] Moreover, Atom probe microscopy indicated that it was an aggregate composed of 30–40 nm ZIF-67 crystals.[25] Figure 1a shows the ZIF gel and fabricated Cu joint prepared in this study. The ZIF gel sintered body was composed of the ZIF bulk layer with a thickness of ≈2–3 µm, which was attached to the upper and lower Cu substrates (Figure 1b). Additionally, densely aggregated particles of ≈300–500 nm in size were present in the interior. The observed microstructure was significantly different from that reported by Chen et. al.[25] because the ≈40 nm ZIF-67 nanocrystals in the original gel grew to a diameter of ≈300–500 nm and strongly adhered to each other. This indicates that the applied pressure and variation in temperature to 250 °C causes significant microstructural changes. These effects on the sintering behavior of the ZIF gel are clarified in the subsequent section. Furthermore, the sintered body adhered to the uneven Cu substrate surface well (Figure 1b) and it also had a homogeneous composition as shown in Figure S2 in the Supporting Information. This yielded a Cu joint body with exceptionally high strength exceeding 30 MPa, which is comparable to that of conventional structural adhesives (Figure 1c).[40]
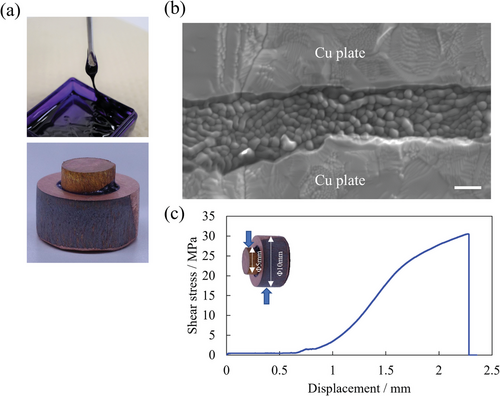
2.2 Fracture Surface Analysis
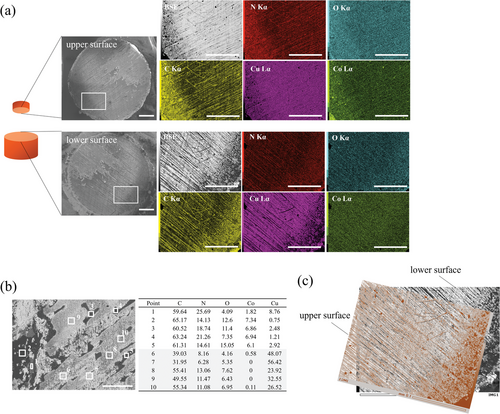
To investigate the mechanism of the high shear strength, SEM-energy dispersive X-ray spectroscopy (EDX) analysis of the fracture surfaces was performed. Figure 2 shows the SEM-EDX results for the fracture surfaces. The backscattered electron (BSE) image shows a representative region of the fracture surface, which can be divided into two regions with distinct brightness levels: 1) the bright region corresponding to the Cu substrate in the ground with almost no Co and 2) the dark region derived from the ZIF with a relatively high Co content (Figure 2a). This is confirmed by the detailed EDX point analysis shown in Figure 2b. Figure 2c shows a superposition of the BSE image of the upper and lower fracture surfaces. The contrast patterns for the upper and lower fracture surfaces coincide, indicating that macroscopic shear failure is governed by cohesive failure in the MOF bulk layer instead of interfacial failure between the ZIF bulk layer and the Cu substrate.
2.3 Characterization of the ZIF Gel
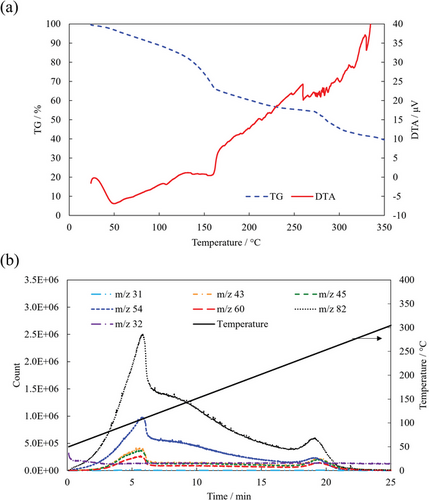
Based on the fractured surface analysis results, it is essential to characterize the ZIF gel and elucidate the sintering behavior, which induces the transformation of the ZIF gel to the ZIF bulk layer, to clarify the mechanism of the exceptionally high adhesion strength observed in this study. First, the ZIF gel is characterized by thermogravimetric/differential thermal analysis (TG/DTA) as shown in Figure 3. A gradual weight loss was observed from room temperature to ≈120 °C owing to the release of the solvent, followed by a large weight loss from 120 °C to ≈150 °C. A slight variation in the weight and exothermic reaction occurred between 200 and 250 °C, indicating that ZIF-67 crystallization occurred in this temperature range. Subsequently, a large weight loss was observed at ≈270 °C, corresponding to the thermal decomposition of ZIF-67 crystals. Figure 3b shows an extracted ion chromatogram of the ions originating from each component obtained by gas chromatography/mass spectrometry (GC/MS), with m/z 54 and 82 corresponding to 2-methylimidazole, m/z 43, 45, and 60 to acetic acid, and m/z 31 and 32 to methanol. The m/z 32 peak was detected at the beginning of heating, corresponding to the release of methanol solvent. Solvent release continued over the entire temperature range. A peak corresponding to 2-methylimidazole and acetic acid was observed at ≈120 °C owing to the vaporization of excess 2-methylimidazole and the by-product acetic acid present in the ZIF gel. This was also observed by TG as significant weight loss (Figure 3a). Subsequently, when the temperature was increased above 150 °C, the vaporization of 2-methylimidazole and acetic acid slowed down, but occurred again at ≈250 °C. The latter temperature corresponds to the observed crystallization of ZIF-67 in TG/DTA (Figure 3a). Above 260 °C, almost no components were detected, except for methanol.
2.4 Sintering Behavior of the ZIF Gel
2.4.1 Effect of Applied Pressure
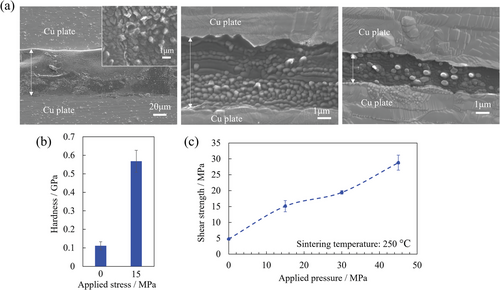
Subsequently, the sintering behavior of the ZIF gel was investigated. Additionally, the effects of applied pressure and temperature, which result in a unique microstructure of the sintered part of the ZIF gel, were evaluated. Cross-sectional SEM images of the sintered part were obtained at applied pressures in the range of 0–30 MPa, as shown in Figure 4a. The film thickness of the sintered part decreased with increasing applied pressure, and the grains changed from a flat to a spherical morphology. The composition was homogeneous at all applied pressures, as shown in EDX maps (Figures S3–S5, Supporting Information). In addition, the shear strength of the joint body linearly increased with the applied pressure (Figure 4c). Additionally, nanoindentation tests were performed on the ZIF bulk layer to elucidate the effect of applied pressure. The hardness of the sintered part at a pressure of 15 MPa was significantly improved compared with the case where no pressure was applied (Figure 4b and Figure S6, Supporting Information), indicating that the hardness of the sintered part increased with the applied pressure. Therefore, it is proposed that the improvement in the shear strength of the joint body was owing to an improvement in the hardness of the sintered part. These results demonstrate that pressure-induced condensation is required to achieve high adhesive strength, as shown in Figure 1c.
The effect of pressure on the sintering behavior of the ZIF gels was further analyzed by thermomechanical analysis (TMA). As shown in Figure 5, compared with pure Cu substrates, rapid shrinkage occurred for the ZIF gels with a discontinuous variation in the displacement curve between 120 and 160 °C at all applied pressures. From the TG/DTA and GC/MS results, this temperature range mainly corresponds to the vaporization of 2-methylimidazole and acetic acid during the sintering process of the ZIF gel. Thus, the discontinuous shrinkage observed in the displacement curve may be attributed to these vaporizations. Moreover, Figure 5 shows that the discontinuous shrinkage increased with increasing applied pressure. Therefore, it is proposed that applied pressure during the sintering of ZIF gels promotes the vaporization of 2-methylimidazole and acetic acid. Thus, considering the enhanced shear strength with increasing applied pressure, as shown in Figure 4c, sufficient vaporization of these substances is essential to obtain a densely aggregated ZIF-67 crystal in the sintered part, which results in a high shear strength of the joint body.
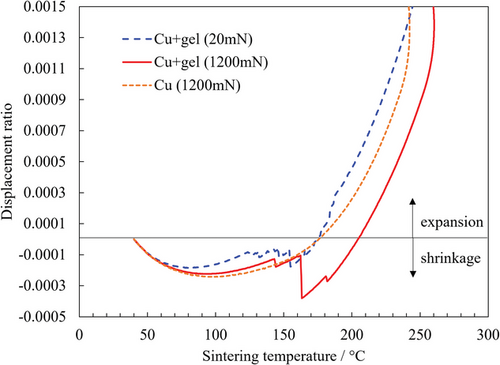
2.4.2 Effect of Sintering Temperature
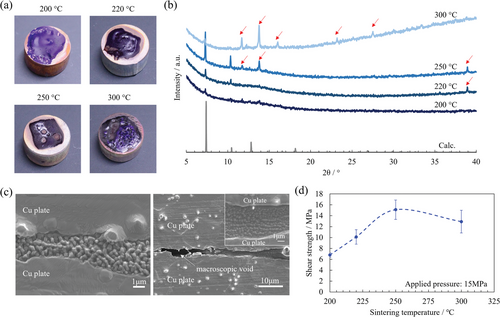
Subsequently, the effect of temperature on the sintering behavior of the ZIF gel was investigated. Figure 6a,b shows the photographs and X-ray diffraction (XRD) patterns of the sintered ZIF gel without pressure, respectively. The surface was relatively smooth when the sintering temperature was 250 °C or lower. The XRD peak intensity of the ZIF-67 crystal increased as the temperature increased to 250 °C, indicating that the crystal growth was progressing, which corresponds with the TG/DTA results (Figure 3a). Figure 6c (left) shows a cross-sectional SEM image of the sintered part at a temperature of 220 °C and an applied pressure of 15 MPa. The crystal growth was insufficient and many voids were observed between the crystal grains compared with the sintered part at 250 °C (central image in Figure 6a). Consequently, the shear strength of the joint body was significantly reduced to ≈10 MPa compared with that at 250 °C (Figure 6d). When the temperature was further increased from 250 to 300 °C, thermal decomposition caused foaming from the surface, which increased the surface roughness (Figure 6a). As observed in the cross-sectional SEM image presented in the inset of the right graphic in Figure 6c, most of the sintered part at 300 °C was similar to that at 250 °C shown in Figure 1b, which exhibited densely aggregated ZIF-67 crystals. However, macroscopic voids of several tens of micrometers were occasionally observed. This is thought to be owing to thermal decomposition. Thus, the shear strength of the joint body at 300 °C was reduced compared with that at 250 °C (Figure 6d). Additionally, another phase was observed in the XRD pattern of the sintered part (Figure 6b), indicated by arrows, which increased as the sintering temperature increased above 250 °C. We could not identify these unknown peaks; however, these peaks were reported in the previous study and might be decomposed substances.[25] The composition of the sintered part was homogeneous even at 220 and 300 °C (Figures S7 and S8, Supporting Information). These results suggest that to obtain a joint body with high shear strength, it is essential to reduce the amount of excess solvent and by-products and to form a densely aggregated structure of relatively large ZIF-67 crystals throughout the sintered part. Thus, the formation of this type of structure at a sintering temperature of 250 °C is proposed to contribute to the high shear strength of the joint body shown in Figure 1c.
2.4.3 Effect of Substrates
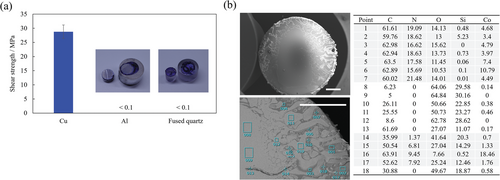
As mentioned previously, the exceptionally high adhesion strength obtained in this study is owing to the ZIF bulk layer and the previous sections have clarified the transformation of the ZIF gel to the bulk layer. Moreover, the failure mode for all the samples investigated in this study was cohesive failure. However, this implies that the interfacial strength between the ZIF bulk layer and the Cu substrate is higher than the cohesive strength of the ZIF bulk layer. Therefore, it is also crucial to clarify the origin of this high interfacial strength. As shown in Figure 7a, the adhesion strength of the ZIF gel exhibited a strong substrate dependence with a significantly lower shear strength observed for substrates such as Al and fused quartz under the same process conditions. Additionally, as shown in Figure 7b, SEM-EDX analysis of the fractured surface of the fused quartz substrate showed that the regions without Co correspond to those without N. However, for the Cu substrate shown in Figure 2b, N was clearly detected, even in the region corresponding to the Cu substrate where Co was not detected. As mentioned previously, imidazole and its derivatives are known to bind strongly to Cu substrates, yielding Cu-imidazole polymers and Cu-coordinated complex films.[33-35] Therefore, the detection of only N on the Cu substrates may indicate that the excess 2-methylimidazole in the ZIF gel formed the Cu polymer, Cu-coordinated complex, or was bound to the Cu substrate directly, thereby functioning as a bridging agent between the ZIF bulk layer and the Cu substrate, which resulted in higher shear strength only with Cu substrates. X-ray photoelectron spectroscopy (XPS) analysis of the fracture surface was performed to detect their coordination bonds. However, they were not detected, probably because the N of 2-methylimidazole were coordinated to Co in ZIF-67 and Cu at the interface, and thus it was difficult to distinguish between the two. Therefore, XPS analysis did not confirm the bond between N and Cu, as shown in Figure S9 in the Supporting Information. Thus, the origin of the high interfacial strength between the Cu substrate and the ZIF bulk layer should be clarified in the future using a different technique. In this study, ZIF-gel selectively adhered to Cu substrates; however, the appropriate selection of the MOF linker may realize suitable adhesion to other substrates.
3 Conclusions
This study focused on the potential application of MOFs as structural adhesives. The ZIF-67 gel sintering technique enabled the joining of Cu substrates with a shear strength exceeding 30 MPa. Fracture surface analysis revealed that this high strength was determined by the strength of the ZIF bulk layer formed by the sintering of the ZIF gel. Moreover, systematic experiments on the effects of temperature and pressure during adhesion revealed that the removal of excess raw material (2-methyimidazole) and the by-product (acetic acid) from the sintered part by vaporization yielded a microstructure with densely aggregated, large spherical ZIF-67 crystals, which is essential to achieve high shear strengths. As for the reason why the interface strength between ZIF bulk layer and the Cu substrate is achieved such that it exceeds the internal strength of the ZIF bulk layer, the results of fracture surface analysis suggested that excess 2-methylimidazole forms a Cu-imidazole polymer or Cu coordination complex on the Cu substate surface, which may serve as a strong bridge between the ZIF bulk layer and the Cu substrate, but this hypothesis has not been verified in the experiments of this paper. This needs to be clarified by future studies. In addition, the thermal expansion behavior of the thin adhesive shown in this paper also needs to be clarified in future studies, as it is important to clarify the thermal expansion behavior for the reliability of the joints in environments with fluctuating temperatures.
The strategy proposed in this study might be applied to other MOF gels and substrates with each-optimized sintering temperatures and pressure, and this is a subject in future work. This study demonstrates for the first time that MOFs, which have typically been investigated as a functional material, have excellent potential as practical structural adhesives for the first time. Thus, it is expected to have a wide impact on the scientific and industrial communities.
4 Experimental Section
Materials
Cobalt acetate tetrahydrate (≥99.0%), 2-methylimidazole (≥98.0%), and methanol were purchased from Fuji Film Wako Pure Chemical Industries Ltd. (Japan) and used as received.
Synthesis of the ZIF Gel
For the ZIF gel, a gel composed of a ZIF-67 precursor was used to achieve an interaction with a Cu substrate. The gel was prepared following the method described by Chen et al.[25] Cobalt acetate (0.6227 g) was dissolved in 25 mL of methanol and 2-methylimidazole (0.821 g) was added and stirred overnight at 27 (room temperature). Subsequently, a small amount of sediment was filtered and the resulting colloidal solution was air-dried at room temperature for 48 h. Consequently, a nonflowing clay-like gel was obtained (Figure 1a), which was formed by the loose aggregation of ZIF-67 nanocrystals with a radius of ≈40 nm, mainly owing to van der Waals forces.[10] The gel crystallizes into the ZIF-67 bulk upon heat treatment.[25]
Characterization of the ZIF Gel
TG/DTA was performed in air from room temperature to 350 °C at a heating rate of 5 °C min−1 using a Rigaku D-DSC8230/TG8120IRH instrument. For the evolved gas analysis-mass spectrometry method, a gas chromatography time-of-flight mass spectrometry JEOL JMS-T100GCV instrument and Frontier Laboratories PY-3030D were used. Heating was performed from 50 to 300 °C at a heating rate of 10 °C min−1 in a He atmosphere. TMA was performed in an Ar flow using a Seiko Instruments EXSTAR TMA/SS6100 instrument with the above-mentioned adhesion test specimen. The heating rate was ≈80 °C min−1, and the pressure was 20 or 1200 mN. The displacement was based on the length of the specimen at 40 °C and the ratio of the displacement to the initial length was calculated. Notably, a positive displacement value represents expansion and negative displacement value represents contraction.
Adhesion
Adhesion was performed by sintering the ZIF gel between Cu substrates. Oxygen-free Cu (Ra = 0.8) substrates with dimensions of φ10 × t5 mm and φ5 × t2 mm were used as the adherend. The adherend was subjected to ultrasonic cleaning with acetone (10 min), soaked in 7% dilute hydrochloric acid (30 s), and dehydrated with acetone (3 min) prior to adhesion. A small hot-press device was used for adhesion. The ZIF gel was applied onto a φ10 copper substrate on the lower die, on which a φ5 copper substrate was placed. Adhesion was performed at a temperature of 250 °C and compression pressure of 45 MPa for 300 s. Additionally, to evaluate the effects of temperature and applied pressure on the sintering of the ZIF gel, additional experiments were conducted under several temperature and pressure conditions.
Characterization of Sintered Parts of the Joint Body
XRD patterns were recorded on a Rigaku Ultima IV with CuKα radiation using a step size of 0.02° and scan speed of 10° min−1. SEM images were obtained using a JEOL JSM-7000F instrument at an accelerating voltage of 5 kV and energy-dispersive X-ray spectroscopy (EDX) was performed using a Hitachi High-Tech Regulus8230 instrument. To prepare a sample for cross-sectional analysis by SEM/EDX, the adhesive was embedded in epoxy resin and emery paper was used to level the surface of the observation layer. Subsequently, ion milling was performed using a Hitachi High-Tech Co., Ltd. ArBlade 5000 instrument at an acceleration voltage of 5 kV and discharge voltage of 1.5 kV for 17 min. To suppress the influence of heat generation, ion-beam irradiation was performed during ion milling by repeated cycles of irradiation and rest for 30 s each. A Hygitron TI1900 instrument was used for the nanoindentation tests. The device was housed in an isolation cabinet to prevent thermal fluctuations and acoustic interference. The maximum load was 200 µN using a Berkovich diamond indenter with a radius of 150 nm and total angle of 142.35°. Measurements were performed 9 or 11 times and average values were obtained.
Shear Test
The shear test was performed at a stroke speed of 1 mm min−1 using the adhesive test specimen described above. The test was performed using a device created by combining a force gauge and electric stage. For each condition, the test was performed three times, and the average value was obtained.
Acknowledgements
The authors thank Dr. Kazuhiko Umemoto, Dr. Yuka Yamada, and Dr. Atsushi Miura at Toyota Central Research and Development Labs. Inc. for fruitful discussion. The authors thank Dr. Taiki Kano at Toyota Central Research and Development Labs. Inc. for technical advice on nanoindentation. The authors thank Dr. Akiko Nozaki and Dr. Shin-ichi Towata at Aichi Synchrotron Radiation Center for technical advice on XRD.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
I.M., M.U., S.M., A.S., and H.T. conceived the project. I.M., A.O., N.T., and Y.M. conducted the experiments. I.M., M.U., and S.M. wrote the paper. All authors discussed the results and have given approval to the final version of the paper.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




