Migration Kinetics of Surface Ions in Oxygen-Deficient Perovskite During Topotactic Transitions
Abstract
Oxygen diffusivity and surface exchange kinetics underpin the ionic, electronic, and catalytic functionalities of complex multivalent oxides. Towards understanding and controlling the kinetics of oxygen transport in emerging technologies, it is highly desirable to reveal the underlying lattice dynamics and ionic activities related to oxygen variation. In this study, the evolution of oxygen content is identified in real-time during the progress of a topotactic phase transition in La0.7Sr0.3MnO3-δ epitaxial thin films, both at the surface and throughout the bulk. Using polarized neutron reflectometry, a quantitative depth profile of the oxygen content gradient is achieved, which, alongside atomic-resolution scanning transmission electron microscopy, uniquely reveals the formation of a novel structural phase near the surface. Surface-sensitive X-ray spectroscopies further confirm a significant change of the electronic structure accompanying the transition. The anisotropic features of this novel phase enable a distinct oxygen diffusion pathway in contrast to conventional observation of oxygen motion at moderate temperatures. The results provide insights furthering the design of solid oxygen ion conductors within the framework of topotactic phase transitions.
1 Introduction
Complex oxides have been at the core of cutting-edge information, energy, and environmental technologies owing to their versatile functionalities, such as various forms of magnetism, superconductivity, ferroelectricity, and ionic conductivity.[1-5] With the large flexibility of anion stoichiometry, oxygen content plays a crucial role in determining the physical properties of complex oxides.[6-10] In particular, oxygen content alters the d-band electron population of transition metals and affects the competitive interplay between strongly correlated electrons, enabling numerous applications, including sensors, batteries, solid-oxide fuel cells (SOFCs), catalysts, and memristive devices.[11-15] Despite the significance and growing interest, deterministic control over the oxygen distribution remains a great challenge due to the complexity of oxygen migration kinetics. To fully exploit these in applications, real-time observations of structural, valent, and electronic changes during oxygen incorporation and extraction processes are vital.
Recently, a distinct tendency for the formation of oxygen-vacancy-ordered superstructures was observed for several perovskite (PV) oxides, for example, SrCoO2.5, SrFeO2.5, CaFeO2, and Nd0.8Sr0.2NiO2.[3, 16-19] For these systems, the oxygen composition can be varied through a topotactic transition without losing the parent lattice framework, making them particularly useful for the study of oxygen diffusion and promising candidates for high ionic conductivity.[11] Furthermore, the surface redox-active centers in transition-metal oxides directly affect the efficacy of energy conversion systems, such as SOFCs and electrocatalysts.[20-22] However, a detailed ionic picture of the oxide surface (or an area near the surface) has remained elusive due to the difficulty in imaging and quantification of local stoichiometry variations.[22-25] In this regard, a precise characterization of oxygen content and diffusion dynamics is highly sought after to address the interplay between local chemical evolution and physical phenomena. Advances in this respect would not only help achieve insights into the oxygen ion migration mechanisms during the topotactic transition, but also provide a perspective for elucidating the ionic phenomena that underpin the operation of related devices.
Here, we report on a real-time study of the oxygen content evolution during the topotactic transition in La0.7Sr0.3MnO3-δ (LSMO) epitaxial thin films by in situ X-ray diffraction (XRD) and in situ X-ray photoelectron spectroscopy (XPS) at elevated temperatures. The limiting cases of the transition are further investigated using polarized neutron reflectometry (PNR), providing a quantitative determination of the oxygen content gradient throughout the entire film. Remarkably, a novel phase is discovered in the vicinity of the film surface after the transition. In combination with atomic-resolution scanning transmission electron microscopy (STEM), a highly anisotropic oxygen ion migration path is revealed at the near-surface region, being essential for optimizing oxygen ion conductivity at moderate temperatures. Surface-sensitive total electron yield (TEY) X-ray absorption spectroscopy (XAS) further identifies the changes in the Mn oxidation state and metal-oxygen hybridization for the distinct topotactic phases, fingerprinting the formation and elimination of the surface phase upon tuning of the annealing conditions. Finally, the corresponding influence on magnetic and electronic transport properties is demonstrated in terms of both oxygen content variations and surface termination.
2 Results and Discussion
LSMO thin films were grown on SrTiO3 (STO) substrates by high oxygen pressure sputter deposition (HOPSD) (see Experimental Section). The as-prepared thin film shows well-resolved Laue fringes in XRD scans at room temperature (Figure S1, Supporting Information), indicating a high-quality epitaxial film. In order to monitor the evolution of the oxygen content during the topotactic phase transition, in situ XRD and in situ XPS measurements were performed at 600 °C in vacuum on pieces cut from the same LSMO sample after preparation.
Figure 1a shows the real-time in situ recording of XRD ω-2θ scans around the STO (002) Bragg diffraction peak. A topotactic transition from PV to brownmillerite (BM) is observed within 5 h of annealing, where the characteristic diffraction peaks evolve in response to a coherent increase in oxygen deficiency (δ). In the early stage of annealing, a depletion of oxygen content results in an expansion of the PV lattice, with the PV-(002) peak shifting monotonically towards lower angles. The width of the PV peak keeps increasing, further indicating a possible inhomogeneous deoxygenation process throughout the film. At 3 h, the maximum expansion of the out-of-plane lattice constant reaches 8%. As the annealing proceeds, a new Bragg peak evolves at 44.6°, while the peak from the expanded PV lattice gradually diminishes. Eventually, the film forms a robust BM structure with superlattice peaks observed in the full range XRD scan (Figure S1, Supporting Information), signifying the emergence of a vacancy-ordered superstructure. It is noteworthy that a faster transition, within 1 h, can be achieved at 750 °C. Reciprocal space maps around the asymmetric (103) peak of STO show that both the PV phase (Figure 1b) and BM phase (Figure 1c) are coherently strained with no lattice relaxation before and after the post-annealing treatment.
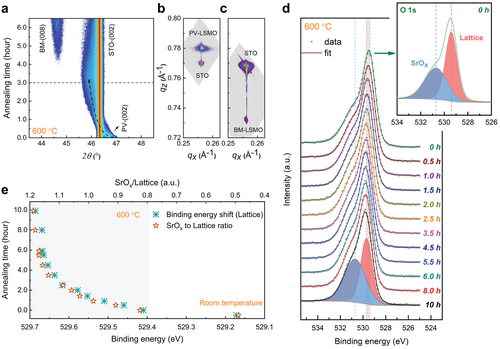
While the overall structural evolution with the oxygen release has been confirmed in the bulk region of the film, a more detailed assessment of the surface chemical stability is still missing. Therefore, in situ XPS measurements were conducted at the same annealing conditions (600 °C, 10−6 mbar), analyzing the oxygen-binding environment in real-time. Figure 1d displays the XPS O 1s core-level spectra recorded at 30–120 min intervals. At 600 °C, a single spectral feature is detected for all the O 1s spectra, accompanied by a shoulder at the higher binding energy side. The asymmetric nature of the O 1s peak arises from the presence of different chemical environments for the surface oxygen species, where the main peak at 529.4 eV can be assigned to the lattice oxygen component in LSMO and the shoulder peak at 530.7 eV to SrOx segregation.[26-29] As the annealing proceeds at 600 °C, the lattice component shows a clear binding energy shift with annealing time, up to 529.7 eV at 10 h. In contrast, the SrOx component exhibits a constant binding energy upon annealing, evidencing that it belongs to an oxygen component decoupled from the LSMO lattice. Analogous behavior is also observed in the time-dependent Sr 3d spectra (see Figure S4, Supporting Information).
Furthermore, the relative contributions of the SrOx and lattice components to the O 1s core-level spectral envelope evolve with annealing time. Figure 1e plots the development of the SrOx contribution normalized to the LSMO lattice contribution. One can clearly observe that the relative increase of the SrOx contribution exhibits the same trend as the binding energy shift of the lattice oxygen component. This indicates that the deoxygenation process in the LSMO lattice is accompanied by SrOx segregation, which develops on the same time scale. Consequently, both the observed evolutions are equally attenuated after 6 h of annealing, a time scale that corresponds well to the emergence of the BM superlattice revealed by real-time XRD. It follows that the evolution of surface oxygen components terminates when the final oxygen-vacancy-ordered state is established.
The surface rearrangement of the Sr cation can act as an effective aliovalent dopant in , which facilitates the formation of defects (e.g., oxygen vacancies). Therefore, the significant question of possible spatial gradients in oxygen vacancy distribution is raised. Rutherford backscattering spectrometry (RBS) enables a quantitative compositional depth profile for each element in the film. Since the measured energy of backscattered Helium ions depends on both the atomic mass and the depth of each element in the sample, the backscattered peaks extend gradually towards lower energy as the ions pass through the depth occupied by the element. As shown in Figure 2a, the RBS spectrum reveals the actual composition ratio (La: Sr: Mn = 0.71: 0.28: 1.00) of the film and a homogeneous distribution for each element-specific energy regime. However, RBS provides low accuracy for light elements and determining the exact amount of oxygen content from the X-ray techniques is only possible indirectly from lattice parameter shifts and remains very challenging. To quantitatively probe the local oxygen concentration, PNR is used to simultaneously measure the structural and magnetic depth profiles, in which the depth-resolved variation of oxygen content and its impact on magnetization are directly mapped.
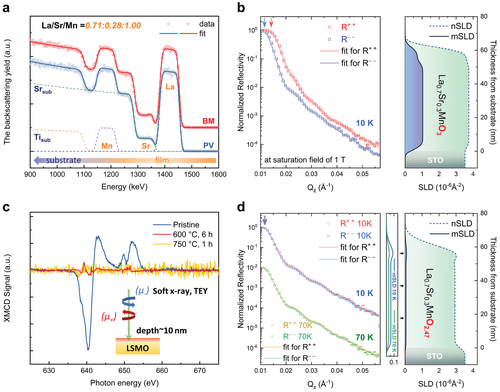
Figure 2b, d displays the measured PNR non-spin-flip reflectivities for the pristine PV phase and transformed BM phase at saturation field, respectively. The corresponding depth profiles of the nuclear and magnetic scattering length densities (nSLD and mSLD) of the films are obtained with the simultaneous fit to the experimental reflectivities measured for two neutron polarizations. Owing to the non-spin-flip interaction, one finds for the spin-up channel (R+ +) the sum of nuclear interaction and the magnetic interaction, and for the spin-down channel (R− −) the difference. In the case of PV-La0.71Sr0.28MnO3-δ, a clear spin splitting between the R+ + and R− − channels thus indicates a strong net magnetic contribution. The extracted depth profile accurately confirms the quotient of the nSLD (3.69 × 10−6 Å−2) and mSLD (1.04 × 10−6Å−2), showing a good agreement with the expected value and a ferromagnetic ground state.[30, 31] This further enables a quantitative determination of oxygen stoichiometry (La0.71Sr0.28MnOx, x = 2.89, error: −0.03/+0.05) for the PV phase. In the topotactically deoxygenated BM phase, no splitting between the two channels is observed in both reflectivities measured at 10 and 70 K, suggesting a negligible net magnetic moment. Such a dramatic change in the observed magnetic depth profile strongly indicates a ferromagnetic to anti-ferromagnetic transition after oxygen depletion. The results are further corroborated by X-ray magnetic circular dichroism (XMCD; Figure 2c), which provides element-resolved information of the net magnetic moment. A large dichroism signal is found at the Mn L3, 2-edge for the PV phase at room temperature, which is consistent with previous results, strongly supports a ferromagnetic state.[32] In contrast, the post-annealed samples exhibit hardly any detectable XMCD signal, which is consistent with the PNR results.
An important aspect, the contrast in nuclear depth profiles for the distinct topotactic phases enables an accurate quantification of the oxygen content variation. As known in neutron reflectivity, the total reflection edge is determined by the largest SLD in the layered structure, including the substrate. Therefore, the SLD of STO substrate (nSLD = 3.53 × 10−6 Å−2) can act as a reference for the evolution of SLDs for LSMO films during the transition. For the pristine PV phase, the sum of nSLD and mSLD is larger than that of the STO substrate, which results in a total reflection edge at larger angles in the R+ + channel, as indicated by the red arrow in Figure 2b. For the topotactically deoxygenated film, the total reflection angle is identical for both spin channels, with a value corresponding to the nSLD of STO. This suggests that the nSLD of the film is significantly reduced due to oxygen removal, resulting in an even lower nSLD than the substrate. The fitted nuclear depth profile confirms the uniformly suppressed nSLD (2.93 × 10−6 Å−2) in the bulk film region, enabling, for the first time, a precise determination of oxygen stoichiometry (La0.71Sr0.28MnOx, x = 2.47, error: −0.01/+0.01) in BM phase oxide thin films.
In contrast to the general observation of reduced SLDs in the top layer, a thin surface layer with increased SLDs with respect to the buried BM-LSMO film is revealed. The thickness of the top layer is 10–15 nm, which is consistent with the Sr enrichment at the surface. The result strongly indicates that the surface chemical evolution during the topotactic transition is collectively influenced by both the anion deficiency and the cation excess. Considering the electrostatic attraction, it is reasonable to recognize the positively charged oxygen vacancies ( at the surface) as an important driving force for the accumulation of negatively charged cations towards the surface in PV oxides.[33] To provide a deeper understanding of the surface structural evolution and migration dynamics at the unit-cell level, atomic-resolution STEM experiments were performed on the same BM-LSMO sample that contains the surface phase.
Figure 3a shows a representative high-angle annular-dark-field (HAADF) STEM image and fast Fourier transform (FFT) patterns taken in the vicinity of the film surface. In this imaging mode, the intensity is approximately proportional to the square of the atomic number, with the heavier cations contributing significantly and the lighter oxygen anions only negligibly to the image intensity. From the arrangement of the atomic columns, we see an important feature that is not typical of the PV-related structures, whereby a c/2 shift along the out-of-plane direction is observed between the neighboring column blocks. Upon further inspection of the lattice spacing, a width of 4.7 Å for the alternating cation atomic columns and a gap of 3.6 Å between the neighboring blocks are revealed, as illustrated in the representative intensity line profile (Figure 3b). Along the vertical direction, the lattice spacing of 2.7 Å is measured for the neighboring atomic rows. These observations clearly evidence the formation of a novel surface (s-) phase in the LSMO thin film during the topotactic phase transition. We noted that this structural feature was not observed in previous Sr segregation or phase separation studies, neither in the form of Ruddlesden–Popper series, BM precursor phase, or dopant-oxides (e.g., SrO-like phases) at the PV surface.[25, 27, 34-37]
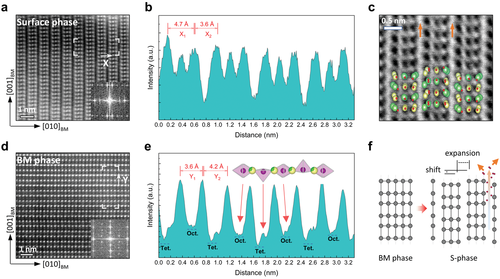
In order to probe the oxygen positions and local vacancy inhomogeneity, annular bright-field (ABF) images were simultaneously collected in the s-phase region (Figure 3c). Notably, one can see that the vertical structural gap forms the brightest contrast in the image, which implies less atomic densities and large-scale oxygen vacancies along the vertical direction. These vacancy channels between the vertically shifted structural blocks provide an anisotropic oxygen diffusion pathway towards the film surface, as it is known that large open frameworks facilitate ionic conduction.[38, 39] Further complementing the experimental results, the s-phase was modeled within density functional theory (DFT) using the Vienna ab initio simulation package (VASP) code.[40, 41] The proposed atomistic model is overlaid on the image. The calculations reveal that the observed structure with shifted stripes has lower energy, by about 65 meV per atom, than the one without the shift (see the supporting information for details).
Beneath the s-phase, a pure BM lattice is formed, where the characteristic doubling of the unit cell is observed, as shown in Figure 3d. Attributed to the ordering of oxygen vacancies, the alternate stackings of stoichiometric octahedral layers (bright stripes) and oxygen-deficient tetrahedral sub-layers (faint stripes) are visualized directly. The associated displacements of cation atomic rows are identified in the line profile along the out-of-plane direction (Figure 3e). In every second MnO plane, the depletion of oxygen reduces the coordination of Mn cations (from MnO6 to MnO4), resulting in a vertical expansion between the La/Sr atomic rows. The local distance between the La/Sr atomic rows turns into 3.6 Å for the octahedral layers and 4.2 Å for the tetrahedral layers. Thus, the presented results demonstrate a unique configuration of two distinct structural variants (i.e the s-phase at the top and the BM phase constituting the inner film) during the progress of a topotactic phase transition in the LSMO film.
The topotactic transformation from the PV phase to BM phase is triggered by oxygen depletion.[3, 9, 11, 16] The oxygen necessarily leaves the oxide through its surface, lending itself to the formation of the observed oxygen vacancy concentration gradients prior to reaching the equilibrium stoichiometry, that is, the inner BM phase. It is known that oxygen vacancies tend to form ordered structures in oxide lattices, a structural relaxation resulting from energy minimalization.[25, 27] The relatively slow transformation of LSMO from the PV to the BM phase at 600 °C allows us to observe and characterize such ordering of oxygen vacancies at the surface region, revealing the presence of the s-phase. Here, we note that in a single experiment performed at 750 °C, a pure BM phase is found to be achieved through the entire film already after 1 h, highlighting the importance of temperature in determining the rate of the transformation.
The vertical oxygen vacancy channels of the s-phase captured by STEM represent a distinct diffusion route in contrast to the horizontal channels observed in the BM phase. The structural blocks of the s-phase facilitate oxygen diffusion at the surface of the LSMO to an extent not readily apparent from the structural features of the bulk PV and BM phases. The capacity of the s-phase to promote faster oxygen dynamics represents an important insight into the topotactic transition at the surface of LSMO at moderate temperatures, and is of interest for applications such as fuel cells and catalysts.
Having resolved the overall structural evolution with the oxygen modulation, the electronic structures of the three distinct phases were further examined by soft XAS, since both the oxygen deficiency and cation enrichment can significantly affect the Mn oxidation state. The evolution of the Mn L3,2-edge and the O K-edge spectra is shown in Figure 4a, b. Note that the obtained Mn L3,2-edge and O K-edge spectra were measured in surface-sensitive TEY mode, with an electron escape depth below 10 nm, so that the signal originates from the near-surface region for each sample.
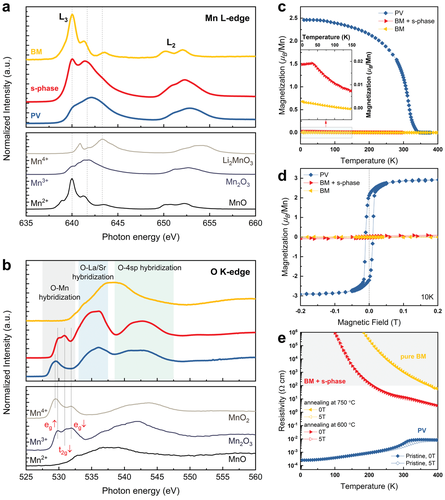
For the pristine PV phase, the Mn L-edge spectra show the expected mixed Mn3+ and Mn4+ valence,[32, 45, 46] which is also consistent with the stoichiometry determined from PNR. For the sample post-annealed at 600 °C for 6 h, the novel s-phase at the surface contributes to the TEY signal (red curve). A shift of the main L3 peak towards lower energy is observed as compared to the PV phase, indicating a change in the Mn oxidation state from a mixed Mn3+/Mn4+ to dominant Mn3+. In addition, a greatly enhanced shoulder peak at 640 eV is observed, which suggests a significant fraction of Mn2+ valence state presented at the surface. Therefore, the overall trend represents that the s-phase has a lower valence state of Mn ions than the PV phase and a Mn2+/Mn3+ combination is adopted among Mn centers.
Another distinct change is the development of the oxygen pre-edge in the O K-edge spectra around ≈530 eV upon structural evolution. In the O K-edge, the pre-edge features originate from the electron transitions between O 1s core levels and partially filled Mn 3d states, which are permitted due to the hybridization between O 2p and Mn 3d states.[32, 44, 47, 48] Thus, the O K-edge spectra provide direct information about the occupancy of the Mn 3d level. The first single peak (≈529.3 eV) observed in the PV phase can be assigned to the combination of unresolved eg and t2g states, while the s-phase exhibits well-separated peaks at higher photon energies. Such trend of the energy shift is consistent with the O 1s binding energy change from Mn4+ (529.2 eV) to Mn3+ (529.7 eV) as measured by XPS (Figure 1e, refs. [44, 47]) Moreover, the deviation in the line shape also reflects a profound change of the MnO covalency. For Mn3+ (3d4) ions, the oxygen pre-edge has three peaks that can be assigned to eg↑, t2g↓, and eg↓, respectively.[49, 50] Here, a pronounced enhancement of the t2g↓ orbital is found in the s-phase, implying a rising Mn2+ contribution, similar to observations for Mn3O4 (Mn3+/Mn2+).[51] In addition, the reduced intensity of eg peaks is commonly interpreted as a signature of electron localization and more ionic environment of Mn.[47] As a consequence, a larger resistivity than that of the PV phase can be expected in the s-phase due to the loss of itinerant eg electrons.
For the sample post-annealed at 750 °C for 1h (yellow curve), one finds a clear signature of the dominant presence of Mn2+ valence state, confirmed by a straightforward comparison with an MnO reference.[42, 43] The result is in excellent agreement with the obtained stoichiometry for the BM phase (La3+ 0.71Sr2+ 0.28Mn2.25+ 1.00O2- 2.47). A comparable shift towards the Mn2+ valence state was also observed in Mn L-edge spectra of H0.3La0.7Sr0.3MnO2.5 film reduced by hydrogen plasma.[45] Furthermore, the O K-edge shows characteristic features of an Mn2+ (3d5) system, in which all the electron spins are aligned in parallel, leading to a higher transfer energy when an opposite spin has to go in. Such an effect pushes the leading absorption edge of several eVs towards higher energies, where it overlaps with the broad features of the 4sp states. Consequently, both the Mn valence and the hybridization feature show a distinct contrast to the PV phase and the newly found s-phase, suggesting a pure BM at such annealing conditions. As such, XAS is shown to be an alternative, non-destructive indicator for the presence of the secondary phase.
Recognizing the different Mn valence among the various phases, the relevant modulations of electronic properties were further characterized. Since, in the LSMO system, the magnetic and electrical transport properties are dominated by the competition between the double-exchange interaction and super-exchange interaction, the nominal Mn valence, which represents the Mn4+/Mn3+/Mn2+ ratio, plays a crucial role in governing these properties.[52, 53]
Figure 4c, d shows the temperature-dependent magnetization M(T) and magnetic hysteresis curves M(H) for the three distinct phases, respectively. A change of the magnetic ground states from ferromagnetism to anti-ferromagnetism is further evidenced by macroscopic magnetometry. In the as-prepared PV phase, a ferromagnetic double-exchange interaction dominates among the mixed Mn4+/Mn3+ ions, exhibiting a high Tc = 330K above room temperature. Upon vacuum annealing, the extraction of oxygen induces a reduction of the Mn valence (emergence of Mn3+−Mn3+ and Mn2+−Mn2+ combinations), where an anti-ferromagnetic order originating from super-exchange interaction starts to govern properties according to the Goodenough–Kanamouri rules.[54] Specifically, the pure BM phase exhibits a minimal magnetic response throughout the entire temperature region, while the sample with an s-phase termination undergoes a magnetic phase transition at 32 K, as shown in the inset. In combination with the magnetic depth profile measured for the same sample (see Figure 2d), one can infer that most of the magnetic signal is originating from the near-surface region (i.e., the s-phase). Since both M(H) curves measured at 10 K (Figure 4d) reveal negligible magnetization or hysteresis, a local antiferromagnetic interaction or long-range antiferromagnetic order is likely for both the s-phase and BM phase. The difference in their Néel temperatures can be rationalized by the different Mn oxidation states and distinct structural types (i.e., Mn2+/3+ for s-phase and Mn2+ for BM phase).
The transformation is also accompanied by a metal-to-insulator transition. As shown in Figure 4e, the largest resistivity difference reaches nine orders of magnitude at 200 K. Here we note that the temperature-dependent resistivity of pure BM-LSMO film is characterized for the first time, thanks to the extended resistance measuring limit up to 200 GΩ (see experimental section). For the film with an s-phase termination, one measures the equivalent resistance of resistors in series (i.e., the s-phase at the top and the BM phase constituting the inner film). An intermediate resistivity is observed in comparison to the pure BM phase, indicating a smaller resistivity for the s-phase. It is worth noting that this more conducting behavior is beneficial for the SOFCs cathodes. The observed multilevel changes in magnetic and electrical properties among the PV, BM, and s-phases corroborate the distinct structural and electronic modulations observed in LSMO thin films throughout the topotactic transition.
3 Conclusion
In summary, we report the first observation of a novel s-phase in LSMO epitaxial thin films during the topotactic transition from the PV to BM phase. Both the real-time XRD and XPS studies suggest a close correlation between structural evolution and oxygen migration kinetics. Using PNR, a direct depth profile of oxygen gradient is achieved in the topotactically transformed state, enabling a precise determination of oxygen stoichiometry variation. The veracity of such an approach is further supported by the direct visualization of the vacancy-induced structural change in the corresponding region. In combination with element-specific spectroscopic information, the distinct electronic structures induced by oxygen deficiency and cation excess are evidenced for the three phases. Given the flexibility of manganese valence states and the anisotropic vacancy channels found in the s-phase, our studies will be of direct interest for the exploration of new candidates for SOFC materials and devices, as well as for a deeper understanding of oxide migration mechanisms.
4 Experimental Section
Film Growth
Epitaxial La0.7Sr0.3MnO3-δ thin films were grown on atomically flat (001) SrTiO3 substrates by HOPSD using a sputter target with the corresponding chemical stoichiometry. The samples were deposited at a substrate temperature of 800 °C and at an oxygen partial pressure of 2 mbar. The vertical distance between the target and the substrate was fixed to 20 mm.
In Situ XRD Measurements
The crystalline structure of the LSMO films was characterized by a Bruker D8 X-ray Diffractometer using monochromatic Cu Kα1 radiation (λ = 1.5405 Å). For the PV to BM conversion, a dedicated vacuum chamber with a heating stage and kapton X-ray windows was installed to provide the possibility for in situ measurements at temperatures up to 800 °C in vacuum of ≈10−6 mbar.
In Situ XPS Measurements
XPS measurements were performed with a PHI Versaprobe II spectrometer using a monochromatic Al Kα source (1486.6 eV). The spectra were obtained using an analysis area of 200 µm in diameter with pass energies of 187.5 eV energy (0.8 eV step) for survey measurements and 23.5 eV (0.1 eV/step) for high-resolution spectra. The O 1s spectrum measured at room temperature was plotted in Figure S3, Supporting Information. For in situ annealing XPS measurements, the sample was heated to 600 °C in vacuum of ≈10−6 mbar step by step in ca. 60 min to remove the surface residuals originating from the exposure of the sample to air. The acquired spectra were deconvoluted using a Shirley-type background and Pseudo–Voigt functions.
Polarized Neutron Reflectivity Measurements
Polarized neutron reflectivity measurements were carried out at the polarized magnetic reflectometer MARIA at the Heinz Maier–Leibnitz Zentrum (MLZ) in Garching, Germany.[55, 56] Non-spin-flip reflectivity spectra were measured at 0.3 T higher than the saturation field, along the in-plane easy axis [010] direction. R+ + and R− − represent the non-spin-flip channels with neutrons parallel and antiparallel to the applied field, respectively. The reflectivity data collected at 10 and 70 K were fitted with the GenX program together in one model to ensure that the chemical scattering length density remains the same.[57] The second fit at 70 K was conducted in which the magnetization was allowed to vary.
Rutherford Backscattering Spectrometry Measurements
The stoichiometry of LSMO films was determined using RBS. The measurements were performed with a collimated 1.7-MeV He+ beam of the Rossendorf van de Graff accelerator with a 10–20 nA beam current at a backscattering angle of 170°.[58]
STEM Imaging and Analysis
The atomic-resolution STEM study was performed on a probe-corrected FEI Titan 80–200 ChemiSTEM microscope, which was operated at an accelerating voltage of 200 kV. The cross-sectional lamella specimens for the HAADF STEM imaging were prepared using an FEI Helios Nanolab 400s focused ion beam (FIB) system. To remove the contamination and the damaged layers, NanoMill Model 1040 system operated at 500 V was used to clean and mill the lamella specimens. The experimental images were analyzed using Gatan Digital Micrograph software.
DFT Calculations
The DFT calculations were performed using the VASP code.[40, 41] The electronic structure calculations and structural relaxations were performed, utilizing projector augmented-wave potentials as implemented in the simulation package.[59] Perdew–Burke–Ernzerhof (PBE) approximation was used to account for the exchange-correlation effects.[60] A plane-wave kinetic energy cutoff of 500 eV and a 2 × 2 × 1 γ-centered k-points mesh were used for the supercell simulations. Structural parameters of the unit cell were obtained by minimization of the Hellman–Feynman forces to values less than 5 meV per Å.
XAS and XMCD Measurements
The soft XAS measurements at the Mn L3,2-edge and O K-edge were carried out at beamline UE56/1-SGM at the synchrotron facility BESSY II in Berlin. The XAS measurements were performed in ultra-high vacuum (≈10−9 Torr) at room temperature, with the incident beam perpendicular to the film surface. The spectra were recorded in the surface-sensitive TEY mode. The corresponding XMCD spectra were acquired with a magnetic field of 300 mT at room temperature.
Magnetic and Electrical Transport Property Measurements
The magnetization of the films was measured using a superconducting quantum interference device equipped with a vibrating sample magnetometer (SQUID-VSM, Quantum Design). The M(T) curves and the M(H) curves were measured with the magnetic field applied in-plane. The magnetotransport measurements were conducted in a van-der-Pauw geometry using a Lake Shore Hall measurement system (resistance range: 40 µΩ–200 GΩ). In the resistivity measurement, the magnetic field was applied along the surface normal direction and perpendicular to the current direction.
Acknowledgements
L.C. gratefully acknowledges the financial support by German Research Foundation (Grant No. ZH 225/10-1). L.C. thank Prof. Manfred Helm for the support and critical reading. The authors thank Dr. Wanli Yang for the fruitful discussions on the XAS data. The authors thank Dr. Wanli Yang for providing the reference XAS spectra. Mn reference spectra were collected at the Advanced Light Source, a U.S. DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. K.Z.R. gratefully acknowledges the financial support by German Research Foundation (Grant No. SFB 917 “Nanoswitches”). L.C. also thank the advice and comments from Prof. Dongsheng Song, Prof. Yinguo Xiao, and Prof. Regina Dittmann. The authors thank HZB for the allocation of synchrotron radiation beamtime.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.