Compost Application Compensates Yield Loss in a Successive Winter Wheat Rotation: Evidence From a Multiple Isotope Labelling Study
ABSTRACT
Introduction
Successive winter wheat (WW) rotations lead to yield decline due to a less favourable microbial community and changes in soil nutrient availability. Research on mitigation options is limited and has great potential to improve farming profitability.
Materials and Methods
Using a quadruple isotope labelling study (13C, 15N, 2H and 18O) and a novel mesocosm experimental setup enabling the growth of wheat in outdoor conditions, we investigated the effects of the rotational position and compost application on the productivity of WW, grown either after oilseed rape or in self-succession, during the flowering (T1) and grain ripening stage (T2).
Results
The initial high soil nutrient content after oilseed rape created a long-lasting soil legacy that gave an advantage to the first WW after oilseed rape (W1) compared to the growth of the second WW after oilseed rape (W2), with significantly higher soil nitrate, ammonium, dissolved organic carbon, and microbial biomass than in W2. Compost significantly compensated for the disadvantage of W2, and by T2, these effects were reflected in enhanced root growth and nutrient uptake in the compost-amended W2. Allocation of freshly assimilated carbon was 46.2% higher in the compost-amended compared to the unamended W2. A similar trend was observed for plant 15N from the 15N-labelled fertiliser. Compost increased the contribution of the topsoil and decreased the contribution of the subsoil to total plant water uptake, which resulted in a 30% higher plant growth and yield gain in the compost-amended W2.
Conclusion
Our findings highlight the capacity of compost to buffer negative plant-soil feedbacks in monotonous crop rotations by influencing key rhizosphere processes, while simultaneously improving wheat growth and yield.
1 Introduction
Due to its high economic importance, winter wheat (WW) is often incorporated into crop rotations by growing two or more WW crops successively after a fallow period (Kwak and Weller 2013). Worldwide, up to 40% of WW is grown in succession, with only a fallow period in late summer as a break (Angus et al. 2015; Yin et al. 2022). The successive cultivation of WW on the same area increases the risk of soil-borne infections, such as total loss disease caused by Gaeumannomyces tritici (Gt), the most important soil-borne fungal pathogen of WW, which causes root rot and significant yield losses (James Cook 2003; Kwak and Weller 2013; Palma-Guerrero et al. 2021). However, this yield decline has also been observed in years without obvious Gt infestation (Arnhold et al. 2023a). Recent studies have shown that the structure of soil microorganisms is influenced by the crop rotation position of WW, suggesting that the yield decline in successive WW crop rotations is a complex phenomenon that is not limited to Gt (Giongo et al. 2024; Kaloterakis et al. 2024a).
Adding non-cereal break crops to the rotation, such as oilseed rape, has been shown to enhance the yield of the following WW (Angus et al. 2015; Weiser et al. 2017). The addition of oilseed rape to the rotation is considered beneficial, improving pathogen suppression, soil aggregation, soil structure and leaving a high postharvest residual nitrogen (N; approximately 70 kg N ha−1) behind, although it might come at the expense of increased environmental N losses (Sieling et al. 2005; Sieling and Kage 2006; Weiser et al. 2017; Hegewald et al. 2018; Hansen et al. 2019; Kerdraon et al. 2019). In successive WW rotations, this beneficial effect is missing. The following WW experiences a long-lasting growth reduction. This is exacerbated by reduced plant and soil carbon (C) allocation and plant nutrient uptake. Previous studies have shown that WW rotations are linked to changes in bacterial and archaeal communities (Giongo et al. 2024; Kaloterakis et al. 2024b, 2025) and C allocation patterns (Kaloterakis et al. 2024b). Whether the beneficial effect of oilseed rape can be achieved in successive WW rotation by adopting certain management practices, such as incorporating organic fertilisers, remains unknown.
The utilisation of organic amendments (OA), such as compost, is considered a potential strategy to enhance the productivity of conventional farming systems in a sustainable manner (Keeling et al. 2003; Lee et al. 2021). This approach aims to promote the accumulation of soil organic matter (SOM) and ensure a sufficient nutrient supply to plants, while simultaneously mitigating nutrient losses, primarily through leaching processes (Agegnehu et al. 2017; Heisey et al. 2022; Duan et al. 2023). The acknowledged multifunctionality of OA in agricultural production is primarily attributed to their elevated SOM content, with compost specifically being recognised for its plant-available N, P and K (Siedt et al. 2021; Nobile et al. 2022). The positive influence of organic acids on soil structure and C storage has been well documented. The role of organic C in this process is that of a soil-binding agent, which improves soil aggregation (Siedt et al. 2021). Compost effectively prevents C mining and loss of stabilised native SOM by providing labile C for microbial uptake, thus contributing to C stabilisation in the soil (Wang et al. 2022). Soil microbes preferentially use the labile C substrate from the compost and reduce decomposition of the relatively stable SOM, causing a negative priming effect (Dijkstra et al. 2013). The magnitude of the associated increase in SOM is contingent upon the soil depth at which the compost is applied. This increase is expected to have a positive effect on water retention and potentially on water uptake by plants, especially in deeper soil layers (Uhlig et al. 2023; Feifel et al. 2024). Furthermore, OA enhance the absorption of water by plants and facilitate their swift recovery from drought conditions. However, the efficacy of this phenomenon is presumed to be contingent upon the composition of the compost substrate (Nguyen et al. 2012; Kowaljow et al. 2017; Soudek et al. 2024).
The capacity of compost to modulate the C transfer between plants and microbes may constitute an effective strategy to mitigate yield losses in successive WW rotations. By increasing the substrate availability for microbes, compost can stimulate enzymatic activities and promote plant nutrient availability and uptake, especially under limited N supply conditions of successive WW rotations. Tilston et al. (2005) reported that the application of green-waste compost led to a reduction in take-all severity and yield losses in WW. This finding indicates that compost application provides plant-available nutrients and supports microbial activity, thereby enhancing plant health. Consequently, the multifunctionality of compost provides an effective soil management practice to address the yield decline in successively cultivated WW by influencing multiple soil health aspects.
However, the key question remains open whether the benefits of compost application influence the processes that are already occurring in the rhizosphere of WW, which in turn are influenced by the soil legacy of the preceding crop, such as rhizodeposition. Plants allocate a substantial portion of the assimilated C below ground through a variety of active and passive processes. These processes include root respiration, root exudation, emission of volatile organic compounds, mucilage production, and root wilting (Brüggemann et al. 2011; Kuzyakov and Xu 2013). For WW, an estimated 20-30% of this assimilated C is deposited in the soil via the roots (Kuzyakov and Domanski 2000; Loeppmann et al. 2019). This labile C is fuelling microbial activity and its associated nutrient cycling in the soil, and this process is particularly intense in the rhizosphere (Jones et al. 2009). Indeed, plants actively recruit microbial taxa that compete with pathogens for available resources in the rhizosphere. These microbial taxa produce inhibitory metabolites that prevent pathogen growth, mobilise nutrients, or influence plant hormonal expression (Philippot et al. 2013).
Recent studies have explored the mechanisms underlying yield decline in successive WW rotations (Kaloterakis et al. 2024a, 2024b), but the influence of compost on the rhizosphere processes of successive WW rotations and potential mitigating mechanisms for the associated yield decline remain unknown. Therefore, our objectives were to: (1) assess the potential of green-waste compost (referred to as compost hereafter) application to compensate for yield losses in successive WW rotations, and (2) assess the effect of compost application on plant and soil C allocation patterns, plant N uptake and water uptake in contrasting WW rotations. We hypothesised that (i) there would be reduced C allocation above and below ground in the successive WW rotation, while compost application would increase C allocation above and below ground and stimulate microbial activity by enhancing C and N cycling enzymatic activity, and (ii) this would result in enhanced root growth, followed by increased N uptake, enhanced subsoil water uptake and finally increased yield. To test these hypotheses, an outdoor mesocosm experiment was conducted, contrasting two rotational positions of WW, that is, WW grown after oilseed rape compared with WW grown in self-succession. We combined biochemical, enzymatic and isotopic analyses to assess nutrient and C availability in the soil of the different rotational positions and to understand the potential mechanisms of underlying compost effects on the successive WW rotation.
2 Materials and Methods
2.1 Experimental Design
The soil used in the experiment was collected from an experimental farm located near Harste in Germany (51°36′23.5″ N, 9°51′55.8″ E) and was classified as a Luvisol with a silty loamy texture. A detailed overview of the agricultural management implemented on the farm has been provided by Arnhold et al. (2023b). The soil was collected from 0 to 30 cm and 30–50 cm soil depth after one season of oilseed rape cultivation and after 1 year of WW following oilseed rape cultivation. After the harvest of the pre-crops, the plant residues were left in the field and the soil was not ploughed before the soil was collected for the experiment. The following rotational positions of WW were simulated in this study, after sowing WW on the collected soil: (1) first WW after oilseed rape (W1), and (2) second WW after oilseed rape (W2), each treatment replicated six times.
We conducted an outdoor experiment (November 8, 2022 to July 18, 2023) using custom-made cylindrical polyvinyl chloride (PVC) sewage pipes (135 cm height, 10.5 cm inner diameter, Figure S1b) with a perforated PVC sleeve at the bottom for drainage. Two opposite sides of the pipes were perforated vertically with resealable 20-mm holes to enable soil sampling during plant growth. The distance between the holes was 5 cm for the first 30 cm of the pipe, followed by 10 cm distance between the holes for the remaining depth of the pipe. Custom-made PVC screws were inserted in every hole and sealed with O-rings. The pipes were placed inside the empty space of a lysimeter pit (120 cm depth × 105 cm width × 105 cm length) in an outdoor lysimeter area of Forschungzentrum Jülich, Germany (50°54′31.9″ N 6°24′11.0″ E). To expose the plants to realistic field conditions, we placed two layers of Styrofoam in the first 42 cm of the pit. Six 10.5-cm holes were created in the Styrofoam to insert the pipes and minimise air exchange between the pit and the environment (Supporting Information S1: Figure S1a). The treatments included the W1 and W2 rotational position without and with compost (W1C and W2C), with 6 replicates each. We used a total of 4 pits and 24 pipes. The experimental unit was the pipe, in which a single plant was growing. A temperature sensor was placed in two of the four pits at a depth of 70 cm to record temperature fluctuations throughout the experiment.
Soil bulk density was adjusted to 1.35 g cm−3 in the topsoil and to 1.45 g cm−3 in the subsoil (30–100 cm). The bottom 30 cm of the pipes was filled with 1.80 g cm−3 quartz sand for drainage. Deionized water was added to adjust soil moisture to 60% water-holding capacity (corresponding to 227 g H2O soil kg−1) at the onset of the experiment. Thereafter, the plants were kept rain-fed throughout the experiment (Supporting Information S1: Figure S2). WW seeds (cultivar “Nordkap”) were germinated on a Petri dish with sterile filter paper for 24 h in the dark at 23℃. Subsequently, one germinated seed was planted into each pipe. The plants were fertilised with 80 kg N ha−1 of calcium ammonium nitrate (CAN, 13.5% NO3–-N, 13.5% NH4+-N; Raiffeisen Waren-Zentrale Rhein-Main eG, Cologne, Germany) at each of the following WW growth stages: Zadoks growth stages 25, 30/31 and 50/51 (Zadoks et al. 1974); hereafter termed “BBCH” from the Biologische Bundesanstalt, Bundessortenamt und CHemical Industry decimal code system), resulting in a total of 240 kg N ha−1 applied throughout the experiment. The fertiliser was mixed with 10 atom% (15NH4)2SO4 (Merck KGaA, Darmstadt, Germany) to reach a target δ15N of 5000‰.
Green-waste compost (GABCO Kompostierung GmbH, Würselen, Germany) was applied at a rate of 40 t fresh mass ha−1 once and thoroughly mixed with the topsoil (0–30 cm) of each pipe before sowing. The application rate is in accordance with the German fertiliser ordinance of May 26, 2017 (BGBl. I p. 1305) of the Federal Ministry of Justice and Consumer Protection. The compost consisted of kitchen and green waste (gardening and landscaping waste, including tree and hedge cuttings, plant leaves and grass clippings). The compost contained (on a fresh mass basis): 30.7% water, 21.6% C, 1.29% total N (C:N ratio of 13), 1.19% organic N, 0.97% mineral N (95% NH4+-N and 5% NO3−-N), 0.3% total P 1% total K, 0.31% total Mg and had a pH (1:5 w/v H2O) of 9.1. The composition of the compost was certified by the German Institute for Quality Assurance (RAL Deutsches Institut für Gütesicherung und Kennzeichnung e.V., Bonn, Germany).
2.2 13CO2 Pulse Labelling at Early Flowering
When the plants reached early flowering (BBCH 60/61 at 205 days after sowing, hereafter called T1), we conducted 13CO2 pulse-labelling. First, the soil surface was covered with a thick gas-impermeable PVC membrane to minimise diffusion of 13CO2 into the soil. Thereafter, custom-made polymethyl methacrylate plant chambers, constructed by the workshop of Forschungszentrum Jülich, were fitted onto the pipes shortly before the labelling. The chamber consisted of a base (3 cm height × 10.5 cm diameter; 0.35 cm wall thickness) and the plant compartment (60 cm height × 20 cm diameter; 0.35 cm wall thickness). Two fans (252 N. DC axial fan, 12 V, 25 × 25 × 8 mm, EBM-Papst Mulfingen GmbH and Co. KG, Mulfingen, Germany) were fixed at opposite sides of the upper part of the chamber for air mixing. A rubber seal port on the top plate of the chamber was used to inject the 13CO2, while another port was used to measure the temperature inside the chamber. 13CO2 pulse labelling was done by injecting 20 mL of 99 atom-% 13C-CO2 (Campro Scientific GmbH, Berlin, Germany) into the chambers. Before 13CO2 pulse labelling, we monitored the decay rate of unlabelled CO2 inside the chamber by injecting 20 mL of pure unlabelled CO2 to reach a mixing ratio of 1500 ppm CO2 inside the chamber. This allowed us to adjust the timing of the CO2 injections and to record the CO2 assimilation time of the plants. Air temperature, relative humidity, and mixing ratio of unlabelled CO2 were monitored with an infra-red gas exchange analyzer (Li-8100, Li-COR, Lincoln, NE, USA). When the concentration dropped to sub-ambient CO2 levels, another injection of 20 mL was made to reach a CO2 mixing ratio of 1500 ppm inside the chamber. For 13CO2 labelling, a total of three injections of 20 mL of 13CO2 each were made in 20-min intervals to ensure that a sufficient amount of 13C was fixed by the plants.
2.3 1H2HO and H218O Labelling and Soil Sampling at Flowering and Grain Ripening
Three days after the 13CO2 labelling, the first soil sampling was conducted by temporarily lifting the pipes out of the pits to gain access to the holes on the side of the pipes and to collect soil. From each of three soil depths (0–30 cm, 30–60 cm and 60–100 cm), we sampled 60 g of soil with 20 g sampled from each of the three holes per depth using metal spatulas. The collected soil was then thoroughly mixed and divided into several subsamples for the various laboratory analyses. Subsequently, 90 mL of 1H2HO (enriched at δ2H = 43000‰) and H218O (enriched at δ18O = 5000‰; Cortecnet Europe, Les Ulis, France) were injected into the soil at a depth of 25 cm and 50 cm, respectively. The amount of water injected corresponded to the amount needed to increase the water-holding capacity from 60% to 100% in a 5 cm layer of soil.
2.4 Sampling at Flowering and Grain Ripening
The plants were harvested when they had reached the grain ripening stage (BBCH 90 at 252 days after sowing, hereafter called T2). The aboveground plant parts were divided into pseudostems (hereafter called stems), leaves, husks and grains. The pipes were then cut into three parts, i.e., 0–30 cm, 30–60 cm and 60–100 cm, and soil was sampled for the different analyses. For both soil sampling time points (T1 and T2), subsamples from all the side holes of each soil depth were pooled and mixed to form a composite and representative sample. They were stored in the freezer at −25°C before processing. For the analysis of soil enzymatic activity, the samples were stored in the fridge at 4°C and analyzed within 1 week.
The roots were also retrieved after washing off the soil through a 1-mm sieve and stored in 30% ethanol. They were scanned at 600 dpi (Epson Perfection V800 Photo, Epson, Japan) and analyzed with the software WinRhizo (Regent Instruments Inc., Quebec, Canada). The following root growth traits were measured: root length, average root diameter (Rdia), root surface area and root volume. Seven root diameter classes were selected: 0–0.05 mm, 0.05–0.1 mm, 0.1–0.5 mm, 0.5–1 mm, 1–1.5 mm, 1.5–2 mm, ≥ 2 mm. Using these root growth traits, the root length density (RLD), the specific root length (SRL) and the proportion of root length were computed for the seven root diameter classes. Estimates of root tissue density (RTD) were made as described in Rose (2017). Plant dry weight was determined after oven-drying at 60°C to constant weight (for a maximum of 3 days). Ball-milled (MM 400, Retsch, Germany) plant and soil samples were weighed into tin or silver capsules (HEKAtech, Wegberg, Germany) for determination of δ13C, δ15N, δ2H and δ18O using an elemental analyzer coupled to an isotope-ratio mass spectrometer (EA-IRMS, Flash EA 2000, coupled to a Delta V Plus; Thermo Fisher Scientific Inc., Waltham, MA, USA). All biochemical analyses were performed as described in detail in Kaloterakis et al. (2024a).
The quantification of isotopes and isotopic calculations were done as described in Kaloterakis et al. (2024b). Calculations for the atom% 2H and 18O of the biomass were corrected for the background (initial) unlabelled 2H and 18O content of the different plant parts using the following values for 2H and 18O, respectively: 39.3‰ and -32.9‰ for the grains, 33.3‰ and -88.7‰ for the husks, 20.5‰ and −128.6‰ for the leaves, 27.2‰ and −99.1‰ for the stems, and 25.1‰ and -88.1‰ for the roots. These values were obtained from earlier experiments, and since the 2H and 18O enrichment levels were very high and the labelling uniform throughout the plants, the comparisons between the treatments are sufficiently precise.
2.5 Statistical Analysis
The following fixed factors were included in the analysis: rotational position (W1 and W2), OA (with and without compost application) and, whenever applicable, soil depth (0–30 cm, 30–60 cm and 60–100 cm) and plant part (grain, husk, leaf, stem and root). The following statistical analysis was performed in R (v4.2.1.; R Core Team 2022). We conducted PERMANOVA with 10,000 permutations using the ‘vegan’ package (Oksanen et al. 2022) to account for deviations from normality and homoscedasticity of the data, using the Benjamini-Hochberg p adjustment procedure to control the false discovery rate. We conducted follow-up between-subjects t-tests with the ‘RVAideMemoire’ package in R (Hervé 2023). The significance threshold was set to α = 0.05. Visualisations of Spearman rank correlation matrices were made with ‘ggstatsplot’ (Patil 2021) for the response variables and for each rotational position of WW with and without OA application. Graphs were generated with the ‘ggplot2’ package (Wickham 2016).
3 Results
3.1 Soil Biochemical Properties at T1 and T2
At T1, the soil NO3− content was significantly reduced compared to the initial content at the beginning of the experiment (Table 1). We found a significant effect of the rotational position, compost application and soil depth on soil NO3− and NH4+ (Table S1). More specifically, the topsoil of W1 had the lowest concentration of NO3−, followed by W1C and W2C. The soil of W2 had a higher NO3− content compared to W1C and W1, respectively, with no significant difference between W2 and W2C (Figure 1a). In the 60–100 cm layer, W2 soil had a higher NO3− content compared to W2C.
| Soil parameter | Unit | Rotational position | ANOVA | |
|---|---|---|---|---|
| W1 | W2 | |||
| NO3− | mg N kg−1 | 18.0 ± 0.06a | 12.5 ± 0.73b | ** |
| NH4+ | mg N kg−1 | 0.14 ± 0.02 | 0.12 ± 0.01 | ns |
| PCAL | mg kg−1 | 40.4 ± 0.2 | 35.5 ± 1.9 | ns |
| KCAL | mg kg−1 | 58.8 ± 0.4a | 27.3 ± 5.0b | ** |
| SO42− | mg kg−1 | 7.0 ± 0.2ba | 1.8 ± 0.1b | *** |
| Mg | mg kg−1 | 72.5 ± 0.8a | 47.0 ± 1.2b | *** |
| soil C:N | 8.75 ± 0.12 | 8.93 ± 0.12 | ns | |
| pH | 6.81 ± 0.003 | 6.79 ± 0.004 | ns | |
| DOC | mg kg−1 | 38.9 ± 0.3a | 30.9 ± 0.6b | *** |
| Cmic | mg kg−1 | 70.4 ± 2.4a | 52.3 ± 4.2b | * |
| Nmic | mg kg−1 | 10.2 ± 0.3a | 5.6 ± 0.4b | *** |
| Cmic:Nmic | 6.9 ± 0.04b | 9.3 ± 0.06a | *** | |
- Note: The soil for these analyses was collected from the 0 to 30 cm soil depth. Data are mean ± SE (n = 3 for rotational position). Different lowercase letters in each column denote significant differences between the rotational positions at p ≤ 0.05 using Bonferroni correction for multiple comparisons. Main effects identified by ANOVA of rotational position are indicated as follows: ns = not significant.
- * p ≤ 0.05
- ** p ≤ 0.01
- *** p ≤ 0.001.
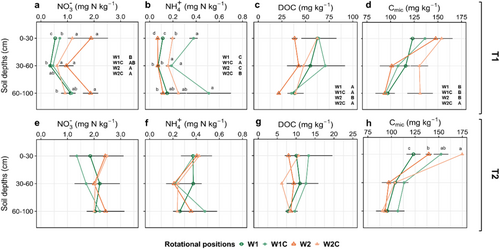
Compost addition increased the soil NH4+ content of both rotational positions, with 235.9% higher soil NH4+ in W1C compared to W1, and 180.3% higher soil NH4+ in W2C compared to W2 (Figure 1b). Compost amendment also increased the overall DOC content of W2C by 45.7% compared to W2, with no obvious differences between W1, W1C and W2. (Figure 1c). In addition, a higher DOC content was evident in the soil W1 but not W2 at T1 compared to the start of the experiment (Table 1), while DOC values significantly decreased at T2 (Figure 1c,g). Cmic exhibited an increasing trend throughout the experiment with higher Cmic values at both T1 and T2 compared to the initial soil Cmic content (Table 1). Cmic was also significantly elevated by 22.5% in the 60–100 cm layer of W2C compared to W1, W1C and W2 (Figure 1d). The Vmax of BGU was significantly increased in the compost-amended W1C (Supporting Information S1: Figure S3a), while the highest Vmax of LAP was found in W1 compared to W1C, W2 and W2C (Supporting Information S1: Figure S3b). At T2, there were no differences in NO3−, NH4+ and DOC between the rotational positions with and without compost addition (Table S2; Figure 1e,f,g). However, there was a 41.8% and 25.4% higher Cmic in W2C compared to W1 and W2 in the topsoil (Figure 1h).
3.2 Belowground Allocation of 13C and 15N at T1 and T2
An 18.1% higher absolute excess of 13C (hereafter called 13C excess) of the soil in W2C compared to W1 was found at T1 across all depths (Figure 2a). Soil was significantly enriched in 13C in the compost amended rotational positions, which was not the case for the 13C excess of DOC and Cmic (Table S1). However, we found higher 13C excess of DOC in W2C and W2 compared to W1 (Figure 2b), with no differences in 13C excess of Cmic and 15N excess of the soil between the rotational positions (Figure 2c,d). At T2, soil depth and not rotational position or compost application had a significant main effect on the abovementioned response variables (Supporting Information S1: Table S2; Figure 2e,f,g), except the higher 15N excess of the soil in W1 and W1C was observed compared to W2C (Figure 2h).
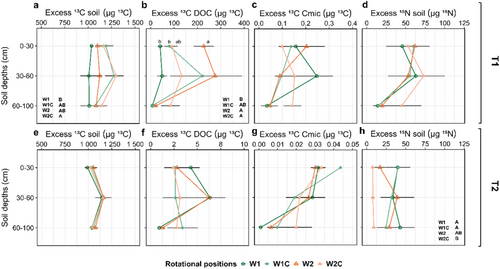
3.3 Allocation of 13C, 15N, 2H and 18O Within the Plant and Biomass Accumulation
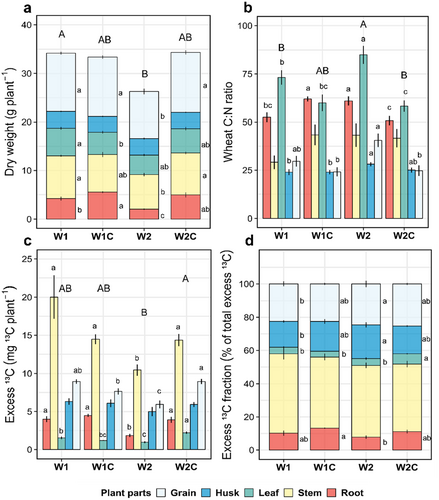
Compost addition to W1C increased root biomass and reduced leaf biomass compared to W1 (Supporting Information S1: Table S3; Figure 3a). W2 exhibited a 23.2% reduction in biomass compared to W1, which was evident for all plant parts except the leaves and the husks (Figure 3a). The addition of compost significantly compensated for the reduction in biomass of W2 in all plant parts (Figure 3a). Plant C:N ratio was also strongly affected by rotational position and compost addition (Supporting Information S1: Table S3). We observed a 19.2% and 22.3% reduction in the C:N ratio of W1 and W2C compared to W2 (Figure 3b). Compost addition did not change the overall plant C:N ratio of W1C plants compared to W1, but increased the C:N ratio of their roots. Compost addition resulted in 46.2% higher 13C excess in the biomass of W2C compared to W2 (Supporting Information S1: Table S4; Figure 3c). Notably, the 13C excess of W2 grains was significantly lower than that of W1, W1C and W2C. This was also the case for all plant parts except the leaves and husks. We also found an increase in the relative allocation of 13C to grains and husks in W2 compared to W1 (Figure 3d). Compost addition also increased the relative allocation of 13C to roots in both W1C and W2C compared to their unamended counterparts, W1 and W2.
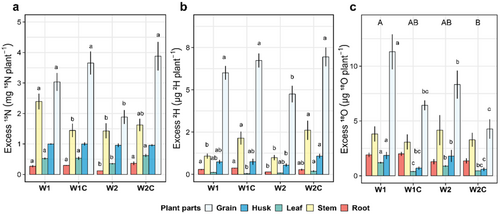
W2 showed a decreased 15N excess in all plant parts, except for the husks, compared to W1 and W1C (Figure 4a). Compost addition increased the 15N excess in all plant parts in W2C, except for stems and husks, compared to W2. A similar trend was observed when measuring the 2H excess of the different plant parts of the rotational positionfs. The grains of W1, W1C and W2C had a 26.4%, 41.8% and 46.2% higher 2H excess compared to W2, respectively (Figure 4b). Compost addition increased the 2H of the stems in both W1C and W2C compared to their unamended counterparts. We also observed a significant decrease in the overall 18O excess of W2C compared to W1 (Figure 4b). Compost addition reduced the amount of 18O incorporated into the grains of W1C and W2C compared to W1 and W2, respectively. Plant biomass was positively correlated with the 13C, 15N, 2H and 18O excess in W1 and W2, with and without compost addition (Supporting Information S1: Figure S4).
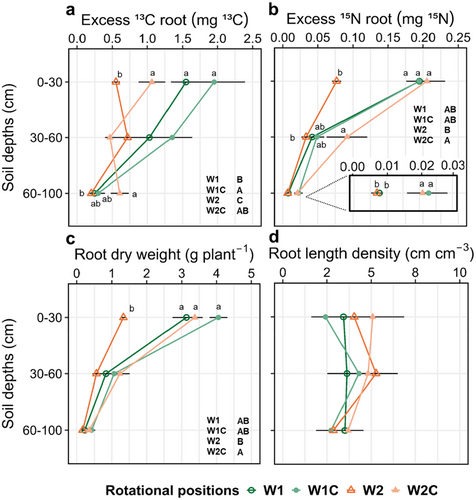
The 13C excess of the W2 roots in the topsoil was on average 64.4%, 71.7% and 44.3% lower compared to W1, W1C and W2C, respectively (Supporting Information S1: Table S5; Figure 5a). In the 60–100 cm soil layer, compost addition increased the 13C excess of the roots of W2C compared to W2. A similar trend was observed with respect to the 15N excess with W2C, with increased values compared to W2 throughout the soil profile (Figure 5b). Root dry weight was significantly reduced in the topsoil of W2 compared to W1, W1C and W2C (Figure 5c). In the 60-100 cm soil layer, compost addition increased root dry weight in W2C compared to W2. Finally, RLD was similar across all rotational positions (Figure 5d).
4 Discussion
Using a multiple stable isotope labelling technique and an outdoor mesocosm setup, we assessed how the rotational position and compost affect the productivity of WW. Our results suggest that successive WW was negatively affected by a lower soil nutrient content and microbial biomass compared to WW after oilseed rape. Compost mitigated this effect and improved root growth, nutrient uptake and water use in the successive WW rotation.
4.1 Initial Soil Characteristics as the Basis for Legacy Effects on Plant Performance
The soil legacy of the preceding crop of WW had a significant impact on the soil nutrient status and thus, on nutrient availability and uptake of the following WW. In our experiment, a higher mineral N content (both NO3- and NH4+) and a generally higher nutrient content was measured in the soil of W1 compared to W2, together with a higher Cmic (Table 1). This confirmed the beneficial management practice of cultivating oilseed rape before WW. Previous studies have shown that the soil after oilseed rape contains high levels of mineral N that is directly available for plant uptake (Groeneveld et al. 2024; Kaloterakis et al. 2024a). In these studies, the higher microbial biomass buildup at the tillering stage in W2 compared to W1 was suggested to induce N immobilisation and, as a result, reduced N availability for WW. A potential dysbiosis in the soil of W2 was also discussed as a confounding factor for this early growth reduction in successively grown WW.
Although the residues of oilseed rape are rich in N, the higher content of other nutrients, such as SO42−, Mg, plant available P and K, provided the basis for a better early establishment of W1 in the field compared to W2. The initial Cmic of W1 was significantly higher than W2, which was associated with a higher DOC content. These two variables respond similarly in cases where accelerated decomposition produces more labile C (glucose) in the soil that can be directly used by the microbes and be incorporated into Cmic. Together with the higher mineral N content in W1, this suggests that microbes are neither C- nor N-limited and do not immobilise N early in the growing season.
4.2 Evidence of Soil Legacy at Later Growth Stages and Compensation of Initial Disadvantages of W2 by Addition of Compost
Flowering is a very important stage that determines the grain yield of WW and, as such, it was important to assess the soil biochemical conditions and the potential growth-promoting role of the compost addition. At T1, the soil NO3- content was already very low, indicating high N uptake rates by WW and/or high N losses due to denitrification, which was not the focus of our study. The higher NH4+ content at T1 for the compost-amended W1C and W2C showed that compost stimulated N mineralisation and provided a slow and steady supply of nutrients that could be utilised by plants and microbes. In fact, it is estimated that only a small portion of OA is mineralised within the first year after its application, depending on its composition (Agegnehu et al. 2017). The low C:N ratio of 13 of the green-waste compost applied in this study should make the compost more readily available for microbial (especially bacterial) use, which was probably the reason why higher N mineralisation occurred in compost-amended soils, a possible cause of the significant compensation for yield losses in the self-successive WW rotation. The higher NH4+ availability in W2C compared to W2 is linked to its higher Cmic, which was also the highest among all rotational positions. This means that even though W2 started with a lower Cmic and DOC compared to W1, compost amendment compensated for this initial difference, which potentially led to a higher NH4+ in W2C. This is further supported by the higher DOC in W2C compared to W2.
According to our first hypothesis, compost application stimulated glucose release, as shown by the high BGU activity, but only in W1C and not in W2C. LAP activity was the highest in W1, suggesting that LAP stimulated organic N mineralisation and the production of NH4+ (Yang et al. 2023). Kaloterakis et al. (2024a) found a higher BGU activity in W2 compared to W1 at tillering and attributed this to the quality of the preceding crop residues (higher N content of the residues of oilseed rape compared to WW). In our study, the BGU activity was more linked to compost mineralisation at T1 than decomposition of the preceding crop residues. In contrast to mineral fertilisers, compost provides nutrients in organic forms that need to be mineralised first before they become available for plant uptake, acting as a slow-release fertiliser (Amlinger et al. 2003; Al-Bataina et al. 2016). Furthermore, compost addition to soil is known to induce higher exudation rates that in turn stimulate nutrient solubilisation and uptake by plants (Rosa et al. 2021; Kumar et al. 2024). Another potential explanation for the increased NH4+ in the compost-amended treatments may be that compost application boosted root growth. The larger root system of W2C may have increased the C input either through increased rhizodeposition or increased root litter decomposition (Remus et al. 2022), which fuelled microbial activity, N mineralisation and thus, NH4+ production.
4.3 Belowground Allocation of 13C and 15N at the Flowering and Grain Ripening Growth Stages
At T1, we traced 13C in different belowground pools and found that soil depth had a strong influence on the transfer of the 13C label in the Cmic pool, with lower values in the 60-100 cm soil layer, which is in contrast to previous studies (Van de Broek et al. 2020; Kaloterakis et al. 2024b). The amount of 13C label that ends up in the Cmic pool is dependent on the C use efficiency of the microbes, which changes at different depths (Li et al. 2021). Soil depth strongly affected the 13C excess in the DOC pool, which is linked to the root distribution across the soil profile but also exudation intensity and root litter turnover (Kaloterakis et al. 2024b).
According to our first hypothesis, we found that a significantly higher amount of the 13C label ended up in the soil of compost-amended WW, especially W2C. This shows that WW benefited from the compost by stimulating root growth and, as a result, increased 13C translocation below ground. This higher 13C in the soil of W2C compared to W2 was utilised by the larger microbial pool of W2C, indicated by higher Cmic values. At T2, the 13C label had already been consumed by the soil microbes, resulting in no differences in the 13C excess of the soil. The increased 13C of the DOC in W2 compared to W1 at T1 contradicts the findings of (Kaloterakis et al. 2024b), who observed higher 13C of the DOC in the sandy loam of W1 compared to W2 at late flowering and linked this to the increased and sustained belowground allocation of photosynthates. Here, the higher 13C of the DOC in W2 might be the result of accelerated C turnover as a response to short-term nutrient availability. Increased root decay in W2 due to accumulation of soil pathogens, a common observation in monocropping systems, could be an alternative explanation (Peralta et al. 2018). Over time, the 13C in the soil pool was utilised by soil microbes (used for growth and maintenance), resulting in no differences at T2. The significantly lower 15N excess of the soil in W2C compared to W1, W1C and W2 suggests that W2C plants took up the labelled N earlier in the season, or that N losses were higher in W2C compared to the other rotational positions. W1 and W1C had a higher soil 15N excess and had incorporated similar amounts of 15N into their biomass as W2C, favouring the possibility of earlier 15N uptake or increased N losses. WW is known to utilise the available N more efficiently when compost is applied (Keeling et al. 2003), most likely due to the supply of other important plant-available nutrients.
4.4 Compost Addition Mitigated Yield Decline of W2 by Improving Plant Growth, Nutrient and Water Uptake From the Subsoil
The initial soil legacy of W2 persisted through the late growth stages of WW, resulting in a substantial yield decline compared to W1. This finding aligns with the conclusions of previous studies that reported a decline in the growth of successively cultivated WW (Arnhold et al. 2023b; Kaloterakis et al. 2024b). W2 exhibited reduced root growth, as indicated by the reduction in RDW (Figure 5c). This was not associated with distinct changes in RLD between W1 and W2 (Figure 5d). In line with our finding, Arnhold et al. (2023a) did not observe differences in the RLD between W1 and W2 at tillering in a field experiment. However, they found higher RLD in W1 at late flowering in a year with high summer precipitation. In the present experiment, W1 allocated a greater amount of freshly assimilated C to the roots than W2, which was followed by a more efficient utilisation of the 15N-labelled fertiliser. The addition of compost led to a significant increase in the 13C content of the root in both the topsoil and subsoil of W1C and W2C (Figure 5a). The enhanced root growth in W2C led to an improvement in the N uptake, as evidenced by the significantly higher 15N excess of the roots at all three measured soil depths (Figure 5b).
The plant biomass exhibited a strong correlation with the excess of all four isotopes applied and measured in the plant biomass, as indicated by the correlation analyses. The positive plant-soil feedback observed in WW following oilseed rape was associated with a significantly higher 13C excess in W1 compared to W2. Despite the increased root biomass observed in W1C following compost addition, no concomitant yield enhancement was detected when compared to W1. The incorporation of compost into the W2C treatment was found to be a highly advantageous management strategy, effectively mitigating the observed decline in yield of W2. Compost has been demonstrated to enhance WW yield and mitigate take-all severity at both lower and higher application rates than the 40 t ha−1 employed in this study (Tilston et al. 2005; Demelash et al. 2014). The incorporation of compost into soil has also been demonstrated to exert a favourable influence on nutrient levels, thereby facilitating the mineralisation of N and, consequently, increasing the availability of NH4+ for uptake by plants and microbes. As hypothesised, this treatment promoted root growth, nutrient uptake, and plant performance, resulting in increased levels of 13C and 15N measured in plant biomass and particularly in the grains. The increased N uptake in W2C as compared to W2 was also reflected in the substantial decrease in the plant C:N ratio for all plant parts with the exception of the husks, which is analogous to the reported increased N uptake at tillering by W1 as reported by Kaloterakis et al. (2024a). Thus, compost application increased the N use efficiency in W2C compared to W2.
In comparison with W2, W1 exhibited an increased tendency of water uptake from the topsoil and subsoil, as evidenced by the elevated 2H and 18O excess, respectively. This phenomenon is likely attributable to the superior functionality of the root system of W1, which is presumably the result of the soil legacy of oilseed rape. The addition of compost resulted in a significant increase of 2H excess, i.e., in water from the topsoil in W2C compared to untreated W2 (Figure 4b). In contrast, water uptake from the subsoil was, at least at times, significantly lower in W2C compared to W2, reflected in the significantly lower 18O excess in the grains and husks (Figure 4c). This observation is linked to the enhanced root growth and nutrient uptake by W2C, especially in the topsoil (Figure 5c), as well as to the increased water-holding capacity of the topsoil compared to the subsoil and the unamended control, highlighting the multi-faceted beneficial properties of compost amendment.
5 Conclusions
In this study, we compared two different compost-amended and unamended rotational positions of WW to assess the potential of compost to mitigate yield losses in successive WW rotations. The beneficial soil legacy of oilseed rape led to enhanced performance of the following WW compared to the soil legacy of WW. This effect was substantial and evident at the flowering and grain-ripening stages. According to our hypotheses, compost application notably mitigated the initial disadvantage of W2 by promoting belowground allocation of freshly assimilated C, enzymatic activity, nutrient mineralisation, root growth, N and water uptake by WW, thereby effectively compensating for the substantial yield loss of the successively grown WW. Our results provide empirical evidence and a mechanistic understanding on the beneficial role of compost amendment in mitigating yield decline in successively grown WW. We showed that compost is a promising management practice to increase yield in successive WW rotations and positively mitigates the negative plant-soil feedback in monotonous WW rotations. Compost application influenced key rhizosphere processes and has the potential to foster wheat yield and sustain farming profitability, while utilising available green waste resources in alignment with sustainable practices of circular agriculture. However, further experiments on the long-term effect of compost application on continuous WW rotations are needed to assess the duration of the yield loss compensation.
Author Contributions
Nikolaos Kaloterakis: conceptualisation, data curation, formal analysis, investigation, methodology, software, validation, visualisation, writing – original draft. Mehdi Rashtbari: data curation, writing – review and editing. Bahar S. Razavi: funding acquisition, project administration, resources, writing – review and editing. Rüdiger Reichel: writing – review and editing. Nicolas Brüggemann: conceptualisation, funding acquisition, methodology, project administration, resources, supervision, validation, writing – review and editing.
Acknowledgements
We acknowledge the support of Jessica Arnhold, Dennis Grunwald and Heinz-Josef Koch in providing the soil for the experiment, as well as the lab support of Sirgit Kummer and Holger Wissel in soil and plant C and N analyses. This study was funded by the German Federal Ministry of Research, Technology and Space (BMFTR) in the framework of the funding initiative “Rhizo4Bio - Importance of the Rhizosphere for the Bioeconomy”, project “RhizoWheat” (grant number 031B0910B). Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data will be uploaded to the BonaRes Repository for Soil and Agricultural Research Data.




