Influence of native rhizobacteria co-inoculation and formulation of bacterial inoculants on the growth and yield of common bean (Phaseolus vulgaris L.)
Abstract
Introduction
Incorporation of inoculum in different carrier materials may increase the efficacy of bacterial inocula.
Materials and Methods
Field experiments were conducted using two strains of rhizobium and phosphate-solubilizing bacteria (PSB) and their respective combinations using different carrier materials in common bean-growing regions in three soil types in Kenya. The field experiment was laid out in a split-plot arrangement with the strain inoculations as the main plot while the subplots consisted of the carrier materials (filter mud, peat moss and yeast extract mannitol broth [YEMB]). Each main plot included two controls: uninoculated negative control and uninoculated controls that received N and P fertilizer. The experiment was conducted for two cropping seasons. Data were collected on the nodulation, shoot and root biomass and yield.
Results
Co-inoculation of the common bean with Rhizobium phaseoli + Bacillus aryabhattai strains had significantly higher number of nodules (55 nodules per plant) compared to single R. phaseoli inoculation (38 nodules). The co-inoculation of the rhizobia and the PSB yielded statistically at par with the application of diammonium phosphate (18:46:0) across the soil types and seasons. The use of filter mud as a carrier material led to a higher number of nodules for most of the rhizobia strains inoculation and their respective co-inoculation with the bacillus strains. Significantly higher yield was obtained with the filter mud (1.64 Mg ha−1) while there was no significant difference in the yield of common bean between peat moss and YEMB as carrier materials for the bacterial strains.
Conclusion
The solid carrier material, specifically filter mud, showed potential for use in the formulation of inoculants. Specific co-inoculation of rhizobia (R. phaseoli and Rhizobium pusense strains) and PSB (Paenibacillus polymyxa and B. aryabhattai) increased the growth, nodulation and yield of common bean more efficiently than the control.
1 INTRODUCTION
Plant growth-promoting rhizobacteria (PGPR) has huge potential in the production of biofertilizers that can be harnessed to increase crop yields through their different mechanisms of action (Hayat et al., 2010). Use of PGPR mixtures whose functions are known and that could act synergistically is of interest as they offer multiple modes of action (Backer et al., 2018; Santoyo et al., 2021). Inocula containing different beneficial microorganisms affect the functional groups of soil microorganisms in different ways (Hayat et al., 2010). In general, co-culturing was found to reduce production costs and to solve problems related to the process parameters, use of nutrients and oxygen demand between co-cultures (Hickert et al., 2014). While the co-culturing of different microorganisms within the same media seems to be a frequently used approach in some fermentation processes, it has not been widely used in the formulation of plant-beneficial microbial inoculants (Vassilev et al., 2015).
Despite several benefits brought by inoculation technology, a major limitation is survival of inoculum in soil and rhizosphere. Formulation is a key aspect for producing inoculants and can determine the success or failure of an effective biological agent (Ruíz-Valdiviezo et al., 2015). To transfer viable bacteria in optimum population from the laboratory to the field, suitable carrier materials are an important factor in both biofertilizer production and crop response (Brahmaprakash & Sahu, 2012). Therefore, the challenges related to suitable biotechnological processing and efficiency need to be overcome for their use as biofertilizers to be successful (Berger et al., 2018).
Various bacterial inoculant formulations have been developed using either liquids or solids as carrier materials. However, the efficiency of inoculants in biofertilizer formulations greatly depends on the choice of carrier materials (Sohaib et al., 2020). Therefore, to improve the efficiency of the bacterial inoculant biofertilizers, there is a need to design them with the appropriate formulation (Brahmaprakash & Sahu, 2012). A suitable carrier material must have a stable pH, high water retention, cost effective, easily available, easy to sterilize and nontoxic for the strain or environment (Ruíz-Valdiviezo et al., 2015).
Use of cheap and locally available carrier materials would ensure long-term inoculant survival and enhance plant growth once the biofertilizer is applied (Gupta et al., 2022). Carrier materials with good physicochemical properties are also a source of nutrients to the microbes, which ensure their better survival (Sohaib et al., 2020). Peat formulations are the most used in the inoculation industry (Chaudhary et al., 2020). However, the overuse of peat has environmental implications since it is a nonrenewable natural resource. The use of peat has also led to a rapid decrease of its reserves leading to increases in price, thus limiting its use (Tabassum et al., 2017). Therefore, there is a need to test the efficacy of other locally and easily available carrier materials. The use of agricultural waste as inoculant carriers is one option that can be explored since they are readily available and cheap (Yadav & Raverkar, 2021). Sugar waste has been found to allow a higher growth of legume-nodulating bacteria in media compared to yeast extract mannitol (YEM) standard media used for legume-nodulating bacteria (Singh et al., 2014). Strains Sinorhizobium mexicanum ITTGR7T, Rhizobium calliandrae LBP2-1T and Rhizobium etli CFN42T with perlite sugarcane bagasse as a carrier led to the largest number of nodules in common bean (Ruíz-Valdiviezo et al., 2015). Other studies reported that liquid inoculants resulted in better nodulation, grain N and P uptake and available soil N and P than carrier-based inoculants (Dudeja et al., 2011). The effect of carrier materials of inoculants from natively isolated bacterial strains from Kenya has not been explored and documented. This has made it difficult to obtain effective biofertilizers from the native bacterial strains that can be made available to farmers to boost bean production. This study therefore determined the effect of inoculant carrier material and co-inoculation of rhizobia and phosphate-solubilizing bacteria (PSB) on common bean growth and yield.
2 MATERIALS AND METHODS
2.1 Strains and description of the experimental sites
The strains Rhizobium pusense (B4), Rhizobium phaseoli (B3), Bacillus aryabhattai (strain 2-Sj-2-1-13-T; NCBI reference sequence: KJ009477.1) and Paenibacillus polymyxa (strain E681; NCBI reference sequence: NC_014483.1) were tested in field trials using different formulations in common bean-growing regions in three agroecological zones: upper midland 1, 34°44.812′ E, 0°16.489′ N (Kakamega; eutric nitisols); lower highland 3, 36°5.562′ E, 0°19.436′ S (Nakuru; mollic andosols) and upper midland 3, 35°0.158′ E, 1°1.145′ N (Trans Nzoia; rhodic nitisols) (Jaetzold et al., 2007). The strains B. aryabhattai and P. polymyxa isolated from the common bean nodules were obtained from Soil Microbial Ecology Laboratory, Egerton University from the isolation work done earlier by Korir et al. (2017). Similarly, the rhizobia strains (R. pusense and R. phaseoli) were isolated from bean-growing areas of Busia and Bungoma Counties by Wekesa et al. (2021). In vitro studies of these strains have been shown to have a higher indole acetic acid production and phosphate solubilization efficiency and promoted growth of common beans in the greenhouse (data not published). The experiment was conducted in two cropping seasons (2020 short rains (SRs), October–December and 2021 long rains (LRs) seasons, April–July). During the second season (2021LRs), the same fields were used, and the specific treatments used in the experimental plots as in 2020SR season, with the same plot receiving the same treatment as the first season.
2.2 Soil sampling and analysis
Soil from the three study sites was dug out at a depth of 0–20 cm and collected in zip-lock bags and stored at 4°C in the laboratory. The soils were air-dried, prepared and analysed using standard soil testing procedures as described by Okalebo et al. (2002). Soil pH was determined using a glass electrode pH metre at 1:2.5 soil/water ratio. Available P was extracted using the Olsen extractant and determined using the ammonium vanadate method using a spectrophotometer at 470 nm. Organic carbon was determined by Walkley and Black sulphuric acid–dichromate digestion followed by back titration with ferrous ammonium sulphate, while nitrogen was determined using the Kjeldahl method. Soil texture was determined using the hydrometer method while the bulk density was determined using the oven-drying method after soil samples were collected using core rings. Exchangeable sodium, potassium, calcium, magnesium and selected micronutrients (iron, zinc and copper) were also determined using procedures as described by Okalebo et al. (2002).
2.3 Preparation of inoculant formulations
For the liquid carrier material, the Rhizobium isolates were grown to 109 CFU mL−1 in YEM broth on a rotary shaker for 72 h at 28 ± 2°C. Likewise, the PSB isolates were grown in nutrient broth at 30°C for 48 h to get a population density of about 109 CFU mL−1. The liquid inoculum was stored in screw cap bottles kept at 4°C.
For the solid formulation, filter mud and peat moss were used as carrier materials. Filter mud, a biproduct in sugar processing, is collected from the bottom of the crushing machine. Filter mud is locally available in most of the sugar factories in Kenya. The filter mud was sourced from Chemilil sugar factory in Kisumu County. The peat moss was obtained from the Ondiri Wetland in Kiambu County. After drying and sieving (2 mm mesh), the filter mud and peat moss were wetted to about 45% moisture-holding capacity, and 20 g was packed into 4 × 6 in. autoclavable polyethylene bags and sealed to maintain the moisture content (Balume et al., 2015). The bags were autoclaved for 60 min at 121°C (Somasegaran & Hoben, 1994). Complete sterilization was checked by placing a sample of the carrier material in Petri plates containing nutrient agar, incubated at 28 ± 2°C for 3–5 days. When microbial growth was detected, the carrier material was further sterilized as described above. In an aseptic condition, 1 mL of strain broth (turbidity: 1 × 109 cells mL−1) of each of the bacterial strains was injected per 20 g of sterilized carrier using sterile syringes and mixed carefully. The syringe-punctured area was wiped with 70% alcohol, and an adhesive seal was applied. The inoculant rhizobia bags were incubated at 28 ± 2°C for 6 days for curing. During the curing period (6 days), the carrier was mixed daily to ensure even distribution of the inoculum.
2.4 Land preparation and experimental design
The fields were ploughed and ridged at a spacing of 40 cm apart. The plot size was 2 m × 2 m (4 m2) and spacing of 40 cm between rows and 10 cm between plants. Each plot had six rows and 21 hills per row giving a total of 126 hills per plot. The field experiment was laid out in a split-plot arrangement with the strain inoculations as the main plot while the subplots were the inoculant formulation. Each main plot included two controls, uninoculated negative control and uninoculated controls that received N and P fertilizer by application of diammonium phosphate (DAP) at the rate of 125 kg ha−1 (22.5 and 57.5 kg ha−1 N and P, respectively; 50 g of DAP applied per plot). Subplots consisted of the carrier materials such as filter mud, peat moss, YEM broth and uninoculated negative control. The experiment was replicated three times.
2.5 Inoculation and planting
-
Main plots = treatments, that is, strain/strains combinations + controls (DAP and negative control).
-
TR-1 = R. pusense (B4) + B. aryabhattai.
-
TR-2 = R. pusense (B4) + P. polymyxa.
-
TR-3 = R. phaseoli (B3) + P. polymyxa.
-
TR-4 = R. phaseoli (B3) + B. aryabhattai.
-
TR-5 = R. pusense (B4).
-
TR-6 = R. phaseoli (B3).
-
Subplots: FM, filter mud, PM, peat moss, BR, broth, CTRL, uninoculated negative control.
2.6 Plant sampling and data collection
Plant samples were taken at two growth stages. At 50% flowering, six plants were randomly sampled from the inner rows of each plot avoiding the outer rows to evaluate nodulation (number of effective nodules), root and shoot dry weight. The roots were washed under gentle running tap water to remove soil adhering to the roots. The nodules were then separated from the roots using a scalpel blade. The effectiveness of the nodules was checked by cutting them longitudinally and checking on the interior colour. Only nodules with pink coloration were counted and recorded. The shoots and roots were oven-dried at 65°C for 72 , and their dry weights were recorded.
At harvest maturity, harvesting was done when plants and seeds were dry. All the plants in the three inner rows were counted (55–60 plants) and the pods were collected for further processing. The pods were then threshed and the grains from the harvested area of each plot were cleaned and weighed at 11% moisture content to estimate the grain yield of a plot per hectare. Accordingly, the seed weight of a plot divided by the number of plants harvested from that plot was multiplied by the number of plants calculated per hectare.
2.7 Data analysis
Data were analysed using SAS Statistical Package Version 9.3 (SAS 2013). The data was first tested for normal distribution and the data for nodule counts was normalized by logarithmic transformation (Log10 x + 1) before analysis. To determine the effects due to inoculation and formulation, analysis of variance at a 95% confidence limit was done and means were separated using the Tukey's test at α = 0.05 whenever there were significant effects (p ≤ 0.05).
3 RESULTS
3.1 Soil physicochemical properties
The selected soil physicochemical and biological properties are presented in Table 1. The soil pH for the three study sites was medium alkaline. Andosol had low nitrogen (0.17%), while ferralsols and nitisols had adequate nitrogen levels, 0.25% and 0.28%, respectively. The phosphorous level was highest in the ferralsol and least in the nitisol, although the levels were high in all the sites (>15 mg kg−1; Mallarino & Blackmer, 1992). Organic matter was moderate in andosols and adequate in both ferralsol and nitisol. The micronutrients were generally adequate except for copper in ferralsol and nitisol and zinc in nitisol which were low.
| Soil parameter | Andosols | Ferralsols | Nitisols |
|---|---|---|---|
| Soil pH | 7.84 | 8.28 | 7.97 |
| Total nitrogen (%) | 0.17 | 0.25 | 0.28 |
| Total organic carbon (%) | 2.01 | 2.79 | 3.17 |
| Phosphorus (Olsen) (mg kg−1) | 97 | 132 | 83 |
| Potassium (Cmol kg−1) | 1.66 | 2.18 | 1.40 |
| Calcium (Cmol kg−1) | 25.2 | 40.0 | 61.4 |
| Magnesium (Cmol kg−1) | 5.31 | 5.56 | 5.56 |
| Manganese (Cmol kg−1) | 0.70 | 0.29 | 0.11 |
| Copper (mg kg−1) | 3.06 | 0.62 | 0.36 |
| Iron (mg kg−1) | 33.9 | 12.1 | 10.7 |
| Zinc (mg kg 1) | 5.02 | 13.3 | 0.15 |
| Sodium (Cmol kg−1) | 0.81 | 1.55 | 0.47 |
| Electrical conductivity (mS cm−1) | 0.46 | 0.81 | 0.54 |
| Sand (%) | 30 | 40 | 24 |
| Clay (%) | 52 | 32 | 58 |
| Silt (%) | 18 | 28 | 18 |
| Rhizobia population (108 CFU g−1 of soil) | 0.51 | 0.52 | 0.07 |
3.2 Analysis of variance
The analysis of variance showed that there was significant site and season effect on the number of nodules per plant, the root weight, shoot weight and yield of common bean. The inoculation also had a significant effect (p ≤ 0.001) on the number of effective nodules, root and shoot weight and yield of common bean. There was a significant site × inoculation effect on the number of effective nodules and the shoot dry weight of common bean. For the season × inoculation interaction, the effect was significant (p ≤ 0.01) on the shoot weight alone. The formulation had a significant effect on the yield; furthermore, the inoculation × formulation interaction effect was significant (p ≤ 0.05) on the yield of common bean (Table 2).
| Source of variation | df | Number of effective nodules per plant | Root weight (g plant−1) | Shoot weight (g plant−1) | Yield (Mg ha−1) |
|---|---|---|---|---|---|
| Replicate | 2 | 0.088 | 0.843 | 197.33 | 1.589 |
| Site | 2 | 1.407*** | 27.40*** | 5416.54*** | 8.287*** |
| Inoculation | 7 | 1.891*** | 5.636*** | 371.74*** | 3.095*** |
| Site × inoculation | 14 | 0.099* | 0.507ns | 77.67* | 0.216ns |
| Season | 1 | 3.324*** | 147.117*** | 13,732.07*** | 11.398*** |
| Season × inoculation | 7 | 0.083ns | 0.545ns | 113.75** | 0.216ns |
| Main plot error | 14 | 0.117 | 0.161 | 12.91 | 0.119 |
| Formulation | 2 | 0.047ns | 0.151ns | 23.03ns | 0.346* |
| Inoculation × formulation | 10 | 0.029ns | 0.198ns | 15.97ns | 0.348** |
| Error | 371 | 0.053 | 0.332 | 40.81 | 0.145 |
| Total | 431 | ||||
| CV (%) | 18.3 | 25.8 | 29.1 | 25.0 |
- Abbreviations: CV, coefficient of variation; ns, not significant.
- *, **, and *** significant at p < 0.05, 0.01 and 0.001, respectively.
3.3 Effect of co-inoculation and inoculant formulation on the number of nodules of common bean
Single inoculation of the common bean with the rhizobia and co-inoculation with the PSB led to significantly (p ≤ 0.001) higher number of effective nodules than the DAP application and the uninoculated control. This was consistent across the three study sites and the two cropping seasons (Table 3). For instance, in the andosol, during the 2020SR season, the inoculated and co-inoculated treatments had significantly (p ≤ 0.001) higher number of effective nodules than the DAP application and the uninoculated control (Table 3). The mixed inoculation of R. phaseoli + B. aryabhattai strains had significantly (p ≤ 0.001) higher number of effective nodules (55 nodules) compared to single R. phaseoli inoculation (38 nodules). There was no significant difference between the single R. pusense and R. pusense + B. aryabhattai. However, co-inoculation with P. polymyxa suppressed the number of effective nodules as compared to the single inoculation for both R. pusense and R. phaseoli (Table 3). During the 2021LR season, there were no significant differences in the number of effective nodules between the inoculated treatments. However, there were significant differences compared to the DAP application and the uninoculated control (Table 3). In the nitisols, during the 2020SR season, inoculation of the common bean with the rhizobia and the PSB led to the formation of significantly (p ≤ 0.001) more effective nodules per plant than the uninoculated control and the application of DAP. There was no significant difference among the inoculated treatments except for the co-inoculation of R. phaseoli + B. aryabhattai. During the 2021LR season, the co-inoculation of the common bean with R. phaseoli + P. polymyxa and single R. pusense inoculation led to significantly (p ≤ 0.001) higher effective nodule numbers than the other inoculation and the controls. In the ferralsols, inoculation led to a greater number of effective nodules than the uninoculated control and DAP application during both seasons. In 2020SR season, co-inoculation of the common beans with R. phaseoli + B. aryabhattai led to significantly (p ≤ 0.001) highest number of effective nodules, followed by R. phaseoli + P. polymyxa. The R. phaseoli + B. aryabhattai co-inoculation treatments had significantly (p ≤ 0.001) more effective nodules than the single R. phaseoli inoculation (Table 3). In the 2021LR season, co-inoculation with R. pusense + P. polymyxa led to significantly (p ≤ 0.001) higher effective nodule numbers than single R. pusense inoculation (Table 3). There was no significant difference between the single R. phaseoli inoculation with the respective co-inoculation with B. aryabhattai and P. polymyxa (Table 3).
| Treatment | Andosol | Nitisol | Ferralsol | |||
|---|---|---|---|---|---|---|
| 2020SR | 2021LR | 2020SR | 2021LR | 2020SR | 2021LR | |
| Rhizobium pusense + Bacillus aryabhattai | 51.4ab | 16.5ab | 20.9a | 16.2b | 16.6c | 28.7a |
| R. pusense + Paenibacillus polymyxa | 42.9ab | 20.0a | 20.4a | 11.5bcd | 21.7bc | 29.9a |
| Rhizobium phaseoli + P. polymyxa | 42.3ab | 18.8a | 20.8a | 26.4a | 29.9ab | 21.1ab |
| R. phaseoli + B. aryabhattai | 55.2a | 16.7ab | 19.8a | 16.3b | 38.6a | 24.2ab |
| R. pusense | 54.6a | 20.1a | 20.9a | 24.8a | 23.8bc | 17.6bc |
| R. phaseoli | 38.4b | 19.0a | 20.8a | 15.0bc | 29.0b | 26.8ab |
| Diammonium phosphate (18:46:0) | 12.7c | 6.9c | 5.2b | 8.4cd | 6.9d | 9.0c |
| Uninoculated control | 22.1c | 9.5c | 6.0b | 7.3d | 6.6d | 9.0c |
| CV (%) | 18.3 | |||||
- Note: Means followed by different letters within a column are significantly different at α ≤ 0.05.
- Abbreviations: CV, coefficient of variation; LR, long rain; SR, short rain.
The number of effective nodules was affected by the interaction between the bacterial strains and the carrier materials. However, the interaction was not consistent across soil types with the ferralsols and the nitisol showing interactions between the bacterial strain and carrier material (Figure 1a). However, in the andosol, no significant carrier material by bacterial strains interaction was observed. In the ferralsols, for instance, only the broth formulation of R. phaseoli + P. polymyxa elicited formation of more effective nodules compared to the filter mud and peat moss carrier materials (Figure 1a). In the case of filter mud carrier material, its formulation with R. phaseoli + B. aryabhattai and R. pusense led to more effective nodule numbers, while peat moss formulation did not outperform either the broth or filter mud in any of the bacterial strains (Figure 1a). These results were consistent in the nitisol, where the use of filter mud as a carrier material led to a higher number of effective nodules for most of the rhizobia strains inoculation and their respective co-inoculation with the bacillus strains (Figure 1b).
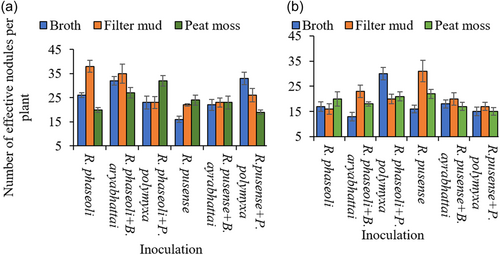
3.4 Effect of co-inoculation on the root dry weight of common bean
Inoculation and co-inoculation of common bean with rhizobia and bacillus strains led to higher root dry weight compared to the uninoculated control, consistent across the three soil types and the two cropping seasons (Table 4). The application of DAP led to a higher root biomass while the uninoculated control had significantly (p ≤ 0.001) lower root biomass (Table 4). In andosol, during the 2020SR season, co-inoculation with R. phaseoli + B. aryabhattai had a mean root weight that was at par with the DAP application. The root biomass resulting from the inoculation with the other bacterial strains was significantly (p ≤ 0.001) higher than the uninoculated control (Table 4). In the 2021LR season, co-inoculation with R. phaseoli + B. polymyxa led to a higher root biomass among the inoculated treatments; however, it was not significantly different from single R. phaseoli and R. pusense inoculation (Table 4). For nitisol, during the 2020SR season, there were no significant differences among the inoculated plants, but they had significantly higher root biomass in comparison to the uninoculated control. In the 2021LR season, co-inoculation of the common bean with R. phaseoli + P. polymyxa and R. phaseoli + B. aryabhattai was performed at par with the DAP application. R. phaseoli + P. polymyxa co-inoculation led to a significantly higher root biomass than the single R. phaseoli inoculation (Table 4). For the ferralsols, co-inoculation of the common bean R. pusense + P. polymyxa led to significantly (p ≤ 0.001) higher root biomass compared to the other inoculated treatments in the 2020SR season. During the 2021LR, there was no significant difference in the root biomass between the various co-inoculation treatments and application of DAP except for the co-inoculation involving R. phaseolus and P. polymyxa (Table 4). However, the single inoculation of either the R. phaseoli or R. pusense had significantly (p ≤ 0.001) lower root biomass than the DAP application but higher than the uninoculated control (Table 4).
| Treatment | Andosol (g plant−1) | Nitisol (g plant−1) | Ferralsol (g plant−1) | |||
|---|---|---|---|---|---|---|
| 2020SR | 2021LR | 2020SR | 2021LR | 2020SR | 2021LR | |
| Rhizobium pusense + Bacillus aryabhattai | 1.17b | 0.53cd | 0.70b | 0.55c | 0.80c | 0.25abc |
| R. pusense + Paenibacillus polymyxa | 1.17b | 0.50d | 0.71b | 0.58bc | 0.87b | 0.31a |
| R. phaseoli + P. polymyxa | 1.27b | 0.63b | 0.73b | 0.65ab | 0.77cd | 0.23bc |
| R. phaseoli + B. aryabhattai | 1.47b | 0.53cd | 0.69b | 0.61abc | 0.80c | 0.25abc |
| R. pusense | 1.30b | 0.57bcd | 0.71b | 0.58c | 0.73cd | 0.22bc |
| R. phaseoli | 1.30b | 0.60bc | 0.72b | 0.57c | 0.77c | 0.27ab |
| Diammonium phosphate (18:46:0) | 1.57a | 0.73a | 0.83a | 0.82a | 1.19a | 0.28a |
| Uninoculated control | 0.87c | 0.40e | 0.53c | 0.50d | 0.70d | 0.20c |
| CV (%) | 25.8 | |||||
- Note: Means followed by different letters within a column are significantly different at α ≤ 0.05.
- Abbreviations: CV, coefficient of variation; LR, long rain; SR, short rain.
3.5 Effect of co-inoculation on the shoot biomass of common bean
Co-inoculation of common bean with the rhizobia and bacillus strains had a significant (p ≤ 0.001) effect on the shoot biomass with all the inoculated plants having higher biomass than the uninoculated control across all the soil types and cropping seasons (Table 5). In comparison to the application of DAP, most of the inoculated plants had similar shoot biomass, particularly in the second (2021LR) season. For instance, in andosol, the R. phaseoli + P. polymyxa co-inoculated plants had the highest shoot biomass (Table 5).
| Treatment | Andosol (g plant−1) | Nitisol (g plant−1) | Ferralsol (g plant−1) | |||
|---|---|---|---|---|---|---|
| 2020SR | 2021LR | 2020SR | 2021LR | 2020SR | 2021LR | |
| Rhizobium pusense + Bacillus aryabhattai | 11.9d | 5.5bc | 5.8a | 3.8b | 6.2ab | 6.8bcd |
| R. pusense + Paenibacillus polymyxa | 13.6c | 5.6bc | 5.6ab | 3.7b | 5.9ab | 7.3a |
| Rhizobium phaseoli + P. polymyxa | 13.7c | 6.2a | 5.8a | 4.2ab | 6.1ab | 7.0abc |
| R. phaseoli + B. aryabhattai | 10.8d | 5.4c | 5.5b | 4.0b | 5.9ab | 6.5d |
| R. pusense | 13.8c | 5.9ab | 5.8a | 4.6a | 6.4ab | 6.6cd |
| R. phaseoli | 15.4b | 5.7bc | 5.8a | 4.1b | 6.2a | 6.8bcd |
| Diammonium phosphate (18:46:0) | 18.6a | 5.8ab | 6.1a | 4.4ab | 6.8a | 7.1ab |
| Uninoculated control | 10.0e | 4.5d | 4.6c | 3.2c | 5.2c | 5.8e |
| CV (%) | 29.1 | |||||
- Abbreviations: CV, coefficient of variation; LR, long rain; SR, short rain.
- Note: Means followed by different letters within a column are significantly different at α ≤ 0.05.
3.6 Effect of inoculation and inoculant formulation on the yield of common bean
Inoculation and co-inoculation with the bacterial strains significantly (p ≤ 0.001) increased the yield of common bean over the uninoculated control. This was consistent across all the soil types and seasons except for the single rhizobia inoculation in nitisol during the 2021LR season (Table 6). Most of the co-inoculation of the rhizobia and the PSB yielded statistically at par with the application of DAP in the three soil types and the two seasons except for the R. pusense + B. aryabhattai in the andosol during the 2021LR season (Table 6). For instance, the co-inoculation led to more yield than the DAP application in the andosol (2020SR), nitisol (2020SR) and ferralsol (2021LR), though the difference was not significantly different (Table 6). Some specific rhizobia–PSB co-inoculation led to significantly (p ≤ 0.001) higher yield than their respective single rhizobia inoculation. For example, in ferralsol, R. pusense + B. aryabhattai co-inoculation led to significantly higher yield than the single R. pusense inoculation in the 2020SR season, while in the 2021LR, co-inoculation of either the rhizobia strains with P. polymyxa led to significantly (p ≤ 0.001) higher yield than their single inoculation (Table 6).
| Treatment | Andosol (Mg ha−1) | Nitisol (Mg ha−1) | Ferralsol (Mg ha−1) | |||
|---|---|---|---|---|---|---|
| 2020SR | 2021LR | 2020SR | 2021LR | 2020SR | 2021LR | |
| Rhizobium pusense + Bacillus aryabhattai | 2.49a | 1.37b | 1.38ab | 1.41a | 1.91ab | 1.36abc |
| R. pusense + Paenibacillus polymyxa | 2.26ab | 1.49ab | 1.27ab | 1.43a | 1.69bc | 1.76a |
| Rhizobium phaseoli + P. polymyxa | 2.28ab | 1.51ab | 1.42a | 1.47a | 1.67bc | 1.64ab |
| R. phaseoli + B. aryabhattai | 2.25ab | 1.41ab | 1.23ab | 1.47a | 1.91ab | 1.25bc |
| R. pusense | 2.30ab | 1.38b | 1.24ab | 1.31ab | 1.47c | 1.15c |
| R. phaseoli | 2.00b | 1.49ab | 1.18b | 1.35ab | 1.48c | 1.18c |
| Diammonium phosphate (18:46:0) | 2.32a | 1.64a | 1.34ab | 1.49a | 2.18a | 1.47abc |
| Uninoculated control | 1.43c | 0.93c | 0.82c | 1.16b | 1.09d | 0.48d |
| CV (%) | 25.0 | |||||
- Note: Means followed by different letters within a column are significantly different at α ≤ 0.05.
- Abbreviations: CV, coefficient of variation; LR, long rain; SR, short rain.
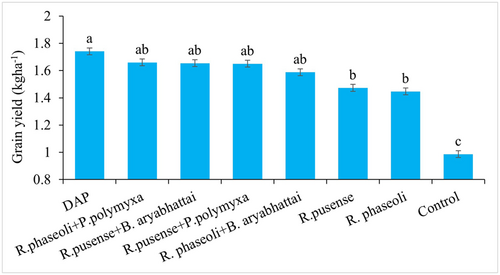
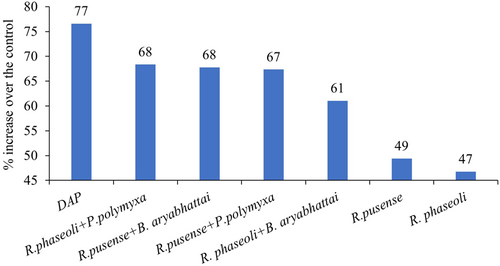
On average inoculation and co-inoculation of common bean with the bacterial strains increased the bean grain yield compared to the uninoculated control (Figure 2). Co-inoculation of the rhizobia with the PSB yielded at par with the application of DAP, while DAP application yielded significantly (p ≤ 0.001) more than the single rhizobia inoculation (Figure 2). No significant difference in yield of common bean was observed among the different bacterial strains inoculation and co-inoculation (Figure 2). Application of DAP led to a 77% increase in yield over the uninoculated control. Co-inoculation with the rhizobia and the PSB led to more than 60% increase in the yield of common bean compared to the uninoculated control while the single inoculation of rhizobia led to 49% and 47% for the R. pusense and R. phaseoli, respectively (Figure 3).
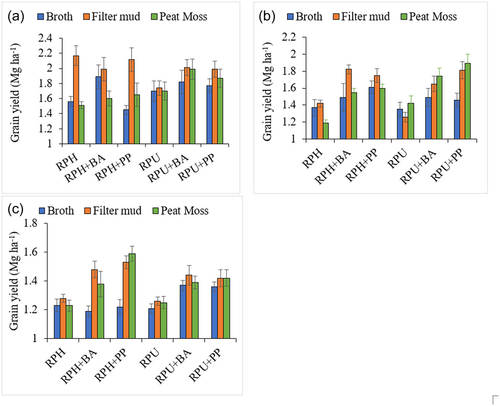
The interaction between the bacterial strain and the carrier material on the yield of common bean showed that there was preference of strain to a carrier material. Inoculation involving R. phaseoli performed best when filter mud was used as the carrier material across all three soil types, although no significant difference was observed between the filter mud and peat moss for some specific strain treatments (Figure 4). For instance, in the ferralsol (Figure 4b), peat moss performed better than filter mud when R. pusense was co-inoculated with B. aryabhattai and P. polymyxa, although the difference was not statistically significant. The broth (liquid) carrier performed the least in the majority of the bacterial strains treatment (Figure 4).
4 DISCUSSIONS
4.1 Effect of inoculant formulation on common bean growth and yield
Results from this study revealed that all three rhizobia and PSB formulations (peat, filter mud or liquid) generally increased nodulation, growth and grain yield, relative to uninoculated treatments. In terms of the effect of the carrier material, the results from the present study showed that generally, filter mud- and peat-based inoculants performed better for enhancing nodulation, root and shoot growth, nutrient uptake and yield of common bean as compared to liquid inoculum. Previous authors have reported similar findings. For instance, Denton et al. (2009) reported that nodulation from liquid inoculants typically provided less nodule than peat inoculants. Similarly, Irfan et al. (2019) reported that filter mud and peat increased maize growth significantly when compared to uninoculated control treatment and liquid inoculation. The improvement of crop growth and yield by the carrier-based inoculants over the liquid inoculants may be attributed to the ability of the carriers to increase bacterial survival rates by preventing them from being desiccated and drying (Brahmaprakash & Sahu, 2012; Vassilev et al., 2020). Moreover, application of liquid inoculum in natural conditions may not be successful due to different environmental constraints such as high soil temperatures, drought and salinity (Malusá et al., 2012). Contrary results have been reported by other authors who showed that liquid inoculants performed better than the solid-based carrier materials. For instance, Dudeja et al. (2011) reported that liquid inoculants of Mesorhizobium sp. and PGPR led to significantly better nodulation and higher N and P uptake than their carrier-based inoculants.
Regarding the performance of the two carrier-based formulations (peat and filter mud), the present study showed that their performance varied. These results are probably due to the differences in the physicochemical properties of the carrier materials (Ma, 2019; Roychowdhury et al., 2015; Sohaib et al., 2020). One such feature is the moisture content and the water-holding capacity. High moisture-holding capacity of carrier material is desirable to support proper growth and multiplication of the bacteria (Shahzad et al., 2017), thereby improving the survival and longevity of the inoculant (Sohaib et al., 2020). Javed et al. (2022) explained that high water-holding capacity enhances the enzymatic processes involved in the breakdown of the organic materials, thus providing essential nutrients for the bacteria.
Peat carrier material has been widely used and reported to be more superior in preserving inoculant population and improving crop performance (Albareda et al., 2008; Bashan et al., 2014). However, results from this study revealed that the filter mud as a carrier material produced greater crop responses relative to the peat solid-based and the broth inoculants for most of the bacterial strains inoculation. This could be attributed to the ideal physicochemical properties of filter mud. Laboratory analysis of filter mud revealed that it has a high water-holding capacity (155%), total carbon (18.4%) and nitrogen (2.0%), thus maintaining and promoting bacterial population (Balume et al., 2015). Additionally, Rawat et al. (2021) reported that filter mud had a 70% moisture content, 2.6% nitrogen, 2.9% phosphorous and 43% organic carbon. Furthermore, the authors reported that filter mud possesses other ideal qualities such as being cost effective, available in large quantities, full of nutrients and sterilizable, hence making it a good carrier material choice.
A previous study showed that strains S. mexicanum ITTGR7T, R. calliandrae LBP2-1T and R. etli CFN42T kept on sugarcane bagasse induced the largest number of nodules in the common bean (Ruíz-Valdiviezo et al., 2015). Balume et al. (2015) found that filter mud was a better carrier when compared to the inorganic locally available horticultural vermiculite. Similarly, filter mud was reported to enhance almost all parameters significantly than uninoculated treatment and liquid inoculation (Irfan et al., 2019). Sugarcane bagasse was shown to be the most appropriate carrier to store the rhizobial strains (2 × 109 cells g−1) and to maintain their survival. Sugar waste was found to allow a higher growth of legume-nodulating bacteria in media compared to YEM standard media used for legume-nodulating bacteria (Singh et al., 2014).
4.2 Effect of co-inoculation on nodulation, growth and yield of common bean
Results from this study showed that inoculation of the common bean with rhizobia increased nodulation compared to the uninoculated control and the mineral fertilizer application. Furthermore, upon co-inoculation of the common bean with the rhizobia strains and the PSB, there was a general increase in the nodulation compared to the single rhizobia inoculation. This is similar to previous results of studies carried out both in the greenhouse and under natural field conditions. For instance, Rajendran et al. (2012) reported an increased nodulation in greenhouse conditions when common bean was co-inoculated with rhizobia together with other nodule-associated bacteria. Furthermore, Matse et al. (2020) reported that inoculation of white clover with Rhizobium in combination with two PGPRs, B. aryabhattai strain Sb and Azotobacter vinelandii strain G31, enhanced significantly nodule number, as compared to single rhizobium inoculation. Similarly, Sánchez et al. (2014) reported that field co-inoculation of common bean with Rhizobium pisi (R40982) + Pseudomonas monteilii (R43453) increased nodule number by 55%, respectively, over single inoculation with R. pisi (R40982). Similarly, significant increases in nodulation of co-inoculated treatments as compared to noninoculated treatments have been observed (De Souza & Ferreira, 2017). Furthermore, Raklami et al. (2019) reported the positive effect of co-inoculation with rhizobia and PGPRs on nodulation of faba bean. Several other studies on the co-inoculation of PGPRs along with rhizobia have been shown to enhance nodulation and symbiotic interaction of many legumes (Fatnassi et al., 2015; Kong et al., 2017; Swarnalakshmi et al., 2020; Zafar, 2011).
In the present study, both the noninoculated control and the nitrogen (DAP) control gave a statistically equal number of effective nodules contrary to what has been reported on the inhibition of nodulation by inorganic nitrogen application (Talaat & Abdallah, 2008). This could be attributed to the fact that the amount of nitrogen used (23 kg N ha−1) was probably not sufficient enough to inhibit nodulation in common bean. In a previous study, Yoseph and Worku (2014), even higher application of nitrogen (50 kg N ha−1) led to higher number of nodules per plant than the noninoculated soybean plants.
Results from this study showed that generally there was an increase in the root and shoot dry weight of common bean due to inoculation with the rhizobia and PSB relative to the uninoculated control. Furthermore, co-inoculation of rhizobia and the PSB led to higher root and shoot biomass of common bean compared to the single rhizobia inoculation for most of the specific rhizobia–PSB strain. The increase in shoot dry weight of common bean may be attributed to increased root proliferation induced by used rhizobia or PSB or both, mobilization of insoluble nutrients, thus promoting nutrient and water uptake by roots (Israr et al., 2016). Additionally, Bello et al. (2018) suggested that growth hormones synthesized by rhizobium have been considered as the other probable means to promote plant growth. Similar to the present findings, a previous study by Benidire et al. (2017) has reported that co-inoculation of rhizobial strains compared to single inoculation improved growth parameters of common bean under field conditions. Similarly, Zafar (2011) reported that co-inoculation with rhizobia and PGPRs enhanced the shoot and root dry weight of common bean. Matse et al. (2020) demonstrated that both the individual inoculation of two Rhizobium strains and their co-inoculation with the two PGPR, B. aryabhattai strain Sb and A. vinelandii strain G31, significantly increased the dry weight of white clover. Furthermore, Raklami et al. (2019) reported the positive effect of co-inoculation with rhizobia and PGPRs on biomass and growth of faba bean. Several authors have reported that co-inoculation stimulates plant growth of many legumes more than separate inoculation (Fatnassi et al., 2015; Fox et al., 2011; Hungria et al., 2013, 2015; Kong et al., 2017; Singh et al., 2014). Relative to the application of inorganic source of N and P in the present study, results showed that co-inoculation of the rhizobia and PSB led to a higher root and shoot dry weight that was statistically at par with the inorganic fertilizer application. Similar results were reported by Cardoso and Ferreira (2021), who found that the co-inoculation of rhizobia and azospirilla treatment resulted in higher shoot biomass, whereas co-inoculation of rhizobia and azospirilla and the co-inoculation of rhizobia, azospirilla and trichoderma provided higher root and shoot biomass, resulting in values similar to those of the N-fertilizer treatment.
Findings from the present study showed that inoculation with rhizobia and rhizobia–PSB co-inoculation increased the grain yield of common bean compared to the uninoculated control. Furthermore, specific rhizobia-–PSB co-inoculation led to higher grain yield that was statistically at par with the application of inorganic nitrogen and phosphorous. However, the single rhizobia yields were significantly lower than the inorganic nitrogen and phosphorous application. Some studies have shown that inoculation of common bean with rhizobia results in equal and/or higher grain yield as compared to N-fertilizer treatment (De Souza & Ferreira, 2017). These results suggest that inoculation with mixed bacteria inocula has a synergistic effect on the yield of the common bean. Benidire et al. (2017) reported that co-inoculation of rhizobial strains compared to single inoculation has the advantage to improve yield components of common bean under field conditions. Hungria et al. (2013) also demonstrated that seed inoculation of common bean with Rhizobium tropici alone increased yield by 8.3%, while co-inoculation with Azospirillum brasilense boosted the yield to 19.6%. Similarly, Yadegari et al. (2010) reported seed yield production from co-inoculation of Rhizobium + Pseudomonas fluorescens P-93 with a significant increase of up to 73% over Rhizobium alone. Benjelloun et al. (2021) reported up to 242% increase in grain yield of chickpea as a result of co-inoculation with Mesorhizobium sp. MA72 combined with Enterobacter aerogenes P1S6. Several other studies have reported on the rhizobia PGPR co-inoculation advantage over single rhizobia inoculation in many legumes (Galindo et al., 2018; Hungria et al., 2015; Raklami et al., 2019; Singh et al., 2014; Stajković-Srbinović et al., 2021).
Most of the tropical soils have low P availability and this has been shown to compromise the biological nitrogen fixation (BNF) of legumes. In the present study, the PBS B. aryabhattai and P. polmyxa were shown to increase the grain yield of common bean. This is probably a result of their phosphate-solubilizing potential availing P for the optimum activity of the rhizobia strains and BNF (Shiri-Janagard et al., 2012). The PGPR effect might also have resulted in better nitrogen and phosphorous nutrient efficiency. A study by Pereira et al. (2020) reported that rhizobacteria strains are capable of increasing the nutrient efficiency in crops, especially under nutrient and water stress conditions.
The present results showed a varied response of the common bean to inoculation across the three study sites. This may be attributed to the soil and ecological factors during the study period such as the temperature, soil moisture, soil type, rhizobia population and their effectiveness (Matse et al., 2020; Solomon et al., 2012). The present study showed that there was a difference in the rhizobia population among the three soil types. This might have influenced the effectiveness of the introduced bacterial stains. Previous studies have also reported site-specific plant response to rhizobia and PGPR inoculants and this might be attributed to differences in effectiveness of rhizobium strains and native rhizobia strain population among locations (Kellman, 2008). Additionally, Hungria et al. (2003) reported that it is common to find site-specific responses to bacterial inoculation. The three study sites also experienced different rainfall amounts during the two cropping seasons, hence varying soil moisture that might have led to significant differences in the growth, nodulation and yield of the common bean. For instance, during the second season (2021LR) in the andosol, there were suppressed rainfall amounts that could have led to reduction in most of the plant growth parameters and yield. Drought stress during plant growth has been shown to negatively affect plant growth ranging from reduced root, shoot and leaf development (Beebe et al., 2013). Limited soil moisture also can lead to poor nutrient uptake by the plant, reduced photosynthesis, reduced pod setting and seed yield (Asfaw et al., 2017; Beebe et al., 2013).
5 CONCLUSIONS
In this study, it is concluded that the inoculant carrier material influences the effectiveness of the inoculants and plant performance. The solid carrier material, specifically filter mud, showed potential for use in formulation of inoculants since it enhanced the effectiveness of specific bacterial strains inoculants on the growth and yield of common bean. Additionally, filter mud is a cheap and locally available carrier material and can thus be sustainably used in the formulation of bioinoculants. Further, specific co-inoculation of rhizobia (R. phaseoli and R. pusense strains) and PSB (P. polymyxa and B. aryabhattai) increased the yield of common bean. Inoculation with the bacterial strains significantly increased the yield of common bean over the uninoculated control. This was consistent across all the soil types and seasons except for the single rhizobia inoculation in nitisol during the 2021LR season. Some specific rhizobia + PSB co-inoculation yielded statistically at par with the application of DAP across the soil types and seasons. The two native rhizobia and two PSB used in this study might promote plant growth via one or a combination of their plant growth-promoting traits. This study recommends that filter mud has the potential to be used as a carrier material for bacterial inoculants since it performs better than the other carrier and is locally and easily available.
AUTHOR CONTRIBUTIONS
Hezekiah Korir, Nancy W. Mungai and Victor W. Wasike: Conceptualization. Hezekiah Korir, Nancy W. Mungai and Victor W. Wasike: Methodology. Hezekiah Korir: Data analysis. Hezekiah Korir: Investigation. Hezekiah Korir: Writing. Hezekiah Korir, Nancy W. Mungai and Victor W. Wasike: Review and editing. Nancy W. Mungai and Victor W. Wasike: Supervision. Hezekiah Korir: Funding acquisition.
ACKNOWLEDGEMENTS
This study was funded by the World Bank through the Centre of Excellence in Sustainable Agriculture and Agribusiness Management (CESAAM) at Egerton University, in Kenya.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that they have adhered to the ethical policies of the journal.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.




