Functional redundancy across space and time in litter-degrading fungal communities
Abstract
Introduction
Microbial-driven litter decomposition contributes significantly to global carbon (C) turnover. Fungi play central roles in the degradation process due to their ability to hydrolyse recalcitrant litter components. The spatiotemporal variations in taxonomic composition of litter-degrading fungi have been well documented. However, associated variations in litter-degradation-related functional composition of fungal communities remain unexplored.
Materials and Methods
In this study, a 16-week field-based buried rice straw experiment was conducted at three experimental sites across subtropical China in combination with laboratory 13C-straw-based DNA stable-isotope probing (DNA-SIP) microcosm experiments. Amplicon sequencing combined with shotgun metagenomic sequencing were the approaches of choice.
Results
The field experiment showed that the taxonomic composition of the straw-degrading fungal community was highly variable while the functional composition was rather stable. The higher permutational multivariate analysis of variation F scores (20.904−48.660) and the steeper slopes (1.92 E-04−4.15E-04) of the distance decay relationship for taxonomic composition than for function across periods (with lower F scores = 7.047−21.601 and gradual slopes = −1.33 E-05 to −1.03E-04) both indicated that the spatiotemporal patterns of functional composition in litter-degrading fungi community were more conserved. The laboratory DNA-SIP confirmed the field observations and showed that the conserved functional composition in litter-degrading fungi was underpinned by a high functional redundancy of Basidiomycota.
Conclusion
Function and taxonomy of litter-degrading fungi were decoupled. The functional composition of the litter-degrading fungal community was highly conserved in space and time, the taxonomic composition less so. The main drivers behind the observed taxonomic decoupling are probably/most likely functional redundancy and metabolic niche selection resulting in conservation of function, with changing environmental conditions and dispersal limitation drove the observed high taxonomic turnover of the community over the course of the litter degradation progression. Our study provides valuable insights in the ecology of fungi and their roles in in global C sequestration for ecosystem sustainable development.
1 INTRODUCTION
Soil organic carbon (SOC) is a keystone indicator of soil health as it reflects soil functions and sustainability (Nunes et al., 2021). However, with the gradual increases in temperature and the intensive crop cultivation in agriculture worldwide, an increasing global SOC losses are looming (Crowther et al., 2016; Nottingham et al., 2020; Schindlbacher et al., 2009). Organic carbon (C) inputs derived from plant litter are an important way of SOC sequestration, which can compensate for soil C losses (Tautges et al., 2019; Whitbread et al., 2003).
Soil microorganisms are known to control the C conversion processes from plant-derived C into SOC (Liang et al., 2017; Zheng et al., 2021). Fungi can produce multiple highly active cellulases and are central to plant litter degradation (Couturier et al., 2018; Dixon, 2013; Kaffenberger and Schilling, 2015; Lustenhouwer et al., 2020; Miao et al., 2017; Miyauchi et al., 2016; Montegut et al., 1991). Previous studies have addressed the taxonomic composition and dynamics of litter-decomposing fungal communities (Fang et al., 2020; Tedersoo et al., 2014; Zhang et al., 2018), however, little is known about the functional attributes of these communities, especially at the field scale. Such information is extremely important for a comprehensive understanding of global fungal-driven ecosystem SOC budgets.
Both the functional and the taxonomic attributes of microbial communities affect plant litter decomposition (Bao et al., 2023). Insight in the biogeochemical cycling processes involving microbial participation (e.g., plant litter decomposition), usually requires information on the functional attributes of the microbial community, rather than just about its taxonomic composition (Louca et al., 2018). Commonly, communities exhibit functional redundancy which improves ecosystem stability against environmental disturbances (Louca, Jacques, et al., 2016; Louca, Parfrey, et al., 2016; Louca et al., 2018). According to this paradigm, a specific biological function can be carried out by multiple different microbial groups (Louca, Parfrey, et al., 2016). As individual microorganisms are susceptible to environmental changes (Jansson and Hofmockel, 2020), the microbial composition of a community may vary strongly across large scales with different environmental conditions, while the functional components involved in specific biochemical reaction processes are nevertheless highly conserved (Louca et al., 2018). Accordingly, in a previous study we found that the functional community composition of straw-degrading bacteria has less environmental variability than the taxonomic composition (Bao, Guo, et al., 2020). However, the environmental variability of the community composition of straw-degrading fungi (especially from the functional perspective) remains unexplored. Based on the common aforementioned phenomenon of functional redundancy that existed in the communities, we hypothesised that the spatiotemporal variability of functional composition in straw-degrading fungal communities is low, while the variability in the taxonomic composition of the community is relatively high.
To test this hypothesis, we first used a 16-week field-based buried rice straw experiment—as a model system for plant litter decomposition—in anoxic paddy fields at three experimental sites (Chongqing [CQ], Changshu [CS] and Yingtan [YT]) with contrasting environmental conditions across the subtropical zone of China. Fungal ITS amplicon sequencing combined with function predictions was then used to analyse the taxonomic and functional spatiotemporal variability of the fungal community. Additionally, laboratory 13C-straw-based DNA stable-isotope probing (DNA-SIP) combined with shotgun metagenomic sequencing approaches were used to verify the amplicon sequencing results of the field litter decomposition experiment.
2 MATERIALS AND METHODS
2.1 Experimental sites and the field straw decomposition experiment
The field-based buried rice straw experiment using litter bags was conducted at three replicated rice field experimental sites in CQ, CS and YT across subtropical China. The mean annual temperature (MAT) and precipitation (MAP) of these three experimental sites were 18.4°C, 16.6°C, 17.6°C and 1106 mm, 1321 mm, 1795 mm, respectively (Supporting Information: Table S1). These sites span a wide geographic distance (~ 1300 km) with different environmental conditions (e.g., soil chemical properties and climatic factors, Supporting Information: Tables S1 and S2), which provides an ideal opportunity to reveal the spatial variations of both the functional and taxonomic composition in litter-degrading fungal communities.
Rice straw materials were collected from the local farmland in 2014 and air-dried. In brief, 20 g of rice straw was cut to 5 cm in length and put into the nylon litter bags (12 × 20 cm in size and with 300 mesh with 41 μm pore size), which permitted the free transfer of fungi between the bags and paddy soils. Before rice cultivation, bags containing straw were randomly buried at a 10 cm depth in a 48 m2 area. The litter bags were respectively collected at 1, 2, 4, 8 and 16 weeks after they were buried. Each collection time point had 12 replicates, and a total of 60 litter bags were collected for each experimental site. In total, there were 180 straw samples in this investigation. Samples of straw for DNA extraction were stored at −80°C.
2.2 DNA extraction
Five grams of straw was cut to 5 cm length, combined with 40 mL of phosphate-buffered saline, and vortexed at 180 rpm for 1 h. After filtering, the remaining straw was washed three times. All the suspensions were collected and centrifuged at 4°C with 5000g for 10 min. Then the genomic DNA was extracted from 0.5-g precipitates prepared above by using the FastDNA® SPIN Kit (MP Biomedicals) including a negative control.
2.3 Fungal ITS amplicon high-throughput sequencing and data processing
The primer set ITS1F (CTTGGTCATTTAGAGGAAGTAA)/ITS2 (GCTGCGTTCTTCATCGATGC) was used to amplify approximately 250−500 bp of fungal ITS1(b) fragments (White et al., 1990). Briefly, the unique 7-bp barcoded oligonucleotide sequence was fused to the forward primer to distinguish different samples. PCR was carried out in 25-µL reaction mixtures with the following components: 5 × reaction buffer 5 µL, 5 × GC buffer 5 µL, dNTP (2.5 mM) 2 µL, forward primer (10 uM) 1 µL, reverse primer (10 µM) 1 µL, DNA template 2 µL, ddH2O 8.75 µL, Q5 ® High-Fidelity DNA Polymerase 0.25 µL. Thirty cycles (98°C for 15 s, 55°C for 30 s, and 72°C for 30 s) were performed with a final extension at 72°C for 5 min. The purified bar-coded PCR products from all of the samples were normalised in equimolar amounts and sequenced using a NovaSeq. 6000 SP Reagent Kit (500 cycles) following the manufacturer's protocols with Illumina NovaSeq. 6000 sequencing platform (Illumina Inc.).
The raw sequence data were processed using the Quantitative Insights Into Microbial Ecology (QIIME) pipeline (Caporaso et al., 2010). Sequences with a quality score below 25 were trimmed. Then, the good quality sequences were assigned to samples based on unique 7-bp barcodes. The reads were then binned into operational taxonomic units (OTUs) using UCLUST (Edgar, 2010) at a 97% identity threshold, and the most abundant sequence from each OTU was selected as a representative sequence. Taxonomy was assigned to fungal OTUs using the UNITE's reference dynamic data set (version 8.3) (Abarenkov et al., 2021).
2.4 Fungal community analyses
Functional characteristics of straw-degrading fungal communities in the field experiment were analysed based on the ITS sequences that were predicted by phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2) (Boutin et al., 2021; Douglas et al., 2020). Briefly, HMMER (http://hmmer.org/) was used for representative sequence alignment. Then, EPA-NG was used to map the sequences to a reference phylogeny. Finally, GAPPA v0.7.1 was used to convert the file into newick format (Czech et al., 2020) and the castor R package was used for hidden-state prediction of the gene family abundances (Louca and Doebeli, 2018). Data used for these functional predictions was from the 1000 Fungal Genomes Database (http://1000.fungalgenomes.org). After prediction, straw-degrading related (i.e., involved in the degradation of the main straw components of cellulose, hemicellulose, lignin, and cello-oligosaccharides) enzyme (Supporting Information: Table S3) encoding genes were selected. The straw-associated fungal taxonomic and functional composition was visualised by nonmetric multidimensional scaling analysis (NMDS) based on the Bray-Curtis dissimilarity calculated from the OTU table and the selected straw-degrading related enzyme encoding genes. The distance decay relationship (DDR) of the fungal taxonomic and functional compositions were calculated as the slope of a linear least squares regression on the relationship between the geographic distance versus the dissimilarity of fungal taxonomic and functional composition based on Bray-Curtis dissimilarity calculated from the OTU table and the selected predicted functional genes related to straw decomposition (Martiny et al., 2011). Random Forest (RF) model was used to evaluate the potential contributions of environmental conditions (three properties of altitude, MAP, and MAT in Supporting Information: Table S1 and eight properties in Supporting Information: Table S2) to the taxonomic and functional compositions (the first axis of PCoA based on Bray-Curtis dissimilarity distance calculated from OTU table or the selected predicted functional genes related to straw decomposition) of straw-degrading fungi among the three experimental sites across the 16 weeks decomposition periods (Jiao et al., 2018). A multiple regression model with variance decomposition analysis was used to validate the outcome of RF analyses by using the lm and calc. relimp function in the ‘relaimpo’ package in R (Grömping, 2006). The accuracy of the model was computed for each tree and averaged over the forest (5000 trees). Percentage increases in the MSE (mean squared error) of variables were used to estimate the importance of environmental conditions, and higher MSE% values imply more important predictors.
2.5 Microcosm-based DNA stable isotope probing, shotgun metagenomic sequencing of DNA in heavy buoyant fractions, and data processing
To further verify our hypotheses, we determined the taxonomic and functional composition of straw decomposition fungi by DNA-SIP and metagenomic shotgun sequencing in two representative soils (from CS and YT) with distinct soil chemical properties. Details of the 13C-straw amended microcosms, DNA stable isotope probing experiment, the shotgun metagenomic sequencing of the heavy buoyant DNA fractions, and data processing have been fully described in Bao et al. (2021). In brief, 0.1 g of 13C-labelled rice straw (ca. 70 at %) and 10 g soil were added to each bottle (13C). The headspace of serum bottles was flushed with N2 to obtain an anaerobic condition, and then bottles were sealed and incubated in the dark at 27°C for 90 days. Bottles amended with natural 12C-rice straw (ca. 1.08% of 13C to ΣC) (12C) were established as the control of the 13C treatment. Each treatment was replicated three times. Gas samples and soil samples were respectively collected at 8, 24, 56 and 90 days after they were incubated. Thus, there were 12 gas samples and 12 soil samples collected for each experimental site and a total of 24 gas samples and 24 soil samples in this investigation. The concentrations of CO2 were analysed by gas chromatography with ECD (Agilent 7890 A, Agilent Technologies).
DNA stable isotope fractionation was conducted as described by Jia and Conrad (2009). Briefly, 3.0 μg DNA with an initial CsCl buoyant density of 1.720 g/mL was prepared for gradient fractionation; then the solution was centrifuged at 177,000g for 44 h under vacuum. After ultracentrifugation, the solution was separated from bottom to top into 15 equal fractions. DNA was separated from CsCl by PEG 6000 precipitation and dissolved in 30 µL of TE buffer. The ‘heavy’ DNA fractions were used for Shotgun metagenomic sequencing. The ‘heavy’ DNA fractions were fragmented to an average size of approximately 300 bp. A paired-end library was prepared. Adaptors containing the full complement of sequencing primer hybridisation sites were ligated to the Blunt-end fragments. Paired-end sequencing was performed on an Illumina HiSeq. 4000 platform (Illumina Inc.) at Majorbio Bio-Pharm Technology Co., Ltd.
The data were analysed on the online platform of Majorbio Cloud Platform (www.majorbio.com) (Ren et al., 2022). Briefly, adaptors were stripped using SeqPrep. Then, the low-quality reads (length with a quality value < 20 or having N bases or <50 bp) were filtered using Sickle with default parameters. The de Bruijn graph-based assembler SOAPdenovo was employed to assemble short reads. Scaffolds with a length > 500 bp were then broken into contigs. Open reading frames (ORFs) from each metagenomic sample were predicted using MetaGene. The predicted ORFs with lengths of 100 bp or more were retrieved and translated to amino acid sequences using the NCBI translation table. All sequences from gene sets with a 95% sequence identity were clustered as the nonredundant gene catalogue by the CD-HIT. Reads after quality control were mapped to the representative genes with 95% identity using SOAPaligner and then the gene abundance in each sample was evaluated. BLASTP was employed for taxonomic annotations by aligning nonredundant gene catalogues against the NCBI NR database. The cluster of orthologous groups of proteins for the ORFs annotation was performed using BLASTP against the eggNOG database. BLASTP search against the Kyoto Encyclopaedia of Genes and Genomes (KEGG) database was used for the KEGG pathway annotation. Genes encoding carbohydrate-active enzymes (CAZymes) that catalyse the hydrolysis of plant residues were chosen as an example to evaluate the fungal straw-degrading function. First, the nonredundant gene catalogues were taxonomically assigned by BLASTP, then the CAZymes functions of the nonredundant gene catalogues with fungal taxonomic assignment were annotated using hmmscan (http://hmmer.janelia.org/search/hmmscan) against CAZy database V5.0 (http://www.cazy.org/) with an e value cutoff of 1e−5.
2.6 Statistical analysis
Permutational multivariate analysis of variation (PERMANOVA) (Anderson & Walsh, 2013) was conducted to test for statistically significant differences in taxonomic and functional composition among experimental sites, using R software (the ‘vegan’ package (Oksanen et al., 2007), Version 2.2-1). The significance of the linear regression slopes between taxonomic and functional composition across geographic distance was tested by permutation tests. The difference of p < 0.05 was considered significant.
3 RESULTS AND DISCUSSION
3.1 Spatiotemporal patterns in the taxonomic and functional composition of plant litter decomposing fungal communities revealed in a field experiment
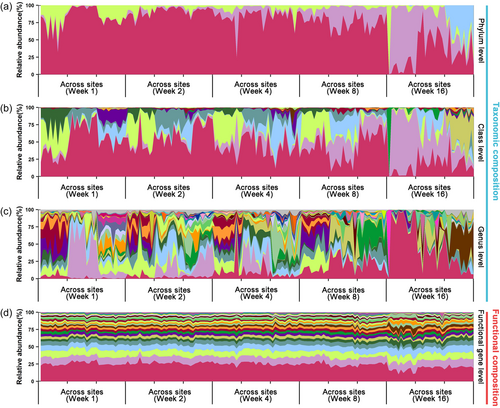
The field-based buried rice straw experiment yielded 8,320,337 quality sequences of the fungal ITS at between 29,777 and 78,987 sequences per sample, with a median value of 46,224 sequences per sample. All samples were randomly rarified to 29,777 sequences (with 83.5% of the total 5557 OTUs annotated to known fungal taxa) for downstream analyses (Shaw et al., 2008). In addition, with the ITS functional prediction, there were 904 enzyme-encoding genes annotated to the fungal communities. The temporal and spatial changes of the relative taxonomic abundances in straw decomposition fungi community were highly variable at the phylum (Figure 1a), the class (Figure 1b), as well as the genus level (Figure 1c). However, the relative functional abundances (quantified by 32 genes related to straw decomposition, Supporting Information: Table S3) were more stable irrespective whether it was within a period or across all sites and periods (Figure 1d).
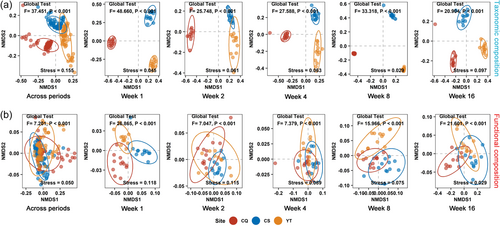
NMDS plots showed that the taxonomic composition (Figure 2a) of the straw-decomposing fungal communities across three experimental sites was more distinct and separate from each other than the functional composition (Figure 2b), both across decomposition periods and within each period. PERMANOVA test results (F scores) further confirmed these results (global test, p < 0.001, Figure 2). All F scores in global tests for the functional composition of straw decomposing fungi among the three experimental sites were lower than those for the taxonomic composition (except for the score for week 16) (Figure 2).
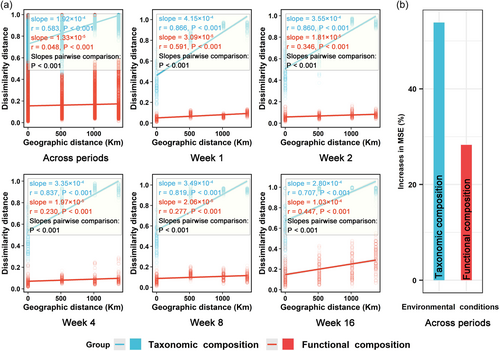
Dispersal limitation induced by spatial separation (Chen et al., 2020; Nekola & White, 1999) is one of the potential explanations for the high taxonomic variability of the straw-decomposing fungal community (Figures 1a−c and 2a). This speculation was supported by the result of DDR, which showed strong (r = 0.583−0.866) and positive (slope = 1.92 E-04−4.15E-04) correlations between the taxonomic dissimilarity of the straw-decomposing fungal community and their geographic distance than for functional composition (slope = −1.33 E-05 to −1.03E-04), both within and across the decomposition periods (Figure 3a). These results indicated that the taxonomic spatiotemporal composition of the straw-decomposing fungal community was highly influenced (with steeper slopes) by geographic distance (i.e., dispersal limitation induced by spatial separation) than the functional composition (with gradual slopes). The finding that dispersal limitation constrained the spatial diffusion of microorganisms thereby improving the microbial taxonomic diversity in these soils is consistent with previous studies in many ecosystems such as paddy soils (Chen et al., 2017), rainforest (Rodrigues et al., 2013), and salt marsh sediments (Martiny et al., 2011). In addition to dispersal limitation, environmental conditions may be one of the other potential explanations for the high taxonomic spatiotemporal variability of the straw decomposition fungi community. This speculation was supported by a RF analysis (Figure 3b). Environmental conditions explained 53.8% of the variance related to fungal taxonomic composition, while explaining only 28.3% of the variance related to functional composition (Figure 3b), implying that the taxonomic spatiotemporal composition of straw-degrading fungal community is more influenced by environmental variability than functional composition. Environmental conditions strongly drive microbial taxonomic composition in this study, as was also reported in many other studies (Mula-Michel & Williams, 2013; Sun et al., 2013; Zhan et al., 2018).
In contrast to the high variation in taxonomic composition, the conserved functional patterns of the litter-decomposing fungal community may be attributed to general microbial functional redundancy (Comte et al., 2013; Louca et al., 2018) and convergent metabolic niche selection in different habitat types (Louca, Parfrey, et al., 2016). On the one hand, microbial communities generally exhibit high functional redundancy which improves their stability in ecosystems as it helps against external environmental perturbations (Allison & Martiny, 2008; Comte et al., 2013; Griffiths et al., 2000). Thus a given function, for example, microbial-driven litter decomposition as studied here, can be performed by various taxonomically distinct, but functionally similar species (Louca, Jacques, et al., 2016; Louca, Parfrey, et al., 2016; Louca et al., 2018). This may be one of the reasons why the taxonomic composition of the straw-decomposition fungal community varies strongly across the experimental sites and decomposition periods, while the spatiotemporal variability of the functional composition is conserved (Figures 1-3). On the other hand, litter decomposition usually starts with fast decomposition of easily degradable components, followed by slower decomposition of recalcitrant compounds and subsequently produced biopolymers, and finally mineralisation of monomers to CO2 under oxic conditions and to CO2 plus CH4 under anoxic conditions (Conrad, 1993; Glissmann & Conrad, 2002). Such general degradation steps can provide similar metabolic niches in different environmental habitats (Louca, Parfrey, et al., 2016) which further act as environmental selectors that select taxonomically distinct, but functionally conserved fungal species (e.g., litter decomposition in this study, Figures 1d, 2b and 3a, and Supporting Information: Figure S2). The convergent metabolic niche selection resulted in a conserved microbial functional composition that agreed with previous studies, for example, in sludge bioreactors (Wells et al., 2009), phenetic trees (Aguilar et al., 2004), and soil microbial nitrogen-cycling (Nelson et al., 2016).
3.2 Conserved spatiotemporal patterns of functional composition in litter-degrading fungi community verified by DNA-SIP
To verify the findings of the field-based buried rice straw experiment and avoid any potential limitations of PICRUSt2 for prediction fungal function, we evaluated the taxonomic and functional composition of straw-decomposing fungi by laboratory 13C-straw-based DNA-SIP in combination with shotgun metagenomic sequencing for two representative sites, CS and YT. In accordance with the findings in the field experiment, the functional composition of the fungal communities associated with straw degradation remained relatively stable both within and across decomposition periods when compared to the taxonomic composition of these communities (Figure 4a). Linear regressions further revealed that there was no significant association between the functional composition of straw decomposing fungi and CO2 emission during straw decomposition across the CS and YT experimental sites (p > 0.05), while a positive and significant association was observed for the taxonomic composition (p < 0.05, Figure 4b). These results confirmed the findings of the field experiment that there was a decoupling between the conserved functions and high taxonomic turnover of the straw-degrading fungal communities (Figures 1-3). Such a decoupling was in line with the findings observed in the studies of sludge bioreactors (Vanwonterghem et al., 2014), straw-degrading bacteria (Bao, Guo, et al., 2020), bromeliad tank environment (Louca, Jacques, et al., 2016), global ocean (Louca, Jacques, et al., 2016), and the human gut (Turnbaugh et al., 2009). These above-mentioned results collectively confirmed that the functional composition of straw-decomposing fungal communities has less spatiotemporal variability than their taxonomic composition during litter decomposition.
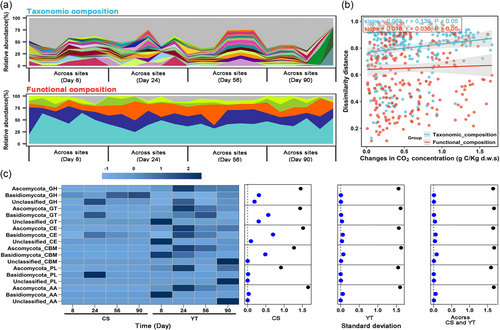
To further reveal the potential taxon or taxa that contribute to the highly conserved functional composition of the straw-degrading fungal community, the variations in the relative abundance (shown as the standard deviation of the relative abundance of CAZymes during the decomposition period) of CAZymes in the dominant fungal taxa were analysed. The average relative abundance of Ascomycota, Basidiomycota, and Unclassified phyla across decomposition stages were 73.1%, 7.5% and 19.4% respectively, at both local (i.e., within sites) and regional (i.e., across sites) scales (Figure 4c). It was found that the variation in CAZymes relative abundances increased in the order of Unclassified fungal phyla (average standard deviation value: 0.042) <Basidiomycota (0.138) <Ascomycota (1.539), at both local and regional scales (Figure 4c). The higher variation in CAZymes relative abundances of Ascomycota indicates that there is a relatively large proportion of species belonging to Ascomycota that have no CAZymes encoding genes, while the lower variation found in Basidiomycota and Unclassified fungal phyla implies that most of the species harbour CAZymes for litter decomposition. Our field results using high-throughput sequencing approaches are in line with with and extend a previous pure cultures-based laboratory studies where 64.4% of the Basidiomycota tested (459 of the 713) could degrade leaf litter, while for Ascomycota that proportion was only 40.6% (204 of the 502) (Osono, 2020). These results suggest that the functional redundancy of litter decomposing Basidiomycota and of some of the members of the Unclassified taxon is higher than that of Ascomycota and that they may be the main contributors to the highly conserved spatiotemporal patterns of functional composition in litter-degrading fungi community found in the present study.
4 CONCLUSION
This study provides compelling evidence for a decoupling between function and taxonomy in litter-degrading fungal communities. Unlike the clear spatiotemporal variability in taxonomic composition, the functional composition of the litter-degrading fungal communities was highly conserved. The observed high taxonomic variability is attributed to the influence of dispersal limitation and environmental conditions, while functional redundancy and metabolic niche selection underpin the responsible for the conserved functional composition. This provides insight in the functional and taxonomic aspects of soil fungi which will benefit a comprehensive understanding of microbial-driven global terrestrial C sequestration, and is extremely important for the sustainable development of agroecosystems.
AUTHOR CONTRIBUTIONS
Youzhi Feng, Yuanyuan Bao, Xiangui Lin and Zhongpei Li designed the experiment. Yuanyuan Bao, Youzhi Feng and Ruirui Chen performed the experiments. Yuanyuan Bao, Youzhi Feng, Xiaodan Cui and Jan Dolfing analysed the data. Yuanyuan Bao, Jan Dolfing and Youzhi Feng wrote the paper. All authors were allowed to review the results and comment on the manuscript and approved the final manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (42207365 and 42177297), the Natural Science Foundation of Jiangsu Province (Grant Nos. BK20221161), and the Chinese Academy of Sciences (CAS) Strategic Priority Research Program Grant XDA28010302.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that they have adhered to the ethical policies of the journal.
Open Research
DATA AVAILABILITY STATEMENT
The sequences of ITS amplicons of field experiments are deposited into the DNA Data Bank of Japan (DDBJ) database (https://www.ddbj.nig.ac.jp/index-e.html) (accession number: DRA017299). All data generated or analysed during this study are included in this published article or are available from the corresponding author upon reasonable request.




