Sustainable agricultural practices contribute significantly to One Health
Abstract
The One Health concept proposes that the health of humans, animals, and the environment are interconnected. Agricultural production is a critical component of One Health as food links the environment to human health. Food not only provides nutrients to humans but also represents an important pathway for human exposure to environmental microbes as well as potentially harmful agrochemicals. In addition, inappropriate agronomic practices can cause damage to the environment which can have unintended adverse impacts on human health. Therefore, improving agricultural production systems and protecting environmental health should not be viewed as isolated goals as they are strongly interlinked. Here, we used the nexus of soil, plant, and human microbiomes to discuss sustainable agricultural production from the One Health perspective. We highlighted three interconnected challenges faced by current agronomic practices: the transmissions of pathogens in soil-human microbial loops, the dissemination of antibiotic resistance genes in agroecosystems, and the impacts of chemical pesticides on humans and environmental health. Finally, we propose the potential of utilising microbiomes for better sustainable agronomic practices to contribute to key goals of the One Health concept.
1 INTRODUCTION
The One Health concept emphasises the connections between humans, animals, and the environment and provides a global strategy that highlights the need for holistic and transdisciplinary approaches to improving the health and well-being of all components of an ecosystem (van Bruggen et al., 2019). Agricultural production systems and practices play central roles in the One Health approach as food represents the link between soil, plant, and animals to human health. Food not only provides nutrients to humans but also acts as a major route for human exposure to potential biotic (e.g., pathogens) and abiotic (e.g., pesticides or other chemical pollutants) risks in the environment. For example, the consumption of fruits and vegetables, which are usually eaten raw, is recognised as an important route for human gut microbiome exposure to pathogens and antibiotic resistance genes (ARGs). Similarly, food production and food consumption are major sources of human exposure to chemical pesticides which have been documented to cause several hundred thousand deaths annually (Nicolopoulou-Stamati et al., 2016). In addition, long-term exposure to chemical pesticides is also associated with chronic toxicity that may cause cancer and birth defects (Nicolopoulou-Stamati et al., 2016). Further, excessive and inappropriate use of agrochemicals has been reported to damage environmental health and lead to problems including loss of biodiversity, resource depletion, soil erosion, and pollution (Garcia et al., 2020). The underlying premise of One Health is that the well-being of humans will be severely compromised in a damaged ecosystem suffering from pollution and ever-diminishing resources (Destoumieux-Garzón et al., 2018). Therefore, multiple governmental organisations and academic societies have consistently recommended the development of innovative sustainable agricultural production approaches from the One Health perspective aiming to ensure food and nutrition security as well as preserve Earth's finite resources (Food and Agricultural Organization of the United Nations, 2020; National Academies of Sciences, Engineering, and Medicine, 2019).
Traditionally, One Health concepts focused on the connection between human and animal health without explicit inclusion of soil health (Zinsstag et al., 2011). However, soil plays a central role in determining both environmental health and human health (Zhu et al., 2019). In an ecosystem, the health of environment and humans are linked by a strong microbial loop through which chemicals, microbes, and resources are exchanged between soil and humans via plants and animals (Figure 1). Therefore, developing effective sustainable agronomic practices with the consideration of the link between soil health and human health is necessary (van Bruggen et al., 2019). In this article, we used microorganisms and chemicals as the link to demonstrate the intricate and dynamic relationships between sustainable agricultural production with human health from the One Health perspective. Specifically, we focused on three major challenges associated with agronomic practices under the One Health concept: the transmission potential of human pathogens in soil microbial loops, the dissemination of ARGs in agroecosystems, and the impacts of chemical pesticides on humans and environmental health. Lastly, we proposed the potential contributions of plant and soil microbiome management in achieving the goals of One Health concept.
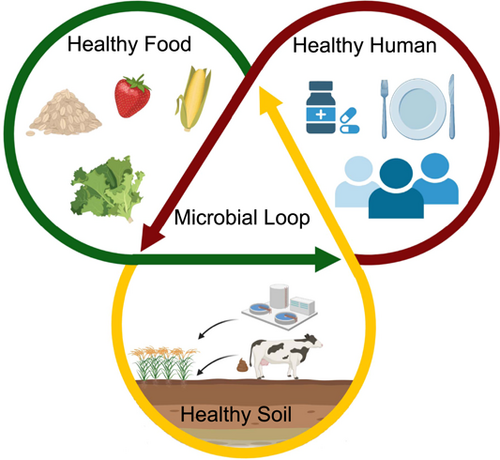
2 SOIL MICROBIAL LOOP—A MICROBIAL LOOP BETWEEN THE BELOW AND ABOVE GROUND
Understanding microbial-mediated interactions between the below-ground and aboveground is crucial (Figure 1). In the interlinked ecological processes of the terrestrial ecosystems, plants fix atmospheric carbon and provide energy/carbon for the heterotrophic (e.g., animal and microbes) components of ecosystems (Walker et al., 2003). Along with litter deposition, terrestrial plants release a large number of organic carbon into the rhizosphere, in the form of soluble root exudates, mucilage, sloughed-off root cells, and tissues, as well as volatiles (Kuzyakov & Razavi, 2019; Walker et al., 2003). These plant-derived carbon support the soil life, such as bacteria, fungi, and soil animals. In return, soil microorganisms and fauna play functional roles in regulating plant nutrient acquisition, plants' resistance to diseases as well as determining primary productivity (Huhta, 2007). A significant number of microbial species in the rhizosphere and bulk soil can colonise the phyllosphere via the xylem and phloem system, or via soil/air dispersal (Liu et al., 2017). The phyllosphere microbiome is known to link with plant health and impact ecosystem functions (Laforest-Lapointe et al., 2016; Liu et al., 2017; Vorholt, 2012). More importantly, the flower, parts of the phyllosphere, also harbours unique microbiomes in the nectar and pollen (Morris et al., 2020). These microbiomes comprise microbial taxa that are also shared by many insect pollinator species mainly in the gut systems, such as Stenotrophomonas, Sphingomonas, and Microbacterium (Anderson et al., 2013; McFrederick et al., 2012). The flower microbiomes influence the nectar odour and the visiting preferences of pollinators (Schaeffer et al., 2017). Interestingly, beneficial microbes can transmit to the seeds, contributing to the seed microbiomes (Mitter et al., 2017). Additionally, pollinators can transmit both beneficial microbes and plant pathogens among plant communities (Mitter et al., 2017).
The soil microbial loop not only involves the microbes that reside in the bulk soil and plants but also colonises on the surface and in the gut of soil fauna. Soil fauna accounts for over 20% of the known animals on our planet Earth and their microbiomes regulate their health and activities which ultimately determine key ecosystem functions raging from soil organic matter decomposition to primary production (Bardgett & van der Putten, 2014). Some soil organisms such as protists, nematodes, and micro-arthropods can directly consume soil bacteria and fungi. However, these organisms normally have preferences in their food types, for example, protists prefer Gram-negative bacteria because they are easier to digest (Chahartaghi et al., 2005; Ruess et al., 2000). As such, these microbe-consumers can reduce the abundance of soil microbiomes and change their diversity and composition. There are also trophic cascade effects on the soil microbiome and plant-microbiome selection. Top predators of the soil such as predatory mites, beetles, and spiders, which mainly feed on microbial consumers mentioned above (protists, nematodes, and microarthropods) can exert distinct cascading effects on the soil microbiome and influence the competitive outcomes between soil bacteria and fungi (Ripple et al., 2016). Overall, complex biotic interactions exist which ultimately impact diversity and composition of soil microbiome that is likely to have cascading effects on plant, animal, and human health. However, exact mechanisms and magnitude of soil food web on plant and animal microbiomes and their health remain poorly understood and warrant systemic studies to improve knowledge.
3 THE TRANSMISSION OF BENEFICIAL MICROBES AND HUMAN PATHOGENS IN AGRICULTURAL SYSTEM
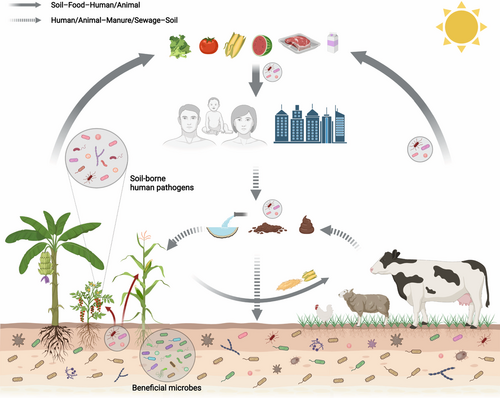
It is well known that soil harbours extremely diverse microorganisms and represents a rich microbial seed bank for the plant microbiome. Interactions between plant and soil microbiomes are crucial for crop growth and agricultural productivity (Hassani et al., 2018; Müller et al., 2016; Singh et al., 2020). Although soil microbiomes offer multiple benefits to the crop host, some potential pathogens that live in soils may transfer to human system via soil–plant–food continuum (Baumgardner, 2012; Steffan et al., 2020). One of the most important challenges in sustainable agricultural production from the One Health perspective is to identify beneficial microbes for the crops and protect crops from harmful microbes. Many recent works focused on identifying keystone taxa of the plant microbiome and harnessing synthetic microbial consortia (e.g., Variovorax, Sphingomonas, Chitinophaga, and Flavobacterium) to improve plant growth and health (Carrión et al., 2019; Finkel et al., 2020; Matsumoto et al., 2021). While these technologies are being developed, it is imperative to evaluate their impact on survival, abundance, distribution, and transmission of soil-borne human pathogens. It is important because soil serves as a reservoir of a large number of human pathogens that are among the most important contributors to infectious diseases related to human suffering and mortality (Gerba & Smith, 2005).
Soil-borne human pathogens can include a variety of microorganisms including bacteria, fungi, viruses, protozoa, and helminths (nematodes) (Gerba & Smith, 2005). Symptoms caused by these pathogens normally show gastrointestinal, wound, skin, and respiratory tract infections in humans, and can further lead to serious diseases like tetanus, anthrax, and botulism (Baumgardner, 2012). According to their residence time, soil-borne human pathogens are commonly categorised as permanent, periodic, transient, and incidental (Table 1) (Brevik et al., 2020). Permanent pathogens such as Clostridium botulinum, Clostridium tetani, and Listeria monocytogenes belong to soil inhabitants which can complete their entire life cycle in soils and sometimes become infectious to humans (Nieder et al., 2018; Samaddar et al., 2021). C. botulinum and C. tetani can produce neurotoxins that paralyse muscles and can lead to death, and L. monocytogenes causes gastrointestinal illness and more serious meningitis through food and water (Steffan et al., 2020). Periodic pathogens are microorganisms that spend part of their life cycle in soil environment. For example, Bacillus anthracis is found in soils throughout the world and cause zoonotic disease anthrax in humans, wildlife, and livestock (Steffan et al., 2020). Transient and incidental pathogens are organisms that may occur naturally in soils (e.g., through animal contact) or are introduced into the soils via anthropogenic sources (Nieder et al., 2018; Samaddar et al., 2021). For example, notorious food pathogen Salmonella enterica and Shiga toxin-producing Escherichia coli can enter agricultural soil via agronomic practices and cause major public health concerns (Samaddar et al., 2021). For instance, it was estimated that Salmonella enterica infection accounts for 93.8 million foodborne diseases every year (Eng et al., 2015).
| Causative agent | Disease | Transmission route | References |
|---|---|---|---|
| Clostridium botulinum | Produce toxins paralyzing muscles | Vegetables, fruits, meats, water | Smith (1978) |
| Clostridium tetani | Tetanus | Vegetables, fruits, meat | Smith (1978) |
| Listeria monocytogenes | Gastrointestinal illness and meningitis | Vegetables, fruits, water | Vivant et al. (2013) |
| Bacillus anthracis | Zoonotic disease anthrax | Meats | Helgason et al. (2000) |
| Salmonella enterica | Gastrointestinal illness | Vegetables, fruits | Schierstaedt et al. (2020) |
| Escherichia coli O157:H7 | Gastrointestinal illness | Vegetables, fruits, water | Mootian et al. (2009) |
| Burkholderia pseudomallei | Pneumonia and melioidosis | Vegetables, fruits | Limmathurotsakul et al. (2016) |
| Legionella longbeachae | Legionnaires' Disease | Water | Steffan et al. (2020) |
| Campylobacter spp | Gastrointestinal illness | Vegetables, fruits, meats, water | Silva et al. (2011) |
| Clostridium perfringens | Gastrointestinal illness | Vegetables, fruits, meat | García and Heredia (2011) |
| Francisella tularensis | Tularaemia | Meats | Baumgardner (2012) |
| Yersinia pestis | Pneumonic and bubonic plague | Meats | Steffan et al. (2020) |
| Histoplasma capsulatum | Histoplasmosis | Vegetables, fruits | Steffan et al. (2020) |
| Coccidioides immitis | Valley fever | Vegetables, fruits | Steffan et al. (2020) |
| Blastomyces dermatitidis | Blastomycosis | Vegetables, fruits | Klein et al. (1986) |
Understanding the pathway of the transmission of human pathogens is crucial for effective management of their presence and virulence (Figure 2). In many cases, pathogens can directly enter human systems through the contamination of food (e.g. fruits and vegetables) and cause human infection (Beuchat, 2002). Whilst transmission of these pathogens, in agricultural systems is still largely unknown, application of animal manure as fertilisers and irrigation with contaminated wastewater provides major pathways for pathogens to entre human food chain (Barak & Schroeder, 2012; Chen & Jiang, 2014) Therefore, effective control of the pathogens in animal manure and irrigation water to reduce the virulence and transmission of human pathogens in agricultural systems should be the first step in achieving One Health goals. Additionally, exposure to contaminated soil through direct contact and inhalation can also serve as an important pathway for soil-borne pathogens to cause human infections and thus should be minimised. Moreover, insects and other animals also play a role in the transmission of soil-borne pathogens (Sela et al., 2005). Practising hygiene and restricting infections in the first place from animal vectors is possible to minimise diseases in humans. Furthermore, soil biotic and abiotic factors like microbial diversity and nutrient availability were recognised to be important drivers influencing survival and success of soil-borne human pathogens (Bultman et al., 2013; Schierstaedt et al., 2020; Semenov et al., 2009). For instance, human pathogens Salmonella enterica and Escherichia coli O157:H7 prefer to live in nutrient-rich soil (Semenov et al., 2009), while the survival of L. monocytogenes depends on soil acidity and microbial communities (Schierstaedt et al., 2020). One Health practice, therefore, also requires cautious monitoring of potential soil-borne human pathogens in different components of ecosystems. We have minimal understanding of distribution and transmission of soil-borne human pathogens, and how this is modified by agronomic practices. Global changes (e.g., land use and climate change) are known to have impacts on soil communities and functions, however, we have little information on how these changes will impact soil-borne human pathogens and their transmission and virulence.
4 ANTIMICROBIAL RESISTANCE IN AGRICULTURAL SYSTEMS
Although antibiotics have a vital role in maintaining human and animal health, the emergence and transmission risk of antimicrobial-resistant pathogens have attracted significant attention from health professional, policymakers, and general public (Hofer, 2019; Kümmerer, 2003; Sugden et al., 2016) Antimicrobial resistance is recognised as one of the biggest threats to global public health and food security which is a central focus of the One Health concept (Hernando-Amado et al., 2019). Particularly, environmental ARGs that are at high risk to be acquired and confer resistance by human pathogens is considered a significant public health concern (Martinez et al., 2015). The shared antibiotic resistomes between human pathogens and soil microbiome and the clinical importance of ARGs in soil environments have been investigated and summarised by several previous studies (Esiobu et al., 2018; Forsberg et al., 2012).
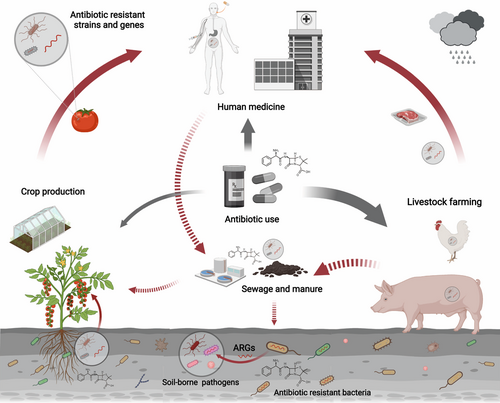
Various antibiotics are widely used in human medication, livestock farming, and aquaculture, and the frequent use of antimicrobial drugs is considered to be the most important factor leading to the increasing AMR (Figure 3 and Table 2) (Hernando-Amado et al., 2019; Holmes et al., 2016). It is estimated that two-thirds of global antibiotic usage is allocated to industrial livestock production. For example, a survey from China has reported that veterinary antibiotics accounted for 84.3% (pig: 52.2%, chicken: 19.6%, and other animals: 12.5%) of the total usage of antibiotics, whereas human antibiotics only accounted for 15.6% (Zhang et al., 2015). Veterinary antibiotics such as β-lactams (e.g., amoxicillin and penicillin), tetracyclines (e.g., doxycycline and oxytetracycline), sulphonamides (e.g., sulfamonomethoxine and sulfaquinoxaline), and fluoroquinolones (ciprofloxacin and enrofloxacin) are often used as veterinary drugs and growth promoters in the livestock industry (Table 2) (Shao et al., 2021; Zhang et al., 2015). The residues of these antibiotics, and the antimicrobial-resistant strains and genes they enrich, may be transferred to agricultural soils through sewage sludge and manure (Zhu et al., 2013), which in turn are then transmitted to humans microbiome via crop microbiomes. In addition, some antibiotics like streptomycin and oxytetracycline can directly enter soil systems via the usage in plant production for control of plant-pathogenic bacteria such as Erwinia amylovora and Pseudomonas syringae (Table 2) (Sundin & Wang, 2018).
| System | Name | Category | References |
|---|---|---|---|
| Human medicine | Cephalexin, Amoxicillin, Penicillin | β-lactams | Kümmerer (2003); Zhang et al. (2015) |
| Erythromycin-H2O | Macrolides | ||
| Ofloxacin, Norfloxacin, Ciprofloxacin | Fluoroquinolones | ||
| Tetracycline | Tetracyclines | ||
| Trimethoprim | Sulfonamides | ||
| Lincomycin | Others | ||
| Livestock farming | Amoxicillin, Penicillin | β-lactams | Zhang et al. (2015); Shao et al. (2021); Zhu et al. (2013) |
| Florfenicol, Lincomycin, Chloramphenicol | Others | ||
| Tylosin, Erythromycin-H2O, Leucomycin, | Macrolides | ||
| Ciprofloxacin, Enrofloxacin, Norfloxacin, Ofloxacin, Pefloxacin, Lomefloxacin | Fluoroquinolones | ||
| Doxycycline, Oxytetracycline | Tetracyclines | ||
| Sulfamonomethoxine, Sulfaquinoxaline, Sulfadiazine, | Sulfonamides | ||
| Crop production | Streptomycin | Aminoglycoside | Sundin and Wang (2018); Stockwell and Duffy (2012) |
| Oxytetracycline | Tetracyclines | ||
| Gentamicin | Aminoglycoside | ||
| Oxolinic acid | Others | ||
| Carbenicillin, Cefotaxime, Timentin | β-lactams | Grzebelus and Skop (2014) |
Due to the excessive use of antibiotics for treatment and growth promotion in animal farming, livestock farms have become an important reservoir of antimicrobial-resistant bacterial strains and genes (Zhu et al., 2013). Therefore, the current One Health approaches to combat AMR focus largely on the reduction of unnecessary antibiotic use in intensive animal farming (Hoelzer et al., 2017). Correlations between the reduction in antibiotic use in animal husbandry and reduction in the abundance of ARGs in animal-based food products have been reported by several studies (Hoelzer et al., 2017; Scott et al., 2018). Previous work has assessed diversity and abundance of ARGs at three large-scale swine farms and found that the abundance of ARGs correlated directly with antibiotic and metal concentrations (Zhu et al., 2013). However, there is limited evidence of corresponding changes of ARGs in human microbiota which suggests the complexity of the transmission of AMR in agricultural microbial loop and suggest considering contributions of plant microbiomes in regulating transfer of ARGs from soil to human. In agricultural ecosystems, mechanisms other than selection pressure presented by antibiotics may also contribute to the development and spread of AMR. For example, heavy metals such as copper, zinc, mercury, and arsenic could act as co-selection factors for ARGs and contribute to the dissemination of AMR in agricultural ecosystems. That is because there is a high level of similarity between prokaryotic metal-resistance system and antibiotic resistance system (Hu et al., 2016; Ji et al., 2012). In addition, agronomic practices, for example, application of chemical herbicides, were found to be associated with the increase in the prevalence of ARGs in agricultural soils (Liao et al., 2021).
Antimicrobial-resistant strains and genes can spread between human systems and agroecosystems through food chain, sewage sludge, and manure, where animal manures act like a double-sided sword influencing agricultural production and the transmission of ARGs (Figure 3) (McEwen & Collignon, 2018). Using sewage sludge and animal manure as organic fertilisers is common in organic farming for the recycling of nutrients. However, these practices can also significantly promote the spread of ARGs in agroecosystems. Since current wastewater treatment processes are not effective in removing ARGs, the vast majority of ARGs in municipal wastewater, a rich reservoir of ARGs, are discharged in sewage sludge (Munir et al., 2011). Different from sewage sludge, animal manures are not only a rich source of ARGs, they can also contain high levels of undigested antibiotics (Kumar et al., 2005). These undigested antibiotics can be taken up by plants from soil and may apply selection pressure for ARGs in the plant microbiome (Kumar et al., 2005). More importantly, the presence of horizontal gene transfer means bacteria can easily transfer ARGs via mobile genetic elements (Figure 3). Both sewage sludge and animal manure are identified as hotspots for bacteria carrying mobile genetic elements and thus enhance horizontal gene transfer in soil and plant microbiomes when used as organic fertiliser (Zhu et al., 2017). For example, it was reported that some pathogenic strains carrying ARGs such as sul1 and sul2 are presumably able to transmit ARGs from the soil/manure to the plant microbiome.(McEwen & Collignon, 2018; Sun et al., 2020). Many studies have investigated the risks of ARG spread related to agricultural organic fertilisation. An important finding was that organically produced lettuce harbours approximately eightfold more abundant ARGs than those produced conventionally with mineral fertilisers (Zhu et al., 2017). Therefore, there is an urgent need to optimise the practices of recycling nutrients for more sustainable crop production. A recent study on the wastewater treatment plant suggested that easily biodegradable substances like sodium acetate (NaAc) can significantly reduce antibiotics, mobile genetic element mediated ARGs, and the abundance of human bacterial pathogens (Zhang et al., 2022). Biochar, a commonly used soil amendment, has also been reported to be effective in reducing the potential of ARG spread when used in organic fertiliser composting (Chen et al., 2018). The emerging materials and approaches have great potential for developing effective and sustainable agronomic practices to reduce the transfer of ARGs in the future, and their potential environmental risks in the microbial loop and ecological and biological mechanisms unpinning the ARG reduction to be further evaluated.
5 IMPACTS OF CHEMICAL PESTICIDES ON HUMAN AND ENVIRONMENTAL HEALTH
To meet the constantly increasing demand for food, conventional agronomic practices have seen excessive use of agrochemicals. This is a challenge because of the negative impact of agrochemicals on environmental and human health, particularly chemical pesticides, which are defined as the group of substances used to protect plants from insects, weeds, or pathogenic microorganisms (Nicolopoulou-Stamati et al., 2016). The most common route for chemical pesticides to cause human health problems is occupational and accidental contact through skin, ingestion, or inhalation (Nicolopoulou-Stamati et al., 2016). Another important pathway for human exposure to chemical pesticides is through food consumption as pesticide residues are ubiquitous in food and beverages (e.g., fruits, vegetables, wine, and water) (Nicolopoulou-Stamati et al., 2016). A large number of literature has reported that exposure to common classes of chemical pesticides is associated with several chronic health issues (Table 3). Although the use of chemical pesticides that are associated with severe health effects has been banned or restricted in most countries (Yadav et al., 2015), the public health concerns related to the use of these pesticides are not eliminated. Pesticides can be stable in soil environment with degradation times ranging from months to years (Cremlyn, 1978). For example, organochlorine pesticides are very stable with half-lives up to several decades (Cremlyn, 1978). The persistence of chemical pesticides in soil is regulated by several factors including the chemical composition of the pesticide, microbial and photochemical transformation, sorption, and plant uptake (Gevao et al., 2003). It has been estimated that even very low doses of pesticides persist in agricultural soil and may pose severe health effects to human populations (Yadav et al., 2015). Because most chemical pesticides are lipophilic, they are capable of bioaccumulating in human body including in blood, fatty tissue, and breast milk via food grown in pesticide polluted soils (Yadav et al., 2015). A well-known example of organochlorine pesticides is the insecticide dichlorodiphenyltrichloroethane (DDT) which is recognised as the cause of many environmental and human health issues and is still widely distributed in almost all organisms on Earth due to its long-persistent and slow biodegrading properties. Beard and Collaboration (2006) In general, organochlorine pesticides are associated with health effects including carcinogenic, causing endocrine disorders, disrupting reproductive system, and impacting lipid metabolism (Eskenazi et al., 2006; Karami-Mohajeri & Abdollahi, 2011; Loomis et al., 2015; Saiyed et al., 2003; Tiemann, 2008; Turusov et al., 2002) Another important class of chemical pesticide is organophosphorus. Initially, organophosphorus pesticides were introduced to agricultural production as a more ecologically friendly alternative to organochlorines. However, organophosphorus were also found to be associated with several adverse health effects including neurotoxic and endocrine-disrupting effects, influencing nutrient metabolism, and having genotoxic effects (Ezzi et al., 2016; Fritschi et al., 2015; Karami-Mohajeri & Abdollahi, 2011; McKinlay et al., 2008). Similarly, other classes of chemical pesticides such as carbamate, triazines have also been implicated in serious health problems (Goad et al., 2004; Karami-Mohajeri & Abdollahi, 2011; Kniewald et al., 2000; Liu et al., 2006; Wen et al., 2021).
| Chemical class | Example | Direct impact on human | Reference |
|---|---|---|---|
| Organochlorine | Dichlorodiphenyltrichloroethane (DDT) | Carcinogenic | Turusov et al. (2002) |
| Disruption endocrine system | Turusov et al. (2002) | ||
| Neurodevelopmental effects | Eskenazi et al. (2006) | ||
| Endosulfan | Interfere sex hormone synthesis | Saiyed et al. (2003); Sun et al. (2020) | |
| Lindane | Carcinogenic | Loomis et al. (2015) | |
| Impact embryonic development | Tiemann (2008) | ||
| Tris (4-chlorophenyl methanol (TCPM) | Impact embryonic development | Tiemann (2008) | |
| Methoxychlor | Impact embryonic development | Tiemann (2008) | |
| Organophosphates | Glyphosate | Probably carcinogenic | Fritschi et al. (2015) |
| Endocrine disruptors | Fritschi et al. (2015) | ||
| Chlorpyrifos | Genotoxic | Ezzi et al. (2016) | |
| Malathion | Probably carcinogenic | Fritschi et al. (2015) | |
| Genotoxic | Moore et al. (2010) | ||
| Cytotoxic | Moore et al. (2010) | ||
| Diazinon | Probably carcinogenic | Fritschi et al. (2015) | |
| Parathion | Possibly carcinogenic | Fritschi et al. (2015) | |
| Tetrachlorvinphos | Possibly carcinogenic | Fritschi et al. (2015) | |
| Carbamates | Carbofuran | Endocrine disruptors | Goad et al. (2004) |
| Impacting reproductive system | Karami and Abdollahi (2011) | ||
| Ziram | Inhibit sperm mobility | Wen et al. (2021) | |
| Triazines | Atrazine | Cause reproductive disorder | Kniewald et al. (2000) |
| Cytotoxic | Liu et al. (2006) |
In addition to directly influencing human health, excessive use of chemical pesticides also indirectly hinders achieving the goals of One Health concepts by damaging environmental health such as causing loss of biodiversity and having negative impacts on soil health. A good example is the use of glyphosate which has been considered a safe herbicide. However, recent studies found that glyphosate is associated with alternations in microbial community compositions of plant endosphere, rhizosphere, and soil (Hirt, 2020; Van Bruggen et al., 2018). In addition, another recent study found that glyphosate treatment even at a lower than recommended dose reduced plant growth-promoting microorganisms such as mycorrhizal fungi and nitrogen-fixing bacteria (Van Bruggen et al., 2018). In addition, consumption of food contaminated with glyphosate may cause inhibition of beneficial human gut microorganisms including Bifidobacterium and Enterococcus (Van Bruggen et al., 2018). Moreover, reported negative impacts of chemical pesticides on soil microbial diversity could lead to loss of important symbiotic microbes for plant nutrients and thus influencing crop quality (Hirt, 2020). Taken together, all the current evidence indicated that there is an urgent need for a novel sustainable concept in crop production that involves a drastic reduction in the use of chemical pesticides.
6 OUTLOOK—UTILISING SOIL AND PLANT MICROBIOMES TO IMPROVE SUSTAINABLE AGRICULTURAL PRODUCTION
We suggest that harnessing soil and plant microbiomes could significantly contribute towards achieving sustainable agricultural production minimising chemical effects on human health (Figure 4). Directly, soil and plant microbiomes can contribute to food production by increasing plant yield and nutritional quality and at the same time minimising environmental footprint of agricultural production (Singh & Trivedi, 2017). Several studies have suggested manipulating soil and rhizosphere microbiomes as a promising strategy for increasing crop productivity. Targeted deployment of beneficial plant growth-promoting microbes has been reported to increase plant growth and nutrient uptake (Egamberdiyeva, 2007; Fiorentino et al., 2018). For example, the inoculating of Arbuscular mycorrhizal fungi has been demonstrated able to increase the productivity and quality of a wide range of plants including tomatoes, onion, cucumber, and tea plants (Gashaw Deressa & Schenk, 2008; Hu et al., 2013; Ortas et al., 2013; Watts-Williams et al., 2014). In addition, soil microbiome functioning is an important driver of ecosystem processes that are critical for the long-term sustainability of food systems and human wellbeing (Chen et al., 2019a; Wagg et al., 2014). Targeted management of soil microbiomes is likely to alter soil microbial interactions, both with each other and with plants, that are associated with improvements in soil stability, aggregation, and moisture dynamics as well as plant's resistance to biotic and abiotic stresses (Delavaux et al., 2017). However, the successful use of microbial manipulation in field conditions is so far very limited. Research is still needed to delineate the complex soil–plant microbial interaction involved in utilising soil microorganisms to optimise sustainable agricultural production practices.
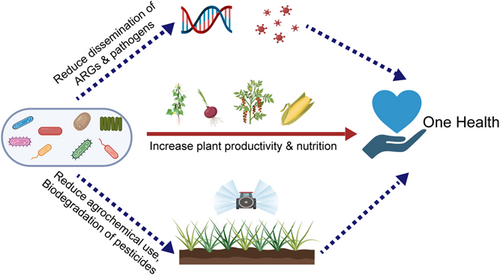
Soil microbiomes can also indirectly contribute to the goals under One Health concepts by serving as possible solutions to the major challenges faced by sustainable agricultural production. The emergence and dissemination of ARGs are one of the most pressing challenges faced by food farming. Animal manure is commonly used as organic fertilisers in sustainable agriculture production for recycling nutrients and reducing the use of chemical fertilisers but is associated with the risk of ARG dissemination (Chen et al., 2017). Recent studies indicated that increasing soil microbial biodiversity by re-introducing indigenous microbes to agricultural soils could serve as a potential solution for this challenge as the proliferation and selection for ARGs in soil environment were negatively correlated with the diversity of soil indigenous microorganisms (Chen et al., 2019b; Klümper et al., 2019) The underlying mechanisms for such observations may be related to the ability of diverse soil microbes to act as biological barriers to prevent the invasion of manure-borne antibiotic resistance bacteria by increasing the cost of resistance (Chen et al., 2019b). Similarly, increased soil microbial diversity was also reported negatively associated with the success of invasion and survival of the enterohemorrhagic human pathogen E. Coli O157:H7 (Van Elsas et al., 2003). This suggests that increasing soil microbial diversity could potentially contribute to reducing the AMR and pathogen loads. Moreover, microbial degradation could be a promising method to ease the problems associated with chemical pesticide use. In natural/agricultural ecosystems, some microorganisms can utilise chemical pesticides as nutrient sources (Kanissery & Sims, 2011). However, the rate of natural microbial degradation is not enough to address the pesticide problem in industrial agricultural production, especially the problems associated with persistent pesticide residuals. Therefore, some alternations to soil microbial communities are imperative to encourage microorganisms to degrade chemical pesticide residuals at a faster rate. Alternatively, booting a number of pesticide degrading microbes in plant microbiomes can achieve twin goals of pest controls and food production with low chemical residues.
Overall, here we summarise the major challenges faced by current agricultural systems and envisaged the possibilities of utilising microbiomes to achieve more sustainable agricultural production. We argue that utilising multidisciplinary approaches and integrating emerging agronomic technologies under the One Health concepts should become an integral part of the strategies to sustainably increase agricultural production for better food and nutrition security. However, current knowledge is sketchy, fragmented, and incomplete. A systematic approach that integrates all components of One Health is needed to identify and predicts agronomic practices that reduce the abundance and transmission of soil-borne human pathogens and ARGs. For example, if relationship between higher soil microbial diversity and low ARG abundance and pathogen load is consistent across soil types and climatic conditions, agronomic practices that promote soil biodiversity (e.g., crop diversity, non-till, and low use of agrochemicals) can be investigated to manage outbreaks. Similarly, we have a limited understanding of global distribution and drivers of soil-borne human pathogens and ARGs. Identifying environmental, climatic, and agronomic drivers can help us to predict their current and future distributions. For example, if we can predict the distribution of pathogens/ARGs under global warming, this will allow time for the development of effective policy and management strategies. However, systematic approaches to research are needed to improve knowledge of all components of One Health. This will also require researchers from trans disciplines (e.g. agricultural, biology, food safety, medical, and social science) to work together on a common cause. This can be facilitated by an effective policy framework and sustainable investment in transdisciplinary research.
AUTHOR CONTRIBUTIONS
Brajesh K, Singh and Zhen-Zhen Yan developed the conceptual framework. All authors contributed to manuscript writing which was led by Zhen-Zhen Yan.
ACKNOWLEDGEMENT
This study was funded by Australia Research Council Discovery grant (DP210102081).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that they have adhered to the ethical policies of the journal.
Open Research
DATA AVAILABILITY STATEMENT
This is a review article thus data availability statement is not relevant.




