Room temperature phosphorescence materials based on small organic molecules: Design strategies and applications
Abstract
Room-temperature phosphorescence (RTP) materials have attracted significant attention due to their applications in various fields such as information storage and encryption, organic light-emitting diode (OLED), sensing, lighting and display, biological imaging, and photodynamic therapy. Traditionally, RTP materials can be efficiently developed using inorganic systems with noble metals or rare earth elements. Recently, many efforts have been devoted to the development of RTP materials based on small organic molecules. The strategies to construct RTP materials include hydrogen bonding, heavy atom effect, n–π* transitions, π–π stacking, donor–acceptor effect, and host–guest doping. Herein, we summarize the recent examples of RTP materials based on small organic molecules primarily focusing on their design strategies and properties. Moreover, their promising applications in information encryption, OLED, as well as bio-imaging and phototherapy are discussed. The challenges and perspectives are given to provide inspiration toward the future development of organic RTP materials.
1 INTRODUCTION
Luminescent materials have attracted extensive attention in a variety of fields, including fluorescence sensing, information encryption, and bio-imaging.1-4 Phosphorescence represents a phenomenon of delayed luminescence that corresponds to the radiative decay of the molecular triplet state. In 1944, Lewis and Kasha proposed the mechanism of phosphorescence,5 demonstrating its origin from the radiative transition of triplet excitons, which is a spin-forbidden process. This is distinct from the generation of fluorescence whose radiative transition arises from spin-allowed singlet excitons. To clarify the related transitions and pathways of phosphorescence, a number of research on the basic theoretical principles and computational aspects of phosphorescence were reported.6, 7 As shown in Figure 1, after absorbing photons, organic luminescent molecules undergo transition from the lower energy ground state (S0) to a higher energy singlet excited state (Sn, n > 1).8, 9 According to Kasha's rule, when the excited molecules return to the lowest singlet excited state (S1) through internal conversion (IC), they emit photons through spin-allowed radiative transitions known as fluorescence. The kICF and kF represent the rates of IC and fluorescence, while knrF represents that of the singlet non-radiative process. Phosphorescence, on the other hand, is generated through a process where luminescent molecules undergo transition from the excited singlet state (S1) to the excited triplet state (Tn) under the influence of inter-system crossing (ISC) (kISC, the rate of ISC) and then return slowly from the excited triplet state (T1) to the ground singlet state (S0) (kP, the phosphorescence decay rate from T1→S0).10, 11 While the phosphorescence radiative processes occur, the non-radiative transitions compete with them (knrP, the triplet non-radiative decay rate). Therefore, phosphorescent materials are known for possessing slower radiative rates and longer lifetimes.12
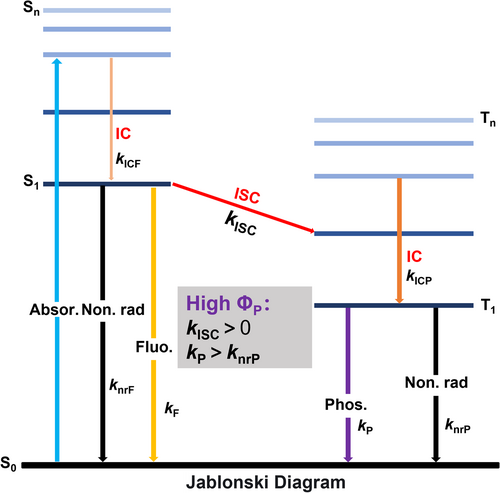
The Jablonski diagrams of fluorescence and phosphorescence.
Due to their unique photophysical processes, phosphorescent materials have found a wide range of applications in organic light-emitting diode (OLED),13-15 biological imaging,16-19 information storage and security anti-counterfeiting,20-22 security ink, and sensors.23-25 Compared to traditional inorganic room-temperature phosphorescence (RTP) materials, purely organic RTP materials have many advantages such as facile synthesis and functionalization, low cost, low toxicity, good environmental compatibility, and biocompatibility.26-28 However, the weak spin orbital coupling (SOC) and fast nonradiative transitions lead to inefficient ISC, which results in low luminescence efficiencies.29 To achieve effective phosphorescence emission for organic RTP materials, several considerations should be taken into account (Figure 1). On one hand, promotion of the ISC process requires a small energy gap (ΔEST) between S1 and T1 levels to ensure kISC > 0, which is dependent on the energy level and electronic configuration of a molecule. On the other hand, suppression of non-radiative relaxation is necessary to ensure the efficient phosphorescence, that is to meet the criteria of kP > knrP.30, 31
Based on the current challenges, a series of design strategies have been developed, such as introducing aromatic carbonyl compounds,32-34 heavy atom (Br, I, etc.) effect,35 and heteroatoms (N, O, S, etc.) with n–π* transitions19, 36 to promote forbidden ISC processes, controlling intermolecular interactions to create rigid micro-environment such as through crystallization,37 aggregation formation,35 doping with rigid matrices,38 and host–guest39 interactions to suppress non-radiative transitions, and insulating phosphors from oxygen molecule.40, 41 Through these strategies, the properties of organic RTP materials have been significantly enhanced.42-47 These enhancements include shifting the emission wavelength toward the near-infrared (NIR) region,48, 49 increasing the phosphorescence quantum yield to exceed 95%,50 and extending the afterglow duration to about 1 h.12
Herein, this review first summarizes the molecular design strategies for RTP materials from small organic molecules that have been developed in recent years (Figure 2). In the next section, the applications of organic RTP materials in information storage and encryption,53-55 OLED,56 bio-imaging, and photodynamic therapy57 are introduced. Finally, this review outlines the current challenges and future prospects for organic RTP materials with the hope to provide some insights for the exploitation of new RTP materials with high efficiencies and long lifetimes.
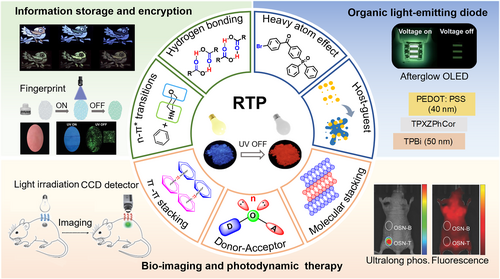
The schematic illustration of the design strategies and applications of RTP materials based on small organic molecules. Reproduced with permission.3 Copyright 2024, John Wiley and Sons. Reproduced with permission.11 Copyright 2017, John Wiley and Sons. Reproduced with permission.20 Copyright 2018, John Wiley and Sons. Reproduced with permission.21 Copyright 2022, John Wiley and Sons. Reproduced with permission.51 Copyright 2023, Springer Nature. Reproduced with permission.52 Copyright 2023, John Wiley and Sons. RTP, room-temperature phosphorescence.
2 STRATEGIES TO ACHIEVE RTP MATERIALS FROM SMALL ORGANIC MOLECULES
2.1 Hydrogen bonding
As one of intermolecular non-covalent interactions, hydrogen bonding can help restrict molecular motion, creating a rigid environment and is therefore widely used to enhance phosphorescence efficiency.22, 29, 32, 58, 59
In 2018, Li et al.60 reported the first non-aromatic pure small organic molecule with persistent RTP (p-RTP) properties using cyanoacetic acid (CAA), which exhibits a long lifetime of 862 ms (Figure 3a). This is attributed to the strong intermolecular hydrogen bonding from the carboxylic group in CAA, which restricts the molecular motion, promoting the ISC process from S1 to T1 state. It is worth noting that RTP was observed in CAA crystals with a spacing distance of 3.0225 Å rather than in solution, indicating the critical role of hydrogen bonding for generation of long-lived RTP. Generally, organic RTPs are derived from aromatic compounds, especially those with carbonyl groups. This research clarifies the relationship between RTP performance and molecular structure as well as packing mode in non-aromatic systems. It offers new insights for the design of organic RTP materials through incorporation of nonaromatic carboxylic acid groups. They can create a rigid environment by forming long-range-ordered structures via hydrogen bonding. This holds significant importance in expanding the scope of RTP molecules.
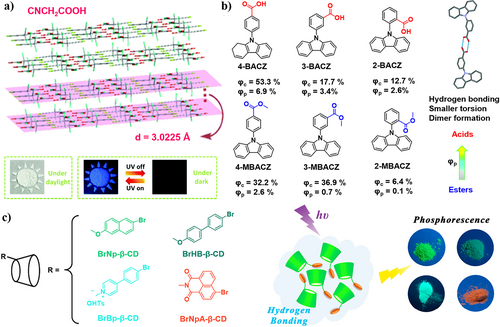
(a) The CAA single crystals that areassembled layer by layer with a spacing distance of 3.0225 Å. Reproduced with permission.60 Copyright 2018, Royal Society of Chemistry. (b) Molecular structures of three carbazole carboxylic acids 4-BACZ, 3-BACZ, 2-BACZ, and their corresponding esters 4-MBACZ, 3-MBACZ, and 2-MBACZ, and the single crystal of the dimer of 3-BACZ. The phosphorescence efficiencies of the crystals (Φc) and powders (Φp) are also given. Reproduced with permission.59 Copyright 2019, Royal Society of Chemistry. (c) Molecular structures of cyclodextrin derivatives BrNp-β-CD, BrHB-β-CD, BrBp-β-CD, and BrNpA-β-CD, and the schematic illustration of the phosphorescence emissions induced by hydrogen bonding as well as the photos with different colors of the phosphorescence emission. Reproduced with permission.61 Copyright 2018, American Chemical Society.
In 2019, Yang et al.59 reported three compounds (2–4)-BACZ that stably emit RTP by the combination of phosphorescent unit carbazole with carboxylic group to enhance SOC. The corresponding three methyl esters (2–4)-MBACZ were fabricated as control compounds with p-RTP efficiencies (ΦP) to be 2.6%, 0.7%, and 0.1% in powder state, respectively (Figure 3b). They exhibited much higher efficiencies (Φc 32.2%, 36.9%, and 6.4%) in crystal state. In comparison to the esters, the carboxylic acid compounds (2–4)-BACZ formed highly rigid dimer structures with smaller torsion in the crystalline state aided by hydrogen bonding, resulting in a significant decrease in vibrational dissipation. As a result, the increasing stability of the triplet excitons greatly enhanced the phosphorescence efficiencies (Φc 53.5%, 17.7%, and 12.7% in crystalline state) and lifetimes of p-RTP (4.3, 13.8, and 12.3 ns), achieving an enhancement of up to 26 times particularly in powder state for 2-BACZ. This result highlights the importance of hydrogen bonding in carboxylic groups, which largely improves the phosphorescence efficiencies of RTP molecules.
In 2018, Tian et al.61 fabricated β-cyclodextrin derivatives BrNp-β-CD, Br-HB-β-CD, BrBp-β-CD, and BrNp-β-CD by incorporating several fluorophores, including 4-bromonaphthalene, 4-bromobiphenyl, 4-(4-bromo-phenyl)-pyridine, 4-bromonaphthalene monoamide, respectively. Their amorphous powders exhibit efficient RTP emissions changing from blue, green to orange owing to the hydrogen bonding between cyclodextrin derivatives (Figure 3c). It helps stabilize the phosphors, suppressing non-radiative relaxation and protecting them from being quenched. This approach shows the advantages of facile preparation, high feasibility, strong phosphorescence emission, low toxicity, and wide application. Moreover, it does not require strict conditions such as deoxygenation or low-temperature, a favorable strategy for efficient RTP emission.
2.2 Heavy atom effect
Introduction of heavy atoms such as chlorine (Cl), bromine (Br), iodine (I), sulfer (S), and selenium (Se), represents another effective strategy to facilitate RTP.46, 62 Heavy atoms, particularly halogen elements, can engage in halogen bonding, which not only exhibits energy compatibility but also features greater directionality compared to hydrogen bonding. It can readily introduce a large SOC and kP (up to 1010 s−1).31
In 2016, Zhao and coworkers reported a class of dibromobenzene derivatives, which display pure organic phosphorescence with ΦP of up to 21.9% and millisecond-scale lifetimes by manipulating heavy-atom interaction.63 To be noted, they found that the ΦP of the molecule with four bromine atoms was higher than that with two bromine atoms, indicating that increasing the intermolecular Br–Br interaction ratio can significantly generate more efficient phosphorescence. The relationship between molecular structure and phosphorescence properties was investigated using single-crystal analysis and Density Functional Theory calculations. Increasing the Br-cluster interactions and decreasing the triple–triple annihilation probability is favorable for phosphorescence generation. This research represents the first organic metal–free system, which enhances phosphorescence efficiencies by manipulating heavy-atom interaction such as bromine atoms substitution. It expands the range of purely organic single-component phosphorescent materials with high luminescence efficiencies.
While under ambient conditions, it seems challenging for organic RTP materials to simultaneously enhance their lifetimes and efficiencies.64 The combination of heavy-atom effect, El-Sayed's law, and the energy-gap principle can contribute to high kP and knrP as well as efficient reverse ISC processes, thereby shortening the lifetimes in spite of increasing the efficiencies.19, 29, 65-67 In 2019, Tang and coworkers68 combined bromo-dibenzofuran or bromo-dibenzothiophene groups with carbazole to form twisted phosphors (Figure 4a). With the aid of heavy atoms, small energy gaps, and spin-electron coupling, they have rationally achieved efficient ISC processes. Through triplet–triplet energy transfer (TTET) process, the triplet excitons were conveyed from high-lying Tn state of bromo-dibenzofuran or bromo-dibenzothiophene groups to carbazole, generating organic p-RTP. The efficient and persistent phosphors have a ΦP of up to 41% (total Φ 73%), with a lifetime of 0.54 s under ambient conditions. Therefore, the efficiencies of RTP materials can be boosted through TTET process, providing a new design strategy for achieving efficient RTP materials with the synergistic implementation of heavy atom effect and other processes.
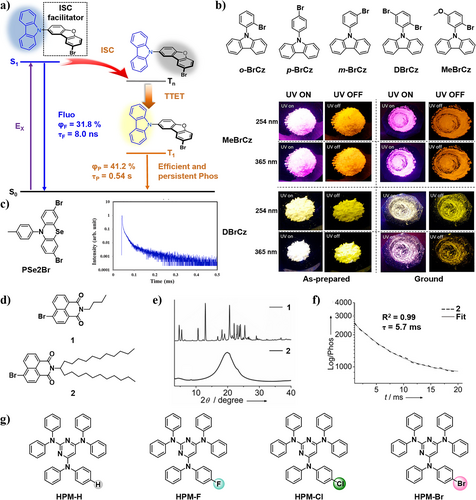
(a) The schematic figure of organic p-RTP molecules obtained from bromodibenzofuran-substituted carbazole by intramolecular TTET process with lifetimes and efficiencies in the crystalline state. Reproduced with permission.68 Copyright 2019, Springer Nature. (b) Molecular structures of o-BrCz, p-BrCz, m-BrCz, DBrCz, and MeBrCz, and luminescent photos of crystals and grinding powders of MeBrCz and DBrCz. Reproduced with permission.69 Copyright 2019, American Chemical Society. (c) The temperature-dependent transient PL spectrum of the crystalline PSe2Br. Reproduced with permission.70 Copyright 2022, Elsevier. (d) Chemical structures of 1 and 2. (e) XRD patterns of 1 and 2 and (f) Phosphorescence lifetime decay profile of 2. Reproduced with permission.71 Copyright 2019, John Wiley and Sons. (g) Molecular structures of HPM derivatives with different substituents HPM-H, HPM-F, HPM-Cl, HPM-Br. The colored spherical shadows indicate the sizes of atoms.72 Copyright 2021, Royal Society of Chemistry. PL, photoluminescence; p-RTP, persistent room-temperature phosphorescence; TTET, triplet–triplet energy transfer; XRD, X-ray diffraction.
Not only the types of the heavy atoms, but also the position of heavy atoms will greatly affect the quantum yields of RTP materials. In 2019, Zhang et al.69 reported a series of efficient metal-free RTP materials including o-BrCz, m-BrCz, p-BrCz, DBrCz, and MeBrCz (Figure 4b). The study revealed that the position of the Br atom substitution substantially impacts the quantum yields, ranging between 1.55% and 1.81% for o-BrCz, m-BrCz, and p-BrCz. Furthermore, it was discovered that both Br atoms and methoxy groups can significantly promote the SOC process between singlet and triplet states, leading to a large increase in the ISC rate constant. With the aid of strong halogen bonds formed by Br atoms, DBrCz exhibited a ΦP of 24.53%, which is 13.7 times higher than that of o-BrCz. To be noted, MeBrCz displayed a phosphorescence lifetime of 148.51 ms with a ΦP of 27.81%. It revealed that the methoxy groups can balance the competition between phosphorescence lifetimes and efficiencies. The compounds DBrCz and MeBrCz can retained their RTP properties even after grinding, and were successfully applied to an AC-LED, achieving a white LED with Commission Internationale d’Eclairage (CIE) coordinates (0.28, 0.29) and a color rendering index (CRI) over 90.
As another kind of heavy atoms, Se can also be incorporated into the molecules to induce RTP via promoting SOC process. In 2022, Lee et al.70 employed both Se and Br atoms to induce SOC process and shorten the excited state lifetimes of organic phosphorescent materials by mixed heavy atoms effect (Figure 4c). The synergistic effect of Se and Br significantly accelerates the transition from T1 to S0 state with a high SOC coefficient of 12.15 cm−1, resulting in a photoluminescence (PL) quantum yield of 32.9%. At the same time, the phosphorescence emission process was also accelerated, with its lifetime as short as 28.0 μs. The author claimed that it was the first report that achieved an excited state lifetime of <50 μs in pure organic phosphorescent emitters via the strategy of heavy atom effect. Due to the mixed heavy atom effect from Br and Se, they have paved the way for advancements in RTP materials with shorter phosphorescence lifetimes, highly favorable for application in OLEDs.
Most organic RTP materials are found in crystalline form because the molecular ordering arrangement enhances ISC process, while examples of solvent-free liquid-phase RTP are scarce. In 2019, Babu et al.71 designed and synthesized an organic liquid phosphor 2 by introducing a long-branched alkyl chain onto bromo-naphthalimide (Figure 4d–f). Owing to the narrow singlet–triplet energy gap (ΔEST) and the presence of the weak Br⋯O halogen bonding, the compound 2 exhibits RTP properties both in crystalline and liquid states. Its reference compound 1 with shorter n-butyl chain also exhibits typical RTP property in crystalline form. It was revealed that there are weak halogen bonds between the molecules, and the phosphorescence lifetime of compound 2 is 5.7 ms. The long flexible alkyl chains do not change the phosphorescent properties of the two compounds, but significantly alter their crystalline properties. The suppressed non-radiative decay in the viscous medium reduced ΔEST, and the presence of weak Br···O halogen bonding facilitated RTP for solvent-free liquid in air. Importantly, compound 2 is the first reported liquid organic small molecule based RTP material. It exhibits the potential to make innovative changes in large-area flexible lighting applications, a new hotspot in the realm of phosphorescent materials.
Despite the extensive research using halogen bonds to enhance SOC, promote the ISC process, and increase the efficiencies of RTP materials, it is essential to acknowledge that halogen-induced molecular stacking may also weaken the efficiencies of RTP materials.73 In 2021, Li and coworkers72 reported a series of hexaphenylmelamine (HPM) derivatives with different substituents HPM-H, HPM-F, HPM-Cl, and HPM-Br through the introduction of different heavy atoms fluorine, chlorine, and bromine (Figure 4g). As the substituent in para-position of HPM derivatives was adjusted from H to F, Cl, and Br, the ultralong lifetimes RTP emission were significantly weakened. Through the analysis of experimental results and theoretical simulations, it is inferred that the π-substituted halogen atoms disrupted the tight molecular stacking of HPM-H, increasing the intermolecular distances and intermolecular repulsion, leading to a substantial weakening of the RTP efficiencies. Considering the negative effect caused by the heavy atoms, it suggests the necessity for carefully design of RTP materials with high efficiencies when incorporation of heavy atoms.
2.3 n–π* transitions
Introduction of heteroatoms such as O, N, and S with lone pair of electrons can generate n–π* transitions, contributing to the ISC efficiencies and triplet excitons populations, which is favorable for RTP generation. The incorporation of n–π* transitions with other interactions has been extensively developed in recent years.
In 2023, Ma et al.51 introduced a non-conjugated heterocycle molecule, morpholine, into benzene and successfully prepared a series of morpholine substituted derivatives 1-morpholinobenzene (MPh), 1,2-dimorpholinobenzene (oMPh), 1,4-dimorpholinobenzene (pMPh), and 1,3,5-trimorpholinobenzene (TMPh) with RTP properties. Their investigations revealed that morpholine is crucial in the generation of n–π* transitions, acceleration of ISC, and generation of triplet excitons. The oxygen atom at the opposite position of nitrogen atom in morpholine can induce multiple intermolecular interactions, which can minimize the non-radiative process and stabilize the triplets. In addition, the introduction of morpholine can possibly generate a variety of C–H…π interactions, favorable for suppression of molecular motion and nonradiative transition process. The synergistic effect of n–π* transitions and hydrogen bonding originating from morpholine can significantly promote RTP emission. Notably, these compounds also exhibited versatile excited state properties (Figure 5a). Among them, oMPh showed dual delayed emission of thermally activated delayed fluorescence (TADF) and RTP. Both pMPh and TMPh showed dual RTP emissions originating from monomer state and aggregated state. Particularly, the phosphorescence lifetime of TMPh, featuring three morpholine groups, extends to an impressive duration of 1.03 s, with a visible afterglow persisting for up to 10 s in the aggregated state. This work illustrated the key importance of the morpholine on the modulation of the lifetimes of RTP materials without utilization of metal, or even any heavy atoms.
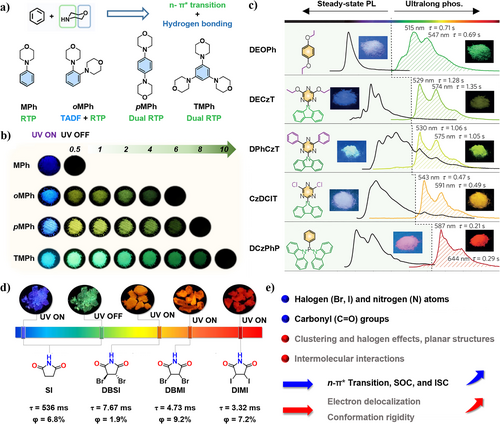
(a) Molecular structures of the phosphors MPh, oMPh, pMPh, and TMPh and (b) their luminescent photographs taken before and after light irradiation at 365 nm under ambient conditions. Reproduced with permission.51 Copyright 2023, Springer Nature. (c) Molecular structures of the compounds DEOPh, DECzT, DPhCzT, CzDClT, and DCzPhP, and their steady-state photoluminescence (left) together with ultralong phosphorescence (right) spectra. Insets: the corresponding photographs taken before (left) and after (right) after light irradiation. DEOPh is excited at 254 nm at 300 K, while other compounds are excited at 365 nm at 300 K. Reproduced with permission.32 Copyright 2015, Springer Nature. (d) Molecular structures, luminescent photographs of the crystals, phosphorescence yields, and lifetimes and (e) the design strategies of the phosphors SI, DBSI, DBMI, and DIMI. Reproduced with permission.74 Copyright 2022, Springer Nature.
In addition to the utilization of n–π∗ transition coupled with hydrogen bonding to boost RTP, the strategy of combining n–π∗ transition and H aggregates formed through π–π stacking has been adopted to generate ultralong RTP materials in recent years.75-78 Given the challenge of producing long-lived excited states due to the ultrafast deactivation of highly active excited states, Chen et al. proposed the modulation of emission lifetimes through strong coupling in H-aggregated molecules, effectively stabilizing the triplet-excited states. They synthesized several organic molecules (DEOPh, DECzT, DPhCzT, CzDClT, and DCzPhP) containing O, N, and P atoms (Figure 5c).32 The molecules adopted planar molecular structures with well-defined substituents to induce parallel alignment, leading to the formation of H-aggregates. This alignment serves to stabilize the excited states and extend phosphorescence lifetimes. The prepared organic molecules exhibited the color of ultra-long phosphorescence from green (515 nm) to red (644 nm) under ambient conditions, with luminescence lifetime up to 1.35 s. This approach of extending the lifetimes represents a major step forward in expanding the scope of organic RTP applications. The creation of RTP materials with red or even NIR emission holds great potential in a wide range of applications in bio-imaging, which will be discussed later in the applications of RTP materials in Section 3.3.
Typically, the reported RTP emitters mainly focus on the blue-green and yellow-green colors. The modulation of RTP color toward red and even NIR, especially for nonaromatic compounds, remains an enormous challenge. In 2022, Yuan et al.74 achieved a remarkable breakthrough by demonstrating unexpectedly red and NIR RTP using a group of halogenated cyclic imides including succinimide (SI), trans-2,3-dibromosuccinimide (DBSI), 2,3-dibromomaleimide (DBMI), and 2,3-diiodomaleimide (DIMI) (Figure 5d). They found that SI crystal exhibits green afterglow with a long lifetime of 536 ms, while the other crystals emit unexpected bright orange to red fluorescence free of afterglows, which are mainly found to be RTP emissions ranging from orange-red to NIR. Through analysis of photophysical properties, single crystals and theoretical calculations, the emergence of red and NIR RTP is ascribed to the synergistic effect of molecular clustering, halogen effect, and π–π stacking (Figure 5e). In particular, the halogen, N atom together with the C=O group not only promote n–π∗ transition and enhance the efficiency of ISC process, but also rigidify the molecular conformation and extend through space conjugation (Figure 5e). Additionally, the planar structure contributes to π–π stacking, resulting in the generation of NIR emission. These represent the first examples of small nonconventional luminophores with red or even NIR RTP. It provides inspiration for the creation of nonconventional luminophores with long wavelength emission.
Theoretical studies on molecular orbitals have indicated the crucial role of the electronic configuration of excitons in ISC and radiative phosphorescence decay. The transition from singlet to triplet states with electronic configurations of (n, π*) and (π, π*) can contribute to effective SOC. Therefore, the design of molecular excited states featuring a combination of (n, π*) and (π, π*) configurations have the potential to significantly enhance the efficiencies of RTP materials. Based on this, Tang et al.29 introduced carbonyl group (C=O) to provide the n orbital in order to trigger ISC from S1 to Tn. Then various conjugated subunits serving as π orbitals were combined to reduce kP and a variety of full-color pure organic phosphors were obtained. Through adjusting the aromatic subunit in phenyl ketone to regulate the characteristics of molecular orbitals and energy levels of excited states, the efficiencies of RTP materials can achieve up to 36.0% with a long lifetime of 0.23 s under ambient conditions.
2.4 π–π stacking
As one type of prevalent intermolecular weak interactions in aromatic molecules, π–π stacking plays crucial roles in promoting RTP since it can strengthen the intermolecular stacking and generate rigid environment, which is helpful to decreasing non-radiative decay.
In 2018, Li, Pu et al. proposed that functional moieties and molecular packing in the solid state can stabilize the excited triplet state, favorable for p-RTP.66 By integrating 9-phenyl-9H-carbazole and sulfonyldibenzene groups, they synthesized a series of 10-phenyl-10H-phenothiazine 5,5-dioxide derivatives (CS-CH3O, CS-CH3, CS-H, CS-Br, CS-Cl, and CS-F) (Figure 6a). By varying the substituent on the 10-phenyl ring, the RTP lifetimes of their crystals increased from 88 ms (CS-CH3O) to 96 ms (CS-CH3), 188 ms (CS-H), 268 ms (CS-Br), 256 ms (CS-Cl), and 410 ms (CS-F). The introduction of electron-withdrawing group (EWG) reduces the electron densities of the phenyl rings and relieves the π–π repulsion, thus shortening the π–π distance and promoting stronger π–π interaction (Figure 6b,c). This further stabilizes the excited triplet state, leading to the ultralong RTP effect in the system. The introduction of another strong EWG, trifluoromethyl, to yield CS-CF3 did not show any extension of the RTP lifetimes. However, CS-CF3 crystals exhibited unique photo-induced phosphorescence upon the changes in molecular stacking. Therefore, subtle changes in molecular structure greatly affect the stacking of molecules in crystals, leading to different RTP behaviors. These investigations suggest that the packing modes, rather than the molecular electronic structures, are primarily responsible for the different RTP behaviors in solid state.
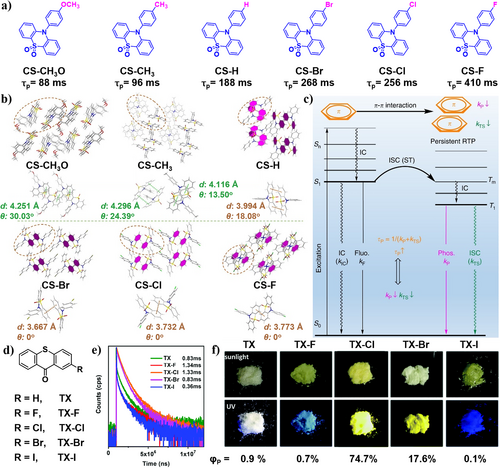
(a) Molecular structures and phosphorescence lifetimes of CS-CH3O, CS-CH3, CS-H, CS-Br, CS-Cl, and CS-F. (b) Packing modes of the crystals for CS-CH3O, CS-CH3, CS-H, CS-Br, CS-Cl, and CS-F: it shows part of the packing, where the centroid–centroid distances and the dihedral angles of the involved phenyl rings are shown and the stronger π–π interactions are labeled in pink. (c) The proposed mechanism for organic p-RTP: the strong π–π interaction decreases the kP and non-radiative transition (kTS) from T1 to S0 state, thus achieving the p-RTP. Reproduced with permission.66 Copyright 2018, Springer Nature. (d) Chemical structures of thioxanthone (TX) with halogenated substituents. (e) Phosphorescence lifetimes at 560 nm for TX, 530 nm for TX-F, and 578 nm for TX-Cl, TX-Br, and TX-I. (f) Photographs of all phosphors (TX, TX-F, TX-Cl, TX-Br, TX-I) under sunlight and UV light and their corresponding phosphorescence quantum yields. Reproduced with permission.62 Copyright 2019, Royal Society of Chemistry. p-RTP, persistent room-temperature phosphorescence; TX, thioxanthone.
In 2019, Yang et al.62 successfully prepared thioxanthone (TX) and its halogenated derivatives (TX-R, R = F, Cl, Br, I), achieving efficient RTP emissions (Figure 6d). In particular, TX-Cl displayed bright orange RTP emission, with an absolute ΦP of 74.7% (Figure 6f). Single crystal analysis showed the formation of H-aggregates through long-range packing, indicating the π–π stacking of molecular column. They proposed that one-dimensional π–π stacking enhanced both ISC and RTP radiative rates. As a result, TX-Cl possesses a phosphorescence lifetime of 1.33 s (Figure 6e). More importantly, the energy splitting induced by one-dimensional π–π aggregation also significantly promotes the RTP radiative rate of T1→S0 through more effective mixing between the excited singlet and triplet states, resulting in efficient RTP.
By the introduction of π stacking units, the original fluorophores can be effectively transformed to phosphors. In 2020, Perepichka et al.79 synthesized a series of azatriangulenetrione (TANGO) derivatives. Through manipulating the molecular orientation within π stacking via adjusting the steric size of the substituents, pure fluorescence was successfully converted to pure phosphorescence (Figure 7a). The t-butyl group induced a rotation of 60°, resulting in almost pure fluorescence (tBuTANGO, ΦP = 11%). In contrast, smaller substituent groups such as H, Cl, Br, and I exhibit pure phosphorescence emission with high ΦP (e.g., HTANGO with ΦP of 42%), resulting from the presence of co-aligned π stacking within the system. Despite the strong π–π interactions in HTANGO and TCTANGO, they manage to avoid aggregation-caused quenching and triplet–triplet annihilation. Without using any heavy atoms, this achievement sets a new benchmark for high phosphorescence efficiency of pure organic solids. Given that triplet–triplet annihilation and extended exciton diffusion are typically associated with exciton quenching, it is impressive to achieve efficient phosphorescence in such densely packed solids through π–π interactions.
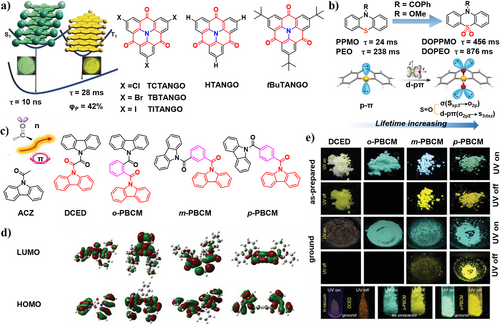
(a) The proposed π–π stacking mechanism for organic RTP and molecular structures of TCTANGO, HTANGO, and tBuTANGO. Reproduced with permission.79 Copyright 2019, John Wiley and Sons. (b) Molecular structures of the compounds PPMO, PEO, DOPPMO, DOPEO, and illustration of the phosphorescence lifetimes enhancement by the introduction of d-pπ bonds. Reproduced with permission.80 Copyright 2019, John Wiley and Sons. (c) Design concept of the parent molecule ACZ and molecular structures of DCED, o-PBCM, m-PBCM, and p-PBCM. (d) Electron density distributions of HOMO and LUMO levels. HOMO, highest occupied molecular orbital; LUMO, lowest unoccupied molecular orbital. (e) Top: Photographs of the as-prepared and ground solids of DCED, o-PBCM, m-PBCM, and p-PBCM at ambient conditions. Bottom: Photographs of the solids of DCED in vacuum and the as-prepared and ground solids of o-PBCM. All of the photos are taken before and after ceasing the 365 nm UV irradiation. Reproduced with permission.57 Copyright 2019, John Wiley and Sons. HOMO, highest occupied molecular orbital; LUMO, lowest unoccupied molecular orbital; RTP, room-temperature phosphorescence.
In 2019, by introducing the d-pπ bond into the thiazine system, An et al.80 synthesized DOPEO, DOPPMO, and prolonged their phosphorescence lifetimes (Figure 7b). Compared with the reference compound PPMO (24 ms), a 19-fold increase in the phosphorescence lifetime has been observed for DOPPMO (456 ms). Theoretical calculations and single crystal analysis showed that the d-pπ bond not only reduces the contribution of (n, π*) to the T1 state but also generates tighter π–π stacking and stronger intra- and inter-molecular interactions, reducing the non-radiative decay rate, resulting in ultralong phosphorescence emission.
The inherent conflict of the lowest excited triplet state (T1) involving (n, π*) and (π, π) in RTP molecules presents a great challenge in the precise modulation of RTP with both high efficiencies and ultralong lifetime. An intriguing illustration of the remarkable capabilities of organic RTP materials is the achievement of white light emission from a single molecule. In 2019, Zhang, Yuan et al.57 reported a class of amide compounds (DCED, o-PBCM, m-PBCM, and p-PBCM) with efficient p-RTP by introducing carbonyl and aromatic π units. The introduction of carbonyl group in DCED improved SOC and promoted the ISC process. The presence of two planar carbazole moieties within a single molecule facilitates the formation of strong intermolecular π–π stacking in the solid state, which largely suppresses vibrational motion (Figure 7c). Further introduction of phenyl rings into the isomers (o-PBCM, m-PBCM, and p-PBCM) allowed for more π orbitals, providing more 3(π, π*) fractions in T1. In addition, the electron clouds of the highest occupied molecular orbital (HOMO) are distributed on the carbazole units, whereas the lowest unoccupied molecular orbital (LUMO) are mainly localized on the benzoyl moieties except for DCED, where the LUMO level is distributed on the whole molecule (Figure 7d). The energy levels of the compounds indicate their intramolecular charge transfer (ICT) feature. As a result, the corresponding ΦP and lifetime (τ) were highly improved to 10.2% and 710.6 ms compared to DCED. White light was observed from m-PBCM due to the dual emission of blue fluorescence and yellow RTP. Both m-PBCM and p-PBCM isomers maintained bright p-RTP even under intense mechanical grinding in the amorphous state under ambient conditions (Figure 7e). By finely adjusting the molecular structure with the carbonyl groups and π units, white light emission through single organic molecule was achieved due to the presence of both fluorescence and phosphorescence within one molecule. It provides new thoughts for the development of white light emitters using single-molecule-based RTP materials.
In 2022, Li et al.77 designed five phenothiazine 5,5-dioxide derivatives with the introduction of the 1,2,3-trifluorophenyl group to strengthen the intermolecular hydrogen bonding and inhibit the non-radiative transition. By changing the substituents in the 2-position from methoxyl group (PTZO-CH3O) to bromine (PTZO-Br), hydrogen (PTZO-H), fluorine (PTZO-F), and chlorine (PTZO-Cl) atoms, their RTP lifetimes were greatly increased from 144, to 179–332, 446, and 745 ms, respectively. The experimental results and theoretical calculations revealed that efficient intermolecular π–π interactions can facilitate p-RTP. In particular, one of the PZZO-Cl single crystals showed non-RTP due to small intermolecular overlap and large intermolecular distance. This result certifies the significant influence of intermolecular π–π interactions on RTP emission.
In 2021, Ray et al.81 reported five donor–acceptor (D-A) (D4-A) molecules TOEPh, TOCPh, TOMPh, TOF, and TOPh. By controlling the orientation of the donor rings with the overlap of the π-cloud and noncovalent interactions, the former four molecules exhibit p-RTP with the latter TOPh only exhibiting a ΦP of ∼11%. Single crystal analysis reveals that the enhancement of the phosphorescence lifetimes (26–245 ms) is caused by the moderate π–π interactions between the donor rings, which adopt J-aggregation with a face-to-face orientation. However, the short phosphorescence lifetime (12 ms) of TOPh was due to the absence of π–π interactions.
2.5 Donor–acceptor effect
Organic molecules with a twisted D-A structure can reduce the overlap between HOMO and LUMO through ICT transitions, generating a smaller ΔEST. This approach offers the advantages of facile preparation, high feasibility, strong phosphorescence emission, low toxicity, and wide application. Moreover, it does not require strict conditions such as deoxygenation or low temperatures, making it a feasible strategy for efficient RTP emission.14, 34, 82-87
In 2018, Tang, Lam et al.88 synthesized three MCBA isomers with D-A structures, where carbazole serves as the donor and benzoate as the acceptor (Figure 8a). By changing the position of the ester group, the torsion angle between D and A units can be adjusted to control the spatial separation of HOMO and LUMO. Moreover, due to the tight intermolecular interactions between D and A units, the non-radiative decay process is suppressed in the crystal state. Through this design, all isomers exhibited p-RTP with the meta-isomer m-MCBA demonstrating an ultralong lifetime of 795.0 ms with a ΦP of 2.1%. Small ΔEST greatly promotes ISC, while the pure ππ* configuration of T1 realized slow phosphorescence emission, resulting from the steric hindrance and conjugation effect of the ester group. This research opens a new avenue for developing efficient and persistent pure organic RTP materials on subtle control of the aggregation behaviors in crystals by molecular design.
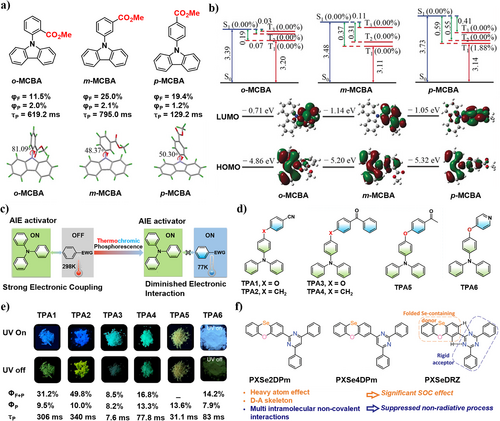
(a) Chemical structures of o-MCBA, m-MCBA, p-MCBA, and their RTP quantum yields, lifetimes, and molecular geometries in single crystals. (b) Calculated energy diagrams, n–π* configuration proportions of S1 and Tn, and HOMO and LUMO orbital distributions of o-MCBA, m-MCBA, and p-MCBA. Reproduced with permission.88 Copyright 2018, John Wiley and Sons. HOMO, highest occupied molecular orbital; LUMO, lowest unoccupied molecular orbital. (c) Designing strategy of TPA-based donor-sp3 linker-acceptor AIE active thermochromic dual phosphorescent molecules. EWG, electron-withdrawing group. (d) Molecular structures of TPA1-6. (e) The photographs of TPA1-6 before and after UV light irradiation together with their fluorescence, phosphorescence quantum yields, and lifetimes. Reproduced with permission.89 Copyright 2021, Springer Nature. (f) Molecular structures and design strategies of the compounds PXSe2DPm, PXSe4DPm, and PXSeDRZ. Reproduced with permission.90 Copyright 2023, John Wiley and Sons. RTP, room temperature phosphorescence; TPA, triphenylamine.
In 2021, Zhang, Sun et al.89 reported six triphenylamine (TPA)-based AIE-active RTP molecules TPA1-6 obtained through D-A strategy (Figure 8c,d). The aggregation induced emission activator TPA serves as an electron donor, which is connected to an acceptor via an sp3 linker (O or CH2). This twisted conformation resulted in an angle of ∼110–120°, preventing the tight π–π stacking and suppressing various molecular motions as aggregates. The as-prepared molecules yielded unexpected ternary emissions with prompt fluorescence and dual phosphorescence (Figure 8e). This ternary emission is due to the presence of a TPA-localized state coupling to a higher acceptor state at room temperature, but was cut off at lowered temperatures. Therefore, this work provides a universal principle for designing a dual-phosphorescence thermochromic material especially from single-component, dual-phosphorescence-based molecules.
In 2023, Su et al. synthesized three highly emissive RTP emitters PXSeDRZ, PXSe4DPm, and PXSe2DPm with high ΦP and short τ by connecting nonaromatic amine donor phenoxaselenine (PXSe) with different rigid electron-deficient groups as acceptors (Figure 8f).90 While the introduction of heavy atom, selenium, enhanced SOC, the D-A skeleton generated separated frontier molecular orbitals, facilitating orbital angular momentum change (ΔL) that can further boost the singlet-triplet SOC effect. As such, PXSeDRZ achieved a record-high external quantum efficiency (EQE) of 19.5%. In addition, voltage-dependent color-tunable emissions and single-molecule white emissions were achieved, which are highly potential for applications in lighting. The current work provides a successful molecular design concept for future RTP-OLEDs fabrication.
Very recently, Ray et al.91 reported organic ambient violet phosphorescent (AVP) materials based on D-A-D structures through connecting two catechol-based donors with the terephthalonitrile acceptor. Because of small ΔEST (0.036−0.046 eV) and conformational effects, the obtained molecules BPT1−BPT3 displayed AVP (∼390−394 nm, τAVP = 73−101 μs) with ΦP of 1.8%−27.4%. Theoretical calculations indicated that all molecules can generate both planar (F1) and twisted (F2) conformations (ground states). The F1 conformation is beneficial to the efficient AVPs, while the twisted conformation (F2) will generate TADF. This research provides new insights for the development of organic phosphorescent materials via the D-A strategy.
2.6 Host–guest doping systems
Host–guest interaction has recently attracted intensive attention for the design of RTP materials owing to their intrinsic advantages such as facile material preparation, low-cost, and molecular structure versatility.92, 93 The host molecules can provide rigid environment for the guest molecules, limiting the molecular vibration and suppressing the strong non-radiation transition caused by moisture, oxygen, and molecular motion.94 Additionally, the host can bridge excitons from the singlet of the guest emitter to its triplet, which minimizes the energy gap between the singlet and triplet energies, thus facilitating the ISC process to realize RTP emission by precisely manipulating the energy level.95-98
In 2021, Li et al.99 fabricated a series of guest molecules Pph, BPph, and DBPph as energy acceptors (Figure 9a). Upon mixing with the host OPph3 as an energy donor, RTP emissions could be achieved through co-crystallization or grinding, promoting energy transfer between them. Among these systems, the BPph-doped system exhibited the best RTP performance, mainly due to effective energy transfer from the host to the guest and strong ISC of the guest molecule BPph. In addition, introduction of pyridyl groups into the guest molecules endowed the doped system with reversible response toward acid-base and acid-heat stimuli, achieving multi-level stimulus-responsive RTP materials from grinding to chemical stimulation.
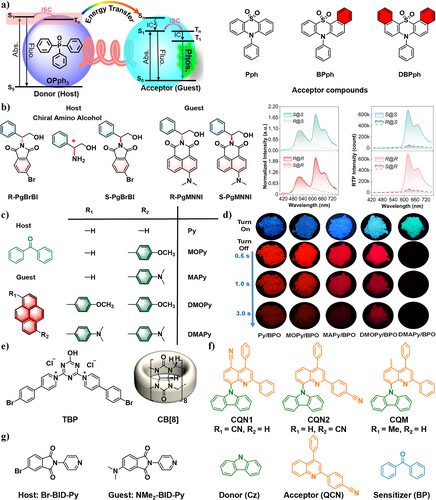
(a) The schematic illustration of energy transfer between donor (host) and acceptor (guest) and molecular structures of the donor (OPph3) and acceptors (Pph, BPph, and DBPph). Reproduced with permission.99 Copyright 2021, John Wiley and Sons. (b) Molecular structures of chiral amino alcohol derivatives: chemically modified chiral analyte guests (R-PgMNNI and S-PgMNNI), and chiral selector hosts (R-PgBrBI and S-PgBrBI) molecules. Normalized steady-state luminescence spectra of two chiral guests (w/w = 100 ppm) in two chiral host solids (e.g., R@R represents R-PgMNNI@R-PgBrBI, guest molecule in front of @) at 298 K (λex = 380 nm). DE (∆t = 0.1 ms) spectra of two guests (w/w 100 ppm) in two host solids at 298 K (λex = 424 nm). Reproduced with permission.100 Copyright 2023, Springer Nature. (c) Molecular structures of the guest and host molecules and (d) Fluorescence (top) and phosphorescence (down) images of guest–host materials. Reproduced with permission.101 Copyright 2022, Springer Nature. (e) Molecular structures of TBP and CB[8]. Reproduced with permission.105 Copyright 2020, Jon Wiley and Sons. (f) Molecular structures of the compounds CQN1, CQN2, CQM, donor (Cz), acceptor (QCN), and sensitizer (BP). Reproduced with permission.108 Copyright 2022, American Chemical Society. (g) Molecular structures of host (Br-BID-Py) and guest (NMe2-BID-Py). Reproduced with permission.109 Copyright 2022, Elsevier. DE, delayed emission; CB[8], cucurbit[8]uril.
By adopting a host–guest doping strategy, Ding et al.101 successfully prepared organic RTP materials with longer wavelengths and lifetimes in 2022. They selected highly conjugated pyrene derivatives as guests (Py, MOPy, MAPy, DMOPy, and DMAPy), whose high coupling ability could lower the T1 energy level. Dibenzoyl peroxide was chosen as the host matrix, which can assist the transfer of guest excitons and restrict the motion of guest molecules to suppress the non-radiative transition. Through this combination, the obtained materials emit strong, visually perceptible, long-wavelength phosphorescence (Figure 9c–d). By increasing the coupling degree of guest molecules, they successfully red-shifted the phosphorescence wavelength from 600/657 to 681/732 nm.
In addition to the development of novel guest emitters to modulate RTP emission, researchers also presented a series of chiral hosts to expand the library of host matrixes and generated chiral-selective RTP enhancement (CPE). In 2023, Zhang et al.100 constructed a host–guest doping RTP system using chiral amino alcohol components (Figure 9b). In this system, CPE was observed with a chiral-related energy transfer mechanism. Specifically, when the R-guest was doped into the R-host, strong red RTP afterglow was produced, while no significant RTP was observed for the S-R host–guest. This may be due to the presence of chiral-related host–guest energy transfer in the excited state, resulting in a remarkable CPE effect. This provides a new approach for a deeper understanding of molecular photophysics and exciton migration in chiral organic solids.
Another kind of host–guest system for RTP emission is frequently constructed from macrocyclic hosts such as cucurbiturils in aqueous phase.102-107 They can form stable inclusion complex structures with various cationic guests in water, effectively suppressing the non-radiative transition and oxygen quenching effect.
In 2020, Ma et al. employed cucurbit[8]uril (CB[8]) as the supramolecular host serving as a confined cavity to include a heavy-atom-modified triazine derivative TBP as the guest (Figure 9e).105 It revealed that a 2:2 quaternary complex structure was obtained with yellow RTP emission and a lifetime of 190 μs. The cavity of CB[8] serves as a confined space to restrict the molecular motion of TBP and protect the triplet exciton from being quenched in water. With the bromine to promote the ISC process, aqueous phase RTP was achieved via this host–guest system. Notably, this yellow RTP emission can be obtained through visible-light excitation, making it a promising candidate for cell imaging.
There is also a research focusing on the effect of host triplet on activating dark triplets of guest by TTET. In 2022, Ray et al.108 reported dual room temperature phosphorescence (DRTP) materials via multiple TTETs, which can generate triplet excitons and stabilize them. Due to the large ΔEST, neither of the two D-A configuration guests (CQN1 and CQN2) can emit TADF or RTP (Figure 9f). By the selection of benzophenone (BP) as a triplet sensitizer (host), a small ΔEST is generated, promoting an efficient ISC and leading to multiple TTET processes. It facilitates generation of efficient DRTP. By using a host-sensitized TTET strategy, the rate of phosphorescence emission can be increased from non-TADF guests.
In 2022, Ma et al.109 reported a host–guest doping system where the guest greatly enhanced the phosphorescence intensity of the host. Due to a small structural difference between Br-BID-Py (host) and NMe2-BID-Py (guest), the energy transfer between them is facilitated (Figure 9g). When doped into the host, the non-phosphorescent guest significantly enhanced the phosphorescence of the host by intermolecular energy transfer in the crystal state. In addition, both the host and the guest have exhibited a stimulus-induced afterglow shift due to the protonation of the pyridine rings, enabling stimulus-responsive applications. This represents one of the rare examples of the host–guest afterglow organic system where the guest enhances the intrinsic afterglow of the host.
2.7 Other strategies
In spite of the above-discussed strategies, other strategies are also developed to construct RTP materials with long lifetimes or high efficiencies, such as crystallization-induced phosphorescence, through space charge transfer (TSCT), etc.
For example, Tang et al.110 discovered “crystallization-induced phosphorescence” in pure organic luminophores such as BP and 4,4′-dibromobiphenyl in 2015 (Figure 10a). Three crystals including CZBP, BCZBP, and DBCZBP were designed using carbazole as the electron donor and BP as the electron acceptor in an electron D-A structure. Consequently, the highest energy C-H stretching vibration is constrained to inhibit non-radiative decay, leading to a decreased knrP and extended τp values. CZBP crystal exhibited p-RTP with an average τp value of 517.87 ms. It displayed white light emission originating from the dual emission of blue fluorescence and orange phosphorescence. Bromine-substituted derivatives BCZBP and DBCZBP also exhibited dual emission with a notable proportion of phosphorescence. Their τp values were shorter compared to CZBP, primarily due to fewer rigid conformations rather than heavy atom effects. Through the interconversion between crystalline and amorphous states, coupled with the presence and disappearance of triplet emission, all luminescent monomeric molecules demonstrated reversible and remarkable mechanochromism.
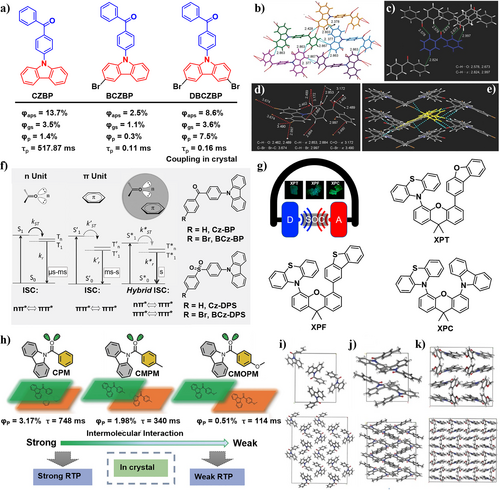
(a) Chemical structures of CZBP, BCZBP, DBCZBP, and their Φp, Φaps, and Φgs: efficiencies of the crystalline and amorphous ground solids. Single-crystal structures and molecular packings of (b) CZBP, (c) BP, and (d, e) DBCZBP with denoted intermolecular interactions. Reproduced with permission.110 Copyright 2015, John Wiley and Sons. (f) Left: Energy level diagram of the relevant photophysical processes for the RTP molecules with n–π* excited state configuration (i.e., containing an n unit), middle: π–π* excited state configuration (i.e., containing a π unit), right: coupled intermolecular n and π units in organic crystals, and the corresponding examples of rationally designed molecular structures for p-RTP. Reproduced with permission.55 Copyright 2016, Elsevier. (g) Molecular structures of XPT, XPF, and XPC via through space strategy. Reproduced with permission.48 Copyright 2021, American Chemical Society. (h) Molecular structures of CPM, CMPM, CMOPM, and the carton modes for their packing in crystal. Single-crystal structures and molecular packing of (i) CPM, (j) CMPM, and (k) CMOPM. Reproduced with permission.111 Copyright 2017, John Wiley and Sons. p-RTP, persistent room-temperature phosphorescence.
In 2016, Chi, Bryce et al.55 proposed that intermolecular electronic coupling within the crystal structure of different subunits plays a crucial role in generation of p-RTP. They emphasized the effect of the different excited-state configurations, namely n–π* and π–π* states. The crystals exhibited not only a high ISC rate originating from the n units but also a low radiative rate from the π unit, leading to a mixed ISC process (Figure 10f). Based on this assumption, two twisted organic molecules Cz-BP and BCz-BP were synthesized containing carbonyl or sulfonyl as the n unit and carbazole base (Cz) as the π unit. As expected, p-RTP was achieved with a significantly long lifetime of 0.28 s and a very high quantum efficiency of 5% under ambient conditions.
In 2017, Li et al.111 studied the impact of molecular stacking in crystalline state on RTP performance. They reported three amide compounds CPM, CMPM, and CMPOM (Figure 10h), which exhibit similar optical properties in dilute solutions but distinct RTP properties in crystallize states. CPM has the strongest emission efficiency and longest RTP lifetime (748 ms at 530 nm). However, the emission efficiencies and RTP lifetimes of CMPM and CMPOM crystals gradually decrease (340 ms at 540 nm and 114 ms at 545 nm). Single crystal analysis revealed that the RTP emission largely depends on the mode of the molecular packing. Obviously, compact face-to-face packing is favorable for long lifetimes and high efficiencies. CPM crystals are even more densely packed, with stronger coupling effects, accompanied by significant RTP phenomenon (Figure 10i–k). Another single crystal CPM-A, with no energy level difference compared with CPM through calculation possesses the same molecular arrangement as CPM, but exhibits looser packing (slightly larger intermolecular distance), resulting in significantly reduced lifetime and intensity (63 ms at 540 nm). This finding further confirms that compact arrangement is beneficial for intermolecular coupling and the stability of triplet excitons.
Purely organic RTP with very fast ISC rate from through-space systems has not been well investigated. In 2021, He, Li et al.48 reported three spatially confined bridged phosphors XPT, XPF, and XPC, where spatial π–π interactions mediated weak TSCT characteristics, achieving almost pure RTP in crystals by competition with fluorescence (Figure 10g). The bridged counterparts of these phosphors had locally excited characteristics of close-lying triplet states. Due to the inherent spatial overlap, degenerated energy levels, and tilted face-to-face arrangement of the 1LE and 3LE states of these bridged systems, the resulting effective through-space SOC leads to efficient ISC, exhibiting overwhelming ISC decay of the singlet-excited state.
In 2024, Fang et al. have innovatively fabricated charge transfer (CT)-based RTP materials from carbazone (Cz) substituted 4-phenyl-1,8-naphthalimide (NMI) derivatives (Figure 11a).112 The NMI compounds display significant intramolecular through bond charge transfer (TBCT) and TSCT properties. While the TBCT process produced fluorescence emission, the TSCT produced charge separation species (NMI•− and Cz•+) first and then transformed into long-lived triplet hybrid local CT state (3HLCT). At room temperature, only blue fluorescence emission can still be seen for NMI derivatives with no phosphorescence. However, upon immersion of the doped poly(methyl methacrylate) (PMMA) films in ammonia, RTP emission with a color change from orange to green was observed. These stimuli-responsive RTP materials may find application in time-resolved luminescence ammonia sensors.
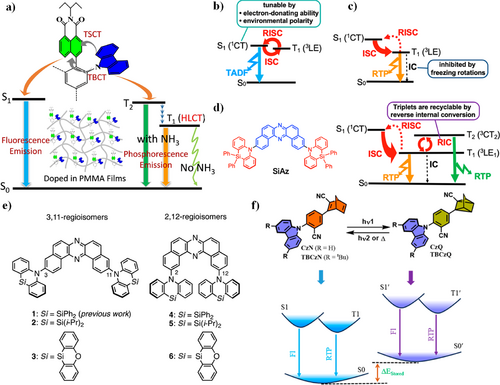
(a) Schematic figure of the photophysical processes of the NMI derivatives doped in PMMA film before and after treatment by ammonia. Reproduced with permission.112 Copyright 2024, American Chemical Society. (b) The Jablonski diagram for typical TADF emitters. (c) The Jablonski diagram for previously studied RTP emitters. (d) The molecular structures and Jablonski diagram of the emitter SiAz. Reproduced with permission.113 Copyright 2024, American Chemical Society. (e) The molecular structures of the emitters. Reproduced with permission. Copyright 2024, Royal Society of Chemistry.114 (f) Molecular structures and switching process of CzN, TBCzN, and energy diagram for the PTRL. Reproduced with permission.115 Copyright 2024, American Chemical Society. HLCT, hybrid local charge transfer; PMMA, poly(methyl methacrylate); PTRL, photothermally reversible luminescence; RTP, room temperature phosphorescence; TADF, thermally activated delayed fluorescence; TBCT, through bond charge transfer; TSCT, through space charge transfer.
Another strategy to boost RTP is achieved by arranging triplet excited states energies to boost reverse IC. As TADF and RTP are connected through a triplet excited state (from T1 to S1 followed by S1 to S0, and from T1 to S0 transition), the switching between TADF and RTP could be achieved through manipulation of triplet-excited state.
In 2021, Data and Takeda et al.113 designed a heavy-atom-free RTP material SiAz by the introduction of less-electron-donating, rigid, and sterically hindered donors into the acceptor (Figure 11b–d). They can suppress the rotation and vibration around the D−A units and activate the RTP emission. By changing the hosts, TADF, RTP, or both emission channels were boosted. The D–A–D compound showed unexpected dual RTP from T1 (3LE1) and T2 (3CT2) in a host matrix, significantly accelerated through a thermally activated reverse internal conversion (TArIC). Based on the excellent performances, they developed an efficient and heavy-atom-free RTP-based OLED devices (4.06%).
In 2022, the same group114 developed another heavy-atom-free organic RTP material based on a series of donor-acceptor-donor (D-A-D) compounds by systematic studies on the regioisomeric and substituent effects on the excited states (Figure 11e). The obtained compounds show dual RTP from T1 (3LE1) and T2 (3CT2) in a host matrix, accelerated through a TArIC process. By varying the hosts, they exhibited distinct differences in TADF, dual TADF and RTP, and dual RTP. Furthermore, the developed RTP-based OLEDs achieved a high EQE of up to 7.4%.
The RTP performance can also be modulated by dynamic breaking and making of C-C bonds through photoswitching. In 2024, Ray et al.115 designed and synthesized norbornadiene (NBD)-functionalized donor–acceptor conjugates (CzN and TBCzN) (Figure 11f). The NBD can photoisomerize to a meta-stable structure quadricyclane by switching fluorescence (blue−violet) and RTP (blue−violet) emission. The RTP switching was achieved upon photoirradiation and heating of the samples in the condensed state by modulating the ΔEST due to the C=C breaking and C–C forming. This smart RTP switch (RTPS) provides new instructions for the development of efficient RTPS materials for multidisciplinary applications.
3 APPLICATIONS OF RTP MATERIALS
In recent years, numerous endeavors have been dedicated to the development of RTP materials with excellent properties. These RTP materials have exhibited advantages such as ultralong lifetimes, extremely large Stokes shift, white light or NIR emission, enabling their applications across a range of fields such as OLEDs,116-118 information storage and encryption,5, 20, 119 sensing, and biological imaging.10, 120-122 Some examples of the applications of RTP materials are illustrated in this section.
3.1 Information storage and encryption
Given the prevalence of misinformation in daily life, information security in modern society is still in urgent demand. Owing to longer lifetime, large stokes shift and low signal-to-noise ratio as well as bio-compatibility, which are superior to fluorescent materials, organic RTP materials have demonstrated significant potential for applications in information storage and encryption.123-126
In 2017, Li et al.53 prepared three different borane compounds PBA-MEO, PBA-Br, and PBA-Cl with similar RTP properties under 254 nm light illumination (Figure 12a). However, in the dehydration process, the PL behavior further changed by combining with boron elements, making it suitable for information storage. As shown in Figure 12b, after loading lower amounts of the three compounds, the pattern word “CHEMISTRY” was written on a printing paper, showing almost the same blue fluorescence under the excitation of 254 nm light. After turning off the UV lamp, a bright “CHEM” and a weak “IS” could be observed. Nevertheless, after heating the paper at 80°C for 2 h, the letters of “CHEM” disappeared, “IS” brightened, and “TRY” appeared. With these easily prepared borane compounds, the information encryption was successfully realized, giving them great potential for practical use.
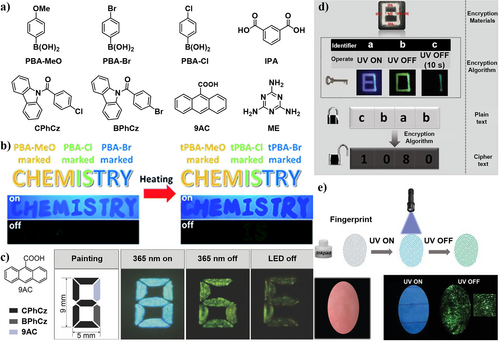
(a) Chemical structures of top left: PBA-MeO, PBA-Br, PBA-Cl, bottom left: CPhCz, BPhCz, and 9AC. right: ME and IPA. (b) Demonstration of encoding for security documents with PBA-MeO, PBA-Cl, and PBA-Br before and after heating. Reproduced with permission.53 Copyright 2017, Royal Society of Chemistry. (c) Dual-encryption application using CPhCz, BPhCz, and 9AC. Reproduced with permission.22 Copyright 2017, John Wiley and Sons. (d) The schematic process of information encryption to decryption. (e) The schematic illustration of the back-free fingerprint identification and luminescent images of fingerprint with special inkpad upon UV on and off. Reproduced with permission.20 Copyright 2018, John Wiley and Sons.
In the same year, using two carbazole derivatives CPhCz, BPhCz, and 9-anthracenecarboxilic acid (9AC), Huang et al.22 prepared a simple dual encryption model under visible light excitation (Figure 12a,c). A pattern “8” was formed by using the powders of the three compounds CPhCz, BPhCz, and 9AC, which displayed a blue number “8” upon excitation at 365 nm. After ceasing the illumination, both CPhCz and BPhCz emitted yellow-green ultralong phosphorescence emission, resulting in the occurrence of a yellow-green number “6,” while 9AC did not emit phosphorescence. Remarkably, a clear letter “E” pattern emerged after excitation by LED light. Through this simple pattern with the combination of three compounds, a dual decryption was intelligently realized from number to letter (Figure 12c).
More efforts were devoted to developing high-level encryption by Yan et al.20 in 2018 through designing an encryption algorithm with three operating modes. They used IPA-ME and its components ME and IPA as the encryption materials, and combined them with the three encryption algorithm “UV-ON,” “UV-OFF (instantly)”, and “UV-OFF (for 10 s),” which represented “a,” “b,” and “c,” respectively (Figure 12a,d). The plain text string “cbab” was translated to meaningful message “1080” by using this encryption algorithm. This information remained clear and stable upon changing the temperature from 220 to 340 K and in humid environments, offering great value in data logic encryption. Moreover, the RTP materials were used to accurately identify personal identity based on fingerprints (Figure 12e). After printing on a file paper, the detailed features of fingerprints could be easily identified under UV light irradiation. Additionally, two different printed samples could be compared in the “UV ON” and “UV OFF” modes. Particularly, the luminescence of the fingerprint could only be detected after excitation, ensuring the accuracy for identifying the personal identity.
In 2019, Huang et al. made a series of triazine derivatives TMOT, DCLCzT containing multiple heteroatoms using simple silk-screen printing technology. This method not only improves the rate of ISC to boost triplet excitons but also constructs multiple intermolecular interactions to restrict the molecular motion in solid states.54 The prepared phosphors were applied in multi-color display and specific wavelength visual detection in the UV region, facilitated by the color-adjustable ultralong organic RTP. Different patterns were created by grinding TMOT and DCLCzT as fixed powders. As the excitation wavelength increased from 254 to 365 nm, the phosphorescent image of TMOT changed from sky blue to green, while that of DClCzT consistently presented as yellow (Figure 13b). By gradually increasing the UV light excitation wavelength in every 10 nm from 300 to 360 nm, distinct color changes in the phosphorescent image of the TMOT could be observed with the naked eye, potentially aiding in UV light detection (Figure 13a).
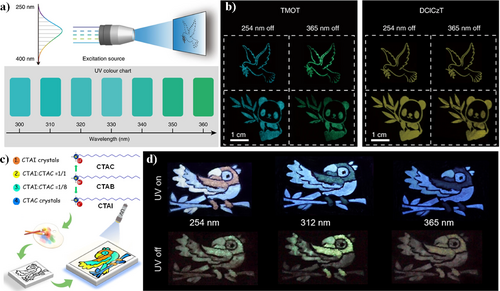
(a) Schematic of the experimental set-up for multicolor display by varying the excitation wavelength from 250 to 400 nm. UV color chart showing the ability of using TMOT crystalline powder to visually detect specific wavelengths in the UV region. (b) The photographs of peace dove and panda patterns, recorded with the TMOT (left) and DClCzT (right) as the ink, respectively. The phosphorescence images were taken after turning off a 254 or 365 nm hand-held UV lamp. Reproduced with permission.54 Copyright 2019, Springer Nature. Schematic illustration (c) and demonstration (d) of the anticounterfeiting application based on color-tunable PL and p-RTP of different ground powders. Reproduced with permission.21 Copyright 2022, John Wiley and Sons. PL, photoluminescence; p-RTP, persistent room temperature phosphorescence.
Advancements have been further achieved in 2022 by Yuan and coworkers21 utilizing quaternary ammonium salts (QAS) to generate CTAC and CTAI with blue and orange emissions, respectively (Figure 13c). By altering the excitation wavelength, alkyl chain length, counter ions, and mechanical stimulation, QAS solids have realized advanced multi-mode anti-counterfeiting applications. As depicted in Figure 13d,a bird pattern was drawn by grinding and mixing CTAI and CTAC solids in different proportions. Under 254 nm UV light, the emission was clearly visible which is composed of CTAI (pink) and CTAC solids (blue) and their 1:1 (white) and 1:8 (blue-white) mixtures. After changing the light source to 312 and 365 nm UV light, the bird pattern appeared in blue-green and blue, respectively. Moreover, green or yellow patterns could be obtained after removing the 254, 312, and 365 nm light sources. The multimode anti-counterfeiting was achieved by tuning the PLs of QAS solids via physical blending. This work affords inspiration for the design of novel nonconventional luminophores.
3.2 OLEDs
The engagement of triplet state and direct radiative transition from the triplet state provide the potential of RTP materials as a new generation emitter in OLEDs.127-130 The main challenge lies in enhancing the ΦP of the RTP molecules when utilized in OLEDs, which determines the sustainability of the devices. Great efforts have been devoted to investigating RTP-based OLEDs in past decades. However, examples of high efficiencies and short-lived RTP-based OLEDs are rare.
Early in 2017, Adachi et al.12 reported the development of a low-power excitation RTP with long persistent luminescence based on a host–guest doping system. As shown in Figure 14a, the selected guest TMB has strong electron-donating group with a very stable radical cation. The selected host PPT is a strong electron-accepting molecule with a high triplet energy, which can provide rigid environment to suppress the non-radiative deactivation. With the aid from the charge recombination of long-lived intermediate charge-separated states, the generated emission can last for more than 1 h. Upon excitation with a standard white LED light, a long emission was generated even at >100oC. This superior system has many advantages such as being transparent, soluble, potentially flexible, and color-tunable. This discovery paves the way for the utilization of such kind of RTP materials in large-area and flexible paints, biomarkers, fabrics, and windows.
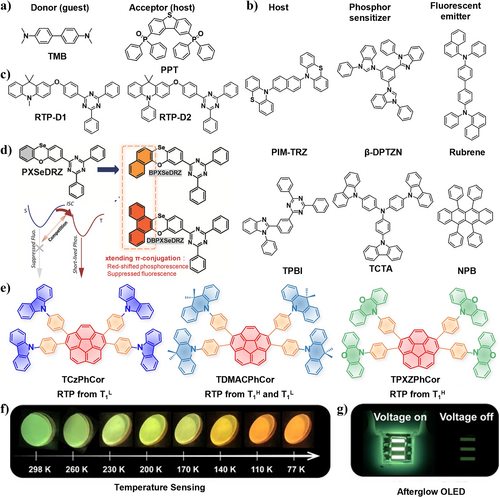
(a) Molecular structures of donor (guest) TMB and acceptor (host) PPT.12 (b) PIM-TRZ, β-DPTZN, Rubrene, TPBI, TCTA, and NPB that are used in OLEDs.56 (c) Molecular structures of the two compounds RTP-D1 and RTP-D2. Reproduced with permission.34 (d) Molecular structures and scheme of the exciton dynamic processes of the compounds BPXSeDRZ and DBPXSeDRZ. Reproduced with permission.90 Copyright 2023, John Wiley and Sons. (e) Chemical structures of multi-D-A corannulenes TCzPhCor, TDMACPhCor, and TPXZPhCor. (f) Temperature-dependent phosphorescence afterglows of 30 wt% TPXZPhCor-doped films (g) Photos of the device showing steady-state and afterglow luminescence under electrical excitation. Reproduced with permission.52 Copyright 2023, John Wiley and Sons. OLEDs, organic light-emitting diodes.
In 2021, Wang, Li, and coworkers fabricated a series of electroluminescence (EL) films using purely organic phosphor-sensitized fluorescence emitter.56 By selecting benzimidazole-triazine (PIM-TRZ), 2,6-di(phenothiazinyl)naphthalene (β-DPTZN), and 5,6,11,12-tetraphenylnaphthacene (Rubrene) as host, phosphor sensitizer, and fluorescent emitter, respectively, they established a unique approach to construct highly efficient OLEDs (Figure 14b). This combination design resulted in a maximum EQE of 15.7%, owing to the PIM-TRZ host with RTP feature, which is crucial for the highly efficient Förster energy transfer from phosphor to fluorescent molecules. The utilization of this low-cost RTP materials effectively harvests singlet and triplet excitons and avoids low PL quantum yields, providing a new avenue for the construction of high-performance OLEDs based on classic fluorescence emitters.
In 2023, Ding et al.34 developed two organic phosphors, RTP-D1 and RTP-D2 using D-A structure that integrates acridine as an electron donor and triazine as an electron acceptor linked by an oxygen (Figure 14c). The D-O-A type structure enabled efficient ISC from singlet excitons to triplet excitons, generating organic room-temperature electrophosphorescence with a record-high EQE of 15.8% (45.8 cd/A, 50.4 lm/W) and CIE coordinates of (0.16, 0.55) for non-doped OLEDs. It was the highest ever reported for organic RTP emitters, marking an important advancement in EL application rather than PL. The increased flexibility of the C-O single bond in small molecules than in polymers resulted in unique aggregation-induced electrophosphorescence. In contrast to the doped polymers, the small molecule RTP-D2 avoided the difficulty in choosing the host and controlling the doping concentration, demonstrating the superiority of the D-O-A geometric structure in achieving efficient aggregation-induced organic room-temperature electrophosphorescence. RTP-D2 was also developed as host-free sensitizers for multiple resonance emitter with an efficient narrowband emission (EQE = 26.4% and FWHM = 26 nm, FWHM, full width at half maxima).
As mentioned in Section 2.5, Su et al.90 designed three highly emissive organic phosphorescence emitters PXSeDRZ, PXSe4DPm, and PXSe2DPm through connecting nonaromatic amine donor (PXSe) with different electron-deficient fragments in a D-A skeleton (Figure 8f). The developed emitters exhibit high Φ and short τ in amorphous films because of the enhanced SOC by the heavy-atom effect of selenium, and the D-A skeleton with suppression of the non-radiative energy dissipation by the rigid molecular structure. The EQE is up to 19.5%, a record-breaking value for the RTP OLED based on PXSeDRZ. These remarkable results illuminate the extensive potential of purely organic RTP materials as highly efficient OLED emitters, especially for lighting applications.
Despite the fact that corannulene derivatives show extensive applications in energy storage and solar cells, they are rarely exploited in OLEDs due to their low exciton utilization. In 2023, Zysman-Colman et al.52 reported for the first time that corannulene-based luminophores exhibit room-temperature multiple phosphorescence by introducing multiple different units such as carbazole (Cz), 9,10-dihydro-9,10-dimethylacridine (DMAC), and phenoxazine as donors to regulate its phosphorescence (Figure 14e). They have demonstrated that these RTP materials can be used in an afterglow OLED and achieved optical temperature sensing stemming from a change in the ratio of multiple phosphorescence yields upon temperature variations, particularly within the range of 77–298 K (Figure 14f). The afterglow OLED with TPXZPhCor shows an EQEmax and a luminance (Lmax) of 3.3 % and 5167 cd m−2 (Figure 14g), respectively, making it one of the most efficient afterglow RTP OLEDs reported to date.
Recently Su et al. further developed two orange RTP emitters BPXSeDRZ and DBPXSeDRZ by extending π-conjugation of the nonaromatic amine donor PXSe (Figure 14d).90 This strategy effectively suppressed the fluorescence radiative transitions by symmetry excited state orbitals in the introduced π–π* transitions. Both of the two emitters exhibited efficient orange-red pure phosphorescence with high ΦP of 66.3% and 66.9% in doped films, thanks to the sufficient ISC transitions. The as-prepared RTP-OLEDs achieved high EQEs of 17.2% and 17.9% based on BPXSeDRZ and DBPXSeDRZ as emitter for the first time. Moreover, stable pure electro-phosphorescence spectra were also obtained in a wide driving voltage. As a result, they also fabricated metal-free phosphor-sensitized fluorescence OLED devices using DBPXSeDRZ as a phosphor sensitizer and tetraphenyldibenzoperiflanthene (DBP) as the conventional fluorescence terminal emitter. The resulted phosphor-sensitized red fluorescence OLED achieved a high EQE of 10.1% and a CIE coordinate of (0.61, 0.38), indicating the efficient triplet excitons utilization in sensitized OLED.
3.3 Bio-imaging and photodynamic therapy
RTP materials have also been widely applied in bio-imaging due to their longer lifetimes and large Stokes shifts that avoid interference from autofluorescence and excitation light, low cytotoxicity, higher signal-to-noise ratio, and superior tissue penetration within cells, especially those emit red or NIR emissions.94, 101, 131-133 Considering the bad solubility of organic RTP molecules in water, the current biomedical application methods mainly adopt nanoscale packaging, meter encapsulation or top-down nanoparticle (NP) methods to prepare crystalline NPs to maintain p-RTP emissions.134
In 2019, Zhang, Yuan et al.57 encapsulated RTP molecules into NPs using lipid-PEG2000 as the encapsulation matrix and a nanoprecipitation strategy and then injected the NPs into the back of a mouse. Without any interference from the mouse's background tissue fluorescence, clear and unique RTP luminescent signals from the two NPs could be distinctly observed (Figure 15a). This luminescence method eliminates the influence of tissue autofluorescence, highlighting the unique advantage of using RTP luminophores for afterglow imaging.
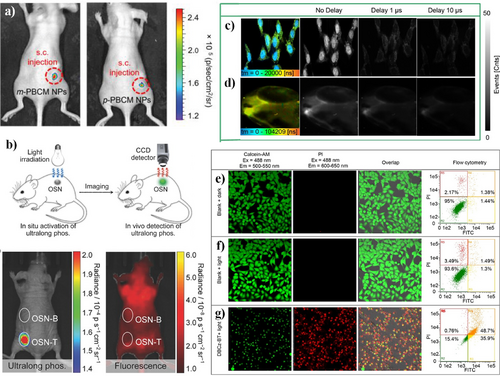
(a) In vivo afterglow luminescent images of mice injected with m-PBCM NPs and p-PBCM NPs. Reproduced with permission.57 Copyright 2019, John Wiley and Sons. (b) Schematic illustration of in situ activation and detection of ultralong phosphorescence of OSNs in living mice. Ultralong phosphorescence and fluorescence imaging of a mouse with the subcutaneous inclusions of OSNs (1.6 × 10−6 m). The circles indicate the location of nanoparticle inclusions. Reproduced with permission.11 Copyright 2017, John Wiley and Sons. Phosphorescence lifetimes imaging in vitro and vivo. (c) Images of living HeLa cells with different delay time. (d) Images of a zebrafish with different delay time. Note that HeLa cells were incubated with PNDs (75 μg/mL) and Hoechst 33342 (a cell nucleus dye, 1.0 μM) at 37°C for 3 h. The excitation wavelength was 405 nm. PNDs (150 μg/mL) were injected into the brain of the zebrafish. Photodynamic therapy application of phosphorescence nanodots. Confocal microscopy images of calcein-AM- and PI-labeled HeLa cells, and the flow cytometry quantification of annexin V-FITC- and PI-labeled HeLa cells (e) in dark control, (f) light control, and (g) incubated with PNDs (75 μg/mL) under irradiation at 475 nm for 20 min. Cells were viewed in the green channel for calcein-AM (λex = 488 nm, λem = 500–550 nm) and red channel for PI (λex = 488 nm, λem = 600–650 nm), respectively. Reproduced with permission.121 Copyright 2019, John Wiley and Sons. OSNs, organic semiconductor nanoparticle; PNDs, phosphorescent nanodots.
In 2017, Huang, Pu et al.11 reported an organic semiconductor nanoparticle (OSN) with ultra-long phosphorescence. By adopting a top-down approach to form NP aggregates, the triplet-excited state of phosphorescent molecules was greatly stabilized, forming ultra-long-lived phosphorescent excitons at room temperature. The luminescence can extend to a timescale detectable by biological imaging after the removal of the external light source. In vivo mouse experiments were conducted by injection of OSN into the back of a mouse, and irradiated with UV light for 1 min (Figure 15b), The images were captured using the in vivo imaging systems live imaging system after removing the light source at t = 10 s. It is clear that the OSN1-T, prepared using the top-down method, produced ultralong phosphorescence, while the ultralong phosphorescence of OSN1-B, prepared using the bottom-up method, was hard to detect. However, due to the interference of the mouse's own fluorescence, fluorescence imaging could not detect these two types of NPs in live mice.
As mentioned above in Section 2.3, RTP materials, especially those emit red or NIR emission, hold great potential for the applications in bio-imaging.135-139 Bolton et al. made one of the pioneering endeavors to develop red-emissive RTP materials using planar naphthalene derivatives. Some other red RTP materials have also been developed by incorporation of boron fluoride, carbazole, and naphthalene diimides. In 2017, Liu, Chi, Yang et al. reported a bright pure organic compound with red p-RTP by introducing an alkoxy spacer between the carbazole and the 4-bromobenzophenone units.19 The red emissive compound C-C4-Br is obtained with a higher level of conformational flexibility. Owing to the excellent biocompatibility and retention of bright phosphorescence in aqueous media, its nanocrystals were applied to the imaging of breast cancer cells, demonstrating effective uptake and bright phosphorescence emission. This research paves the new way for the exploitation of purely organic p-RTP compound in the biological field.
In 2019, Huang et al.121 designed a material with a long emission lifetime (504.6 μs) and high ΦP (14.6%). By encapsulating it with a polymer, well-dispersed phosphorescent nanodots (PNDs) were prepared. The external and internal background fluorescence interference can be eliminated due to its long lifetime, allowing for the application in time-resolved luminescence imaging and photodynamic cancer therapy. As shown in Figure 15c,d, the PLIM was performed in live HeLa cells and zebrafish. From the initial no-delay image, signals from the entire cell can be observed. After a delay of 1 μs, the intense fluorescence signal from the cell nucleus completely disappeared, leaving only the long-lived phosphorescence signal from the PNDs in the cytoplasm. Even after 10 μs, the phosphorescence signal can still be seen, easily identified from the transient background fluorescence. To further verify the photodynamic therapy capability of the PNDs, confocal imaging was performed on HeLa cells labeled with calcein-AM (a green fluorescent live cell tracker) and propidium iodide (a red fluorescent dead cell dye) (Figure 15e–g). After treating HeLa cells with a photosensitizer for 3 h, a large number of apoptotic cells and dead cells floated in the culture medium, and propidium iodide staining showed red fluorescence. After photodynamic treatment, oxidation caused cancer cell apoptosis, with a mortality rate of over 80%.
4 SUMMARY AND OUTLOOK
In summary, this review presents the latest research progress of RTP materials based on small organic molecules. According to the referred literature, the common strategies are outlined for the design of small organic molecule-based RTP materials. These strategies can provide a rigid environment, effectively suppressing the non-radiative transition such as hydrogen or halogen bonding, π–π stackings, host–guest doping95-98, 100, 140 and crystallization.141-143 They can effectively enhance the ISC process and accelerate the radiative transition of triplet excitons, for instance, by the employment of heavy atom effect.6, 144-147 Other strategies include the synergistic suppression of non-radiative transition and promotion of ISC, such as n–π* transitions,148 and D-A systems.14, 34, 83, 84 Despite the common methods, new strategies such as TSCT and coupling of photoswitches with RTP units are being employed. By virtue of these strategies, high phosphorescence efficiencies, long lifetimes, as well as tunable colors of organic RTP materials have been intensively achieved.37, 111-115, 119, 149-152 Moreover, specific examples are elucidated to illuminate the underlying mechanism of these strategies. The promising applications of these organic RTP are discussed in information storage and encryption, OLEDs, as well as bio-imaging and photodynamic therapy.
Despite considerable efforts in the design strategies and application of RTP materials, there remain some issues and challenges that need to be addressed: Firstly, compared with inorganic materials and organic/inorganic hybrid materials, most organic RTP molecules exhibit lower phosphorescence emission efficiencies and shorter lifetimes.143, 153, 154 Therefore, it poses an enormous challenge to develop high-efficiency pure organic RTP materials based on small organic molecules and expand their library. Secondly, it requires to balance the phosphorescence lifetime and efficiency of organic RTP materials in order to broaden their applications.29 Rational design and fine-tuning of molecular structures are still in urgent need. Thirdly, most RTP materials are monochromatic luminescent materials, effective excitation light sources are mostly in the ultraviolet band, and the luminescence color range is relatively narrow.29, 155 It is still a significant challenge to expand the wavelengths range and design color-tunable RTP materials. Specifically, on one hand, the development of visible-light -induced RTP molecules is one direction, which will not only avoid the harm, but is also energy saving. On the other hand, the exploitation of red or NIR RTP molecules with minimal phototoxicity is crucial for the bio-medical application.107 The design of future RTP materials should ensure persistent emissions with various additional functionalities. An interesting and promising direction would be the development of stimuli-responsive RTP materials.156-158 This would enable promising applications in high-level information encryption and sensing devices.
AUTHOR CONTRIBUTIONS
Meixia He: Writing – original draft (equal); writing – review and editing (lead). Cong Ding: Writing – original draft (equal); writing – review and editing (equal). Hexiang Guo: Writing – original draft (supporting); writing – review and editing (supporting). Quan Li: Conceptualization (lead); resources (lead); supervision (lead); writing – original draft (supporting); writing – review and editing (equal).
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support from the Jiangsu Innovation Team Program, National Natural Science Foundation of China (No. 22302036), the Fundamental Research Funds for the Central Universities (2242023K5007), and Start-up Research Fund of Southeast University (RF1028623130).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Biographies

Meixia He is an Associate Professor at the Institute of Advanced Materials and School of Chemistry and Chemical Engineering, Southeast University. She obtained her BS and MS from Shaanxi Normal University (Xi'an, China) under the supervision of Prof. Yu Fang working on fluorescent film sensor. She carried out her PhD and postdoc at Institut de Science et d'Ingénierie Supramoléculaires (I.S.I.S.), University of Strasbourg, France under the supervision of Prof. Jean-Marie Lehn working on dynamic covalent libraries and networks. Her current research interests are stimuli-responsive dynamic functional systems and materials.

Cong Ding received her BS in Chemical Engineering and Technology at School of Chemistry and Chemical Engineering & Materials Science, Shandong Normal University. Currently, she is a graduate student at Institute of Advanced Materials and School of Chemistry and Chemical Engineering, Southeast University. Her current research interests focus on stimuli-responsive organic luminescent materials.

Hexiang Guo received his BS in Chemical Engineering and Technology at School of College of Chemistry and Materials Science, Shanghai Normal University. Currently, he is a graduate student at Institute of Advanced Materials and School of Chemistry and Chemical Engineering, Southeast University. His current research interests focus on stimuli-responsive organic luminescent materials.

Quan Li is Distinguished Chair Professor and Director of Institute of Advanced Materials at Southeast University. He held appointments in USA, Germany, and France. Li received his Ph.D. in Organic Chemistry from Chinese Academy of Sciences in Shanghai, where he was promoted to a youngest Full Professor in February 1998. He is a Fellow of the Royal Society of Chemistry. He has been elected as a member of the European Academy of Sciences and the European Academy of Sciences and Arts. He is also honored as Professor and Chair professor at several universities. His current research interest spans from stimuli-responsive smart soft matter, advanced photonics, and optoelectronic materials for energy harvesting and energy saving to functional biocompatible materials, biomedical materials and nanoparticles to nanoengineering and device fabrication.