Acute lung injury after balloon pulmonary angioplasty results in a similar haemodynamic response and possible clinical advantage at follow-up
Abstract
Acute lung injury (ALI) is a common but poorly defined and understood complication of balloon pulmonary angioplasty (BPA) for chronic thromboembolic pulmonary hypertension (CTEPH). Little data are available on the medium term clinical outcomes of BPA complicated by ALI. We analyzed per-procedure data from 282 procedures in 109 patients and per-patient data from 85 patients. Serial right heart catheterization at baseline, after each BPA and at 3-month follow-up measured pulmonary vascular resistance (PVR), mean pulmonary artery pressure (mPAP), and cardiac output (CO). ALI (ALI+) was identified by chest radiography alone (ALIr+) or in association with hypoxia clinically (ALIcr+). Procedural predictors of ALI and patient outcomes at 3-months were compared no ALI (ALI−). ALI+ occurred in 17/282 (6.0%) procedures (ALIcr+: 2.5%, ALIr+: 3.5%). Prevailing haemodynamics (PVR: p < 0.01; mPAP: p < 0.05) at a procedural and patient level, as well as number of BPA sessions (p < 0.01), total number of vessels (p < 0.05), and occlusions (p < 0.05) treated at a patient level predicted ALI+. Those with ALI had greater percentage improvement in ΔCAMPHOR symptoms score (ALI+: −63.5 ± 35.7% (p < 0.05); ALIcr+: −84.4 ± 14.5% (p < 0.01); ALI−: −27.2 ± 74.2%) and ΔNT-proBNP (ALIcr+: −78.4 ± 11.9% (p < 0.01); ALI−: −42.9 ± 36.0%) at follow-up. There was no net significant difference in haemodynamic changes in ALI+ versus ALI− at follow-up. ALI is predicted by haemodynamic severity, number of vessels treated, number of BPA sessions, and treating occlusive disease. ALI in this cohort was associated with a clinical advantage at follow-up.
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is a sequel of acute pulmonary embolism caused by non-resolution of thrombi in the pulmonary circulation.1 Both proximal mechanical obstruction and distal vasculopathy contribute to the pathophysiology of CTEPH.2 Obstructions can be classified morphologically as total or subtotal occlusions, stenoses, webs and tortuous lesions, or by an abnormal pulmonary flow grade score on angiography.1, 3, 4 Occlusions, or pulmonary flow grade score 0, represent the most severe obstructive disease.
Pulmonary endarterectomy (PEA) is the treatment of choice for proximal obstructive disease in CTEPH and is potentially curative with excellent long-term outcomes.5-7 However, one-third of CTEPH patients are not suitable for surgery.8, 9 The distal vasculopathy of CTEPH can be treated with medical therapy,10 but balloon pulmonary angioplasty (BPA) has emerged as a safe and effective alternative for patients with inoperable CTEPH due to inaccessible obstructions or unfavorable risk-benefit ratio for PEA and recurrent or residual pulmonary hypertension (PH) post-PEA.11
Acute lung injury (ALI) may occur after BPA and although there is no consensus on the precise definition, lung hemorrhage due to wire and/or balloon trauma and lung reperfusion injury (LRI) have been implicated. ALI is the most frequently observed complication seen after BPA and LRI is often the most significant complication.12 LRI manifests clinically as oxygen desaturation and type 1 respiratory failure, usually 24−72 h after BPA but sometimes up to a week later, with radiologically evident wedge opacification in the same distribution of lung treated on chest radiography or computer tomography. In 2001, Feinstein et al.13 reported a series of 18 patients undergoing BPA for CTEPH, of which 11 suffered LRI (23% of procedures) and 3 required mechanical ventilation.
Procedural complications initially limited the adoption of BPA as a treatment option for CTEPH. The evolution of recent practice has greatly reduced the frequency and severity of ALI through identification of predictors of ALI and changes to technique to mitigate the risk.4, 11, 14 Though it remains an important complication, it is unknown if ALI impacts late patient outcome measures of BPA. We report the frequency and predictive factors for ALI in a contemporary cohort of patients undergoing BPA and describe the impact on haemodynamic and clinical outcomes measured at 3-months by comparing clinical response in patients with and without ALI.
METHODS
Consecutive patients with inoperable CTEPH or residual PH post-PEA surgery on stable medical therapy for PH who underwent BPA at the UK National BPA Center, Royal Papworth Hospital, between October 2015 and January 2021 were included in this observational cohort study. None had supplemental oxygen requirement at baseline. Data were collected prospectively and analyzed retrospectively.
Assessment
Patients were maintained on stable PH-targeted medical therapy for a minimum of 3 months before their first procedure, throughout the perioperative period and at 3-month follow-up (FU). Patients underwent serial right heart catheter at baseline, before each procedure and at 3-month FU. Mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR), cardiac output (CO, via thermodilution), and right atrial pressure (RAP) were assessed. NT-proBNP, 6 min walk distance (6MWD), World Health Organization functional class (WHO FC), and Cambridge pulmonary hypertension outcome review (CAMPHOR), an internationally validated patient-reported outcome measure designed in a cohort including CTEPH patients that assesses three domains: activity, quality of life, and symptoms,15 were also assessed at baseline and 3-month FU.
Procedure
The BPA procedural details at our institution have previously been described.16 BPA was performed via the right femoral vein using a 6 French sheath under local anesthesia by the same experienced team of two interventional cardiologists. Unfractionated heparin (70−100 IU/kg) was administered. The type, location, and burden of disease treated was at operator discretion but in a single session, multiple lesions were targeted but only a single lung was treated. Cessation of BPA treatment course was also at operator discretion, often determined when all lesions had been treated or when the risk: benefit ratio of continuing was considered adverse.
Identification and definition of ALI
All patients had oxygen saturations monitored every 2−4 h by pulse oximetry until discharge on Days 2 or 3 and had a departmental, plain, semierect, anterior−posterior (AP) chest radiograph before and within 24 h of completing a BPA procedure. Repeat chest radiography was arranged if oxygen desaturation persisted beyond 24 h. The chest radiograph was reported by a consultant radiologist blinded to the BPA procedural details.
ALI was defined clinically as a fall in oxygen desaturation >4% from baseline and/or radiologically (new wedge opacification in the territory treated on postprocedure chest radiograph). ALI− was defined as procedures with no features of ALI during the patient's in-patient stay and ALI+ had one or both features of ALI. ALI+ was further subcategorized into those with clinical and radiological features of ALI (ALIcr+) and those with radiological features of ALI alone (ALIr+). Other ALI including, wire exit perforation or pulmonary artery dissections that occurred without oxygen desaturation or radiographic changes, were not considered to represent ALI.
Statistical analysis
Baseline and FU haemodynamic, symptomatic, and demographic data were compared by one-way analysis of variance (ANOVA), Student's t-test or χ2 test where appropriate. Per procedure predictive factors for ALI were identified using univariate logistic regression analysis. Variables selected for comparison represented prevailing haemodynamics (mPAP, CO, PVR, RAP) and procedural factors (number of vessels treated, whether an occlusion was treated, whether it was a patient's first BPA and whether a ≥4 mm balloon was used, which was a surrogate of proximity and therefore lung volume subtended downstream of the treated vessel). Per patient predictive factors for ALI over the course of treatment were identified using univariate logistic regression analysis. Multivariate logistic regression of factors identified as significant then analyzed the independent effects of each variable. Variables selected for comparison represented patient demographics (age, BMI, sex), baseline haemodynamics (mPAP, PVR, CO, RAP), other baseline investigations (6MWD, NT-proBNP), and procedural factors (number of vessels treated, BPA treating occlusions, and number of BPA performed).
Per procedure percentage changes in haemodynamics (ΔmPAP, ΔCO, ΔPVR) were compared between ALI+ and propensity-matched procedures from the ALI– group using Student's t-test as well as one-way ANOVA. Controls were matched by age (±10 years), sex, stage of BPA (first to sixth), location, and number of vessels treated. Per patient haemodynamic (ΔmPAP, ΔCO, ΔPVR), symptomatic (ΔWHO FC, ΔCAMPHOR symptoms score), NT-pro BNP (a biomarker released in response to increased cardiac wall tension), and 6MWD percentage changes from baseline to 3-month FU were compared between ALI+ and ALI– groups. ΔWHO FC from baseline to 3-month follow-up was analyzed by logistic regression, using baseline WHO FC and ALI occurrence as covariates. Insufficient appropriate ALI– patients had completed FU to perform propensity-matched analysis. Outcomes were compared by one-way ANOVA and Student's t-test. For all analyses, p < 0.05 was considered significant.
RESULTS
In total, 282 BPA procedures performed on 109 patients were analyzed for incidence of ALI, predictors of ALI, and procedural-level outcomes of BPA complicated by ALI (Table 1). Of these, 85 patients had completed follow-up and were included in the analysis of per patient outcomes of ALI over the course of treatment (for FU data see Table 2). One patient declined further interventions, having suffered clinically significant ALI in consecutive procedures as they did not attend follow-up and are therefore not included in per patient analysis but their data are included in the per procedure analysis.
| ALI+ (n = 15) | n | p Value† | ALIcr+ (n = 6) | n | p Value* | ALIr+ (n = 9) | n | ALI− (n = 94) | n | p Value‡ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Procedures | 3.3 ± 0.8 | 15 | 0.01 | 4.0 ± 1.4 | 6 | 0.05 | 2.9 ± 0.8 | 9 | 2.5 ± 0.9 | 94 | <0.001 |
| Total vessels treated | 7.2 ± 1.8 | 15 | 0.01 | 7.5 ± 2.3 | 6 | 0.13 | 7.0 ± 1.6 | 9 | 5.8 ± 2.1 | 94 | 0.06 |
| Total procedures treating occlusions | 1.9 ± 1.3 | 15 | 0.09 | 1.7 ± 1.6 | 6 | 0.63 | 2.1 ± 1.1 | 9 | 1.3 ± 0.9 | 94 | 0.06 |
| Age | 60.8 ± 10.8 | 15 | 0.15 | 61.8 ± 12.7 | 6 | 0.53 | 60.1 ± 10.1 | 9 | 65.4 ± 11.0 | 94 | 0.32 |
| Sex (male/female) | 6/9 | 15 | 0.49 | 2/4 | 6 | 0.47 | 4/5 | 9 | 47/47 | 94 | 0.71 |
| BMI (kg.m−2) | 24.2 ± 3.6 | 15 | 0.05 | 23.1 ± 2.9 | 6 | 0.03 | 24.9 ± 4.1 | 9 | 26.5 ± 4.9 | 94 | 0.20 |
| mPAP (mmHg) | 49.9 ± 8.3 | 15 | 0.01 | 53.0 ± 8.7 | 6 | 0.03 | 44.9 ± 7.2 | 9 | 41.58 ± 10.1 | 93 | 0.03 |
| PVR (dyn.s. cm−5) | 790 ± 297 | 15 | 0.02 | 947 ± 241 | 6 | 0.006 | 644 ± 241 | 9 | 601 ± 248 | 93 | <0.001 |
| CO (L/min) | 4.29 ± 1.35 | 15 | 0.28 | 3.53 ± 0.99 | 6 | 0.03 | 4.66 ± 1.36 | 9 | 4.51 ± 0.95 | 92 | 0.019 |
| NT-proBNP (ng/L) | 1871 ± 1144 | 14 | 0.44 | 3189 ± 1069 | 6 | 0.05 | 648 ± 340 | 8 | 1107 ± 1234 | 84 | 0.02 |
| 6MWD (m) | 299 ± 119 | 15 | 0.81 | 262 ± 143 | 6 | 0.87 | 370 ± 108 | 9 | 355 ± 117 | 77 | 0.86 |
| WHO FC (1/2/3/4) | 0/4/11/0 | 15 | 0.78 | 0/2/4/0 | 6 | 0.91 | 0/2/7/0 | 9 | 0/29/63/2 | 94 | 0.97 |
| CAMPHOR symptoms score | 11.9 ± 5.7 | 15 | 0.34 | 12.5 ± 4.6 | 6 | 0.29 | 11.4 ± 6.9 | 9 | 10.0 ± 5.7 | 90 | 0.56 |
| Number of PH drugs | 1.5 ± 0.5 | 15 | 0.20 | 1.5 ± 0.5 | 6 | 0.52 | 1.6 ± 0.5 | 9 | 1.3 ± 0.6 | 94 | 0.48 |
| Class of PH drugs (PDE5i/ERA/sGC) (%) | 39/35/26 | 15 | 0.61 | 44/33/22 | 6 | 0.89 | 36/36/29 | 9 | 44/29/23 | 94 | 0.35 |
- Note: p Values: †ALI+ versus ALI−; *: ALIcr+ versus ALI−; ‡: ALIcr+ versus ALIr+ versus ALI. Values expressed as means ± SD. p Values: ‡: one-way ANOVA comparing ALIcr+ versus ALIr+ versus ALI−; †: Student's t-test comparing ALI+ versus ALI−; *: Student's t-test comparing ALIcr+ versus ALI−. χ2 test compared WHO FC and class of PH drugs between groups. p < 0.05 is considered significant (bold).
- Abbreviations: ALI, acute lung injury; ANOVA, analysis of variance; BMI, body mass index; CAMPHOR, Cambridge pulmonary hypertension outcome review; CO, cardiac output; ERA, endothelin receptor antagonist; mPAP, mean pulmonary artery pressure; PDE5i, phosphodiesterase type 5 inhibitor; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; sGC, soluble guanylate cyclase; WHO FC, World Health Organization functional class; 6MWD, 6 min walk distance.
| ALI+ (n = 16) | n | p Value† | ALIcr (n = 4) | n | p Value* | ALIr (n = 12) | n | ALI− (n = 73) | n | p Value‡ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mPAP (mmHg) | 37.3 ± 7.6 | 12 | 0.14 | 43.8 ± 9.8 | 4 | 0.14 | 34.1 ± 3.7 | 8 | 33.6 ± 8.4 | 72 | 0.06 |
| PVR (dyn.s. cm−5) | 525 ± 150 | 12 | 0.009 | 629 ± 146 | 4 | 0.05 | 472 ± 129 | 8 | 384 ± 159 | 70 | 0.10 |
| CO (L/min) | 4.39 ± 1.11 | 12 | 0.07 | 4.66 ± 1.64 | 4 | 0.66 | 4.26 ± 0.85 | 8 | 5.07 ± 1.03 | 71 | 0.10 |
| NT-proBNP (ng/L) | 353 ± 234 | 12 | 0.51 | 383 ± 244 | 4 | 0.80 | 338 ± 245 | 8 | 421 ± 584 | 61 | 0.92 |
| 6MWD (m) | 440 ± 78 | 12 | 0.08 | 462 ± 47 | 4 | 0.03 | 427 ± 93 | 8 | 387 ± 110 | 59 | 0.29 |
| WHO FC (1/2/3/4) | 0/12/0/0 | 12 | 0.01 | 0/4/0/0 | 4 | 0.20 | 0/8/0/0 | 8 | 7/34/25/1 | 66 | 0.06 |
| CAMPHOR symptoms score | 2.9 ± 3.5 | 10 | 0.01 | 1.3 ± 1.2 | 3 | <0.001 | 3.6 ± 4.1 | 7 | 7.3 ± 6.5 | 62 | 0.17 |
- Note: p Values: †ALI + versus ALI−; *: ALIcr+ versus ALI−; ‡: ALIcr+ versus ALIr+ versus ALI−. Values expressed as means ± SD. p values: ‡: one-way ANOVA comparing ALIcr+ versus ALIr+ versus ALI−; †: Student's t-test comparing ALI+ versus ALI−; *: Student's t-test comparing ALIcr+ versus ALI−. χ2 test compared WHO FC between groups. p < 0.05 is considered significant (bold).
- Abbreviations: ANOVA, analysis of variance; ALI, acute lung injury; CAMPHOR, Cambridge pulmonary hypertension outcome review; CO, cardiac output; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; WHO FC, World Health Organization functional class; 6MWD, 6 min walk distance.
Incidence of ALI
In total, ALI+ occurred in 15/109 (13.7%) patients and 17/282 (6.0%) procedures with a distribution of 7/109, 7/108, and 3/65 (p = 0.71) for first, second, and third or more procedures, respectively. All ALI occurred after completion of BPA but within 24 h of the procedure. Radiological features of ALI (ALIr+) occurred in 9/109 (8.3%) patients following 10/282 (3.5%) procedures. Clinical and radiological features of ALI (ALIcr+) occurred in 6/109 (5.5%) patients following 7/282 (2.5%) procedures. Of these patients with clinically overt ALI, 2 patients required noninvasive positive pressure ventilation with oxygen and 5 required supplemental oxygen administration via a mask or nasal cannulae. No patients required invasive ventilation, although two patients did require brief intensive care admission for closer monitoring of oxygenation and supplemental noninvasive oxygen. All patients recovered to discharge; however, ALI was associated with a longer hospital admission (ALI−: 1.5 ± 0.9, ALIr+: 1.2 ± 0.4, ALIcr+: 5.9 ± 3.8 hospital bed days; p < 0.001). Haemoptysis was noted in 2 ALI+ cases (1 ALIcr+, 1 ALIr+) and wire exit with contrast extravasation in only 1 ALIcr+ case. The majority of procedures resulting in ALI were otherwise without clinically overt hemorrhage (haemoptysis or angiographic evidence of contrast extravasation typically seen after a wire perforation, or dissection).
Predictors of ALI—per procedure
Patients with more severe haemodynamics at the time of their procedure were more likely to suffer ALI as a complication of their procedure (Table 3). The strongest association was with PVR, but high mPAP, low CO, and high RAP were also significantly associated with ALI. There were no significant predictors for the radiological features of ALI. Procedural factors including the number of vessels treated, targeting an occlusion, balloon size, or first compared to later BPA session did not affect the per-procedure likelihood of ALI.
| Odds ratio | 95% CI | p Value | |
|---|---|---|---|
| mPAP | 1.050 | 1.007−1.091 | 0.021 |
| 1.088 | 1.023−1.157 | 0.007 | |
| 1.020 | 0.964−1.080 | 0.484 | |
| CO | 0.675 | −0.930 to 0.146 | 0.153 |
| 0.342 | 0.135−0.871 | 0.024 | |
| 0.993 | 0.523−1.886 | 0.983 | |
| PVR | 1.002 | 0.001−0.004 | 0.009 |
| 1.005 | 1.002−1.008 | <0.001 | |
| 1.000 | 0.998−1.003 | 0.718 | |
| RAP | 1.072 | −0.051 to 0.190 | 0.258 |
| 1.191 | 1.001−1.403 | 0.036 | |
| 0.974 | 0.818−1.161 | 0.770 | |
| Number of vessels treated | 1.133 | −0.471 to 0.721 | 0.681 |
| 0.599 | 0.234−1.534 | 0.285 | |
| 1.771 | 0.794−3.949 | 0.162 | |
| Occlusion treated | 1.255 | −0.865 to 1.319 | 0.684 |
| 0.743 | 0.162−3.399 | 0.701 | |
| 1.996 | 0.405−9.839 | 0.396 | |
| First BPA | 1.182 | 0.259−5.384 | 0.829 |
| 1.105 | 0.408−2.995 | 0.845 | |
| 1.052 | 0.290−3.820 | 0.938 | |
| ≥4 mm balloon used | 1.556 | 0.433−5.587 | 0.498 |
| 1.980 | 0.234−16.748 | 0.513 | |
| 1.320 | 0.273−6.375 | 0.730 | |
| ALI+ | ALIcr+ | ALIr+ | |
- Note: Results of univariate logistic regression analysis expressed as odds ratio, 95% confidence interval, and p value. p < 0.05 is considered significant (bold).
- Abbreviations: ALI, acute lung injury; BPA, balloon pulmonary angioplasty; CO, cardiac output; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure.
Predictors of ALI—per patient
The severity of the haemodynamics at baseline was associated with a higher incidence of ALI throughout the course of treatment (Table 4). High baseline PVR showed the strongest association but high baseline mPAP, low baseline CO, and high baseline NT-proBNP levels were also associated with ALI.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p Value | Odds ratio | 95% CI | p Value | |
| Age | 0.967 | 0.925−1.011 | 0.144 | – | – | – |
| 0.978 | 0.915−1.045 | 0.508 | – | – | – | |
| 0.964 | 0.914−1.017 | 0.179 | – | – | – | |
| BMI | 0.884 | 0.765−1.022 | 0.096 | – | – | – |
| 0.819 | 0.641−1.046 | 0.109 | – | – | – | |
| 0.927 | 0.785−1.094 | 0.369 | – | – | – | |
| Female | 1.500 | 0.495−4.548 | 0.474 | – | – | – |
| 1.962 | 0.344−11.184 | 0.448 | – | – | – | |
| 1.250 | 0.316−4.947 | 0.751 | – | – | – | |
| mPAP | 1.065 | 1.008−1.125 | 0.025 | 0.995 | 0.920−1.076 | 0.894 |
| 1.104 | 1.014−1.202 | 0.023 | 1.183 | 0.910−1.538 | 0.209 | |
| 1.037 | 0.970−1.109 | 0.290 | – | – | – | |
| PVR | 1.003 | 1.001−1.005 | 0.008 | 1.002 | 0.999−1.004 | 0.241 |
| 1.005 | 1.002−1.008 | 0.002 | 0.995 | 0.984−1.007 | 0.418 | |
| 1.001 | 0.998−1.003 | 0.497 | – | – | – | |
| CO | 0.659 | 0.371−1.170 | 0.154 | – | – | – |
| 0.243 | 0.085−0.693 | 0.008 | 0.043 | 0.001−2.544 | 0.130 | |
| 1.116 | 0.563−2.212 | 0.754 | – | – | – | |
| RAP | 1.116 | 0.964−1.292 | 0.142 | – | – | – |
| 1.176 | 0.950−1.454 | 0.136 | – | – | – | |
| 1.071 | 0.889−1.290 | 0.472 | – | – | – | |
| 6MWD | 1.001 | 0.996−1.005 | 0.802 | – | – | – |
| 0.999 | 0.992−1.006 | 0.806 | – | – | – | |
| 1.002 | 0.996−1.008 | 0.617 | – | – | – | |
| NT-proBNP | 1.000 | 1.000−1.001 | 0.454 | – | – | – |
| 1.001 | 1.000−1.001 | 0.024 | 1.000 | 0.999−1.001 | 0.367 | |
| 1.000 | 0.999−1.000 | 0.305 | – | – | – | |
| Total vessels treated | 1.370 | 1.042−1.801 | 0.024 | 0.904 | 0.582−1.406 | 0.656 |
| 1.409 | 0.956−2.076 | 0.084 | – | – | – | |
| 1.304 | 0.940−1.808 | 0.112 | – | – | – | |
| Treating occlusions | 1.839 | 1.049−3.223 | 0.033 | 1.620 | 0.820−3.199 | 0.165 |
| 1.309 | 0.586−2.926 | 0.512 | – | – | – | |
| 2.408 | 1.110−5.224 | 0.026 | – | – | – | |
| Number of BPA sessions | 2.158 | 1.272−3.663 | 0.004 | 2.025 | 0.914−4.483 | 0.082 |
| 3.181 | 1.481−6.835 | 0.003 | 3.232 | 1.169−8.937 | 0.024 | |
| 1.564 | 0.796−3.072 | 0.194 | – | – | – | |
| ALI+ | ALIcr+ | ALIr+ | ||||
- Note: Results of univariate and multivariate logistic regression analyses expressed as odds ratio, 95% confidence interval, and p value. p < 0.05 is considered significant (bold).
- Abbreviations: ALI, acute lung injury; BMI, body mass index; BPA, balloon pulmonary angioplasty; CO, cardiac output; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; 6MWD, 6 min walk distance.
The total number of vessels treated, the number of BPA sessions, and whether the patient had occlusions treated were significantly associated with ALI (Table 4). The strongest patient level association was the number of BPA procedures undertaken and was the only variable found to be independently associated with ALIcr+ by multivariate analysis.
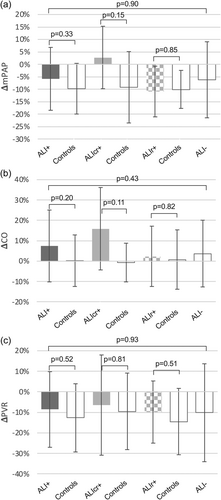
Outcomes of ALI—per procedure
There was no significant difference in procedural level haemodynamic improvements in those with ALI compared to propensity-matched ALI− controls (Figure 1). However, there was a trend to a greater increase in CO and more modest reduction in mPAP in those with ALI, particularly in the ALIcr+ group.
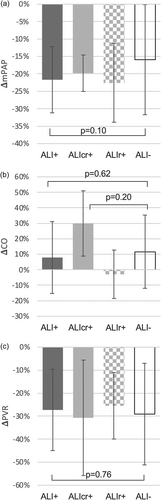
Outcomes of ALI—per patient
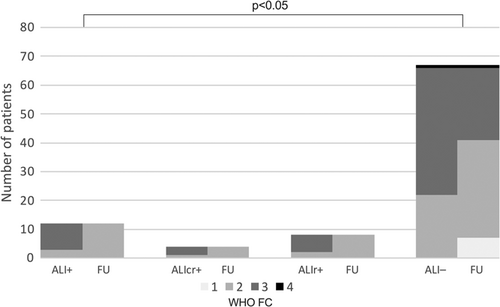
Patients with ALI after BPA did not have a significantly different haemodynamic response from baseline to 3-month FU when compared to those without ALI (Figure 2). Similarly, patients with ALI did not have significantly different improvement in their 6MWD from baseline to 3-month FU (Δ6MWD: ALI+: +42 ± 59 vs. ALI–: +34 ± 61 m, p = 0.67). However, patients with ALI exhibited a greater reduction in NT-pro BNP, that was particularly notable in the ALIcr+ group (ΔNT-pro BNP: ALIcr+: −78 ± 12% vs. ALI–: −43 ± 36%, p < 0.01; Figure 3a,b). Patients with ALI reported a significantly greater symptomatic benefit, measured by both change in CAMPHOR symptom score at FU (Figure 3c) and WHO FC (Figure 4).

DISCUSSION
In this study, we found that radiologically and/or clinically apparent ALI was infrequent and often mild. The risk of ALI was predicted by the severity of the haemodynamics, both at baseline and at each procedure, as well as the overall number of segments treated. In addition, the greater the number of BPA sessions and targeting occlusions increased the risk of ALI. The total number of BPA sessions was the only significant independent predictor of ALI in a multivariate analysis. Looking at outcomes, patients with ALI had a favorable clinical response to BPA, with similar improvement (percentage change) in haemodynamics as those without ALI, but had superior biomarker and reported more symptomatic benefits.
The frequency of clinically significant ALI in our patients compares favorably with data from other centers. Recent meta-analyses have found wide variation between centers, but averaging approximately 13%.17, 18 This is significantly lower than early practice,19 although the frequency of ALI varies depending on the defining criteria and imaging modality used. Our policy is to limit the number of BPA sessions in those with objective and subjective clinical improvement (rather than aiming for a haemodynamic target) and in particular we limit treatment when the risk: benefit ratio is adverse. We also liberally use pulmonary vasodilators before starting BPA. This results in fewer BPA sessions compared to international data and inevitably lowers the incidence of ALI in our cohort. Evidence of good medium to longer term outcomes, even by patients with ALI, may encourage a more aggressive approach.
Haemodynamics predicted the incidence and clinical significance of ALI per procedure as well as per patient, in agreement with the pulmonary edema predictive scoring index (PEPSI).4 However, others have not found high mPAP to predict ALI.3 Surrogates in our study for sum total change in pulmonary flow grade score, namely segment number treated and severity (total occlusions) of diseased vessels successfully treated, were not associated with per-procedure ALI risk, in contrast to findings of lesion-type predicting complications.3, 20 However, at a per-patient level, the total number of vessels and occlusions treated were associated with ALI on univariate analysis and align with PEPSI. The persistent association with number of BPA sessions and ALI could be regarded as a surrogate for disease (and therefore haemodynamic) severity. However, ALI incidence was not more frequent in earlier BPA sessions, when haemodynamics are typically more adverse, although this may have been confounded by our operators' awareness of this association and subsequent initial cautious approach.4
Crucially, while ALI does represent a significant acute clinical stress on patients, as reflected in a more prolonged length of hospital stay, our data provide new evidence that patients' haemodynamic response is not disadvantaged later and symptom and NT-pro BNP responses may even be superior after ALI has resolved; ALI may even be viewed as a marker of a favorable late treatment response. ALI may fundamentally require the successful restoration of blood supply to occur and therefore, if the patient can be successfully supported in the perioperative period, perhaps it is not surprising that medium to long term results are unaffected and good. The discrepancy of observing a similar haemodynamic response (change in PVR, mPAP, and CO) to BPA in those with and without ALI, but greater clinical improvement in those with ALI remains unexplained, but a superior biomarker response suggests a genuinely positive impact on RV function in the ALI+ group.
Mechanism of ALI
Opinion differs as to the mechanism of ALI. Some believe lung infiltrates post-BPA are caused by vascular injury rather than reperfusion edema as seen post-PEA.20, 21 This is supported by the observation that opacification tends to occur in limited regions rather than the entire reperfused territory,20 although this could also be explained by “protective” patchy small vessel vasculopathy in some distal beds. In addition, as we observed, lung injury tends to occur earlier when vascular injury is the cause, whereas reperfusion injury is more commonly observed 24−72 h postprocedure. There are several proposed mechanisms for this vascular injury, including wire perforation, balloon over-dilatation, and pressure overload.21, 22 Nevertheless, in our cohort, only two patients had clinically evident haemoptysis and there was only one instance of angiographic evidence of wire perforation with contrast extravasation, whilst others had wire exit without observable ALI.
Localized lung edema due to reperfusion may also be important—so called LRI. Startling's law states that increased capillary permeability (due to the loss of endothelial tight junction integrity, frequently caused by inflammation) and a rise in capillary hydrostatic pressure: oncotic pressure ratio, results in interstitial fluid shift.23 We and others4 have observed that those with highest mPAP, which often persists immediately post-BPA, develop ALI and endothelial dysfunction caused by both local and systemic inflammation are features of CTEPH.24 Cytokine-mediated systemic inflammation likely explains why edema can sometimes be seen in the contralateral, non-operated lung.4 In addition, conceptually LRI can act over the entire large endothelial area and perhaps is a more plausible explanation for a lobar segment “white out” on chest radiography, than local wire or balloon trauma over a relatively smaller area. However, anti-inflammatory drugs for example, steroids do not appear to prevent ALI after PEA surgery.25 Magnetic resonance imaging may conclusively distinguish whether extravasation of blood or genuine edema are responsible for the lung infiltrates seen, as both have different magnetic properties owing to the iron in blood. It is possible that multiple mechanisms may contribute to the phenomena seen and more research is needed to understand the mechanism to effectively prevent and treat ALI.
Prevention of ALI
Our findings offer further insight into how ALI may be prevented. First, optimizing preprocedural haemodynamics medically, with therapeutics that lower mPAP and PVR should be effective in reducing the probability of ALI and indeed this is so. Medical treatment with riociguat preceding BPA lowers the risk of complications, including ALI.26 In our study, 104/109 of our patients were maintained on medical therapy for at least 3 months before BPA. Ongoing trials are examining the complimentary roles of BPA and medical therapy in the CTEPH treatment protocol.27, 28 Preprocedural pulmonary vasodilatation with oxygen therapy has also been demonstrated to lower mPAP, and similarly may be beneficial.29 Second, by optimizing the procedural factors for example, limiting the number of segments treated until mPAP has been sufficiently lowered reduces the risk of ALI.30 Another approach is to treat all accessible vessels with a stepwise increase in balloon size to avoid vessel over-dilatation.31 Pressure wire guided BPA, limiting the distal pressure to <35 mmHg may also achieve similar results.14 However, if wire induced vessel microtrauma is indeed important mechanistically in ALI, minimizing the number of times a vessel is wired and using familiar low tip load, workhorse wires should reduce the probability of occurrence. Minimizing wire induced vessel microtrauma is consistent with our finding that total number of BPA sessions was the only independently significant predictor of ALI. We have also previously reported reduced procedural-level haemodynamic treatment response beyond three BPA sessions32 and limiting ALI risk by minimizing procedural number may similarly be an important safety consideration too.
LIMITATIONS
This was a retrospective analysis of prospectively collected data at a single high-volume center. Prospective studies are required to confirm our findings. Data was collected for clinical service evaluation rather than dedicated research purposes, and therefore data sets were not always complete and investigators were not blinded. The operators were experienced and not blinded to clinical data, mitigating risk in perceived high-risk cases by altering treatment strategy and this may result in predictive confounding. However, as all ALI occurred after completion of the BPA, procedural factors were not biased in those who had ALI. Severe baseline haemodynamics have been shown to predict response to BPA treatment32; by analyzing percentage change from baseline when comparing groups we attempted to correct for this. Low ALI rates led to limited statistical power in our analysis. We defined ALI radiologically by plain chest radiography within 8 h of the procedure and clinically with regular oxygen saturation monitoring until discharge; more frequent chest radiographs for a more prolonged period may have increased our detection rate. However, most ALI occurs within the first 24 h12 and no patient within our cohort reported hospitalization postdischarge, which may be expected if significant ALI had been missed. In addition, a more sensitive tool such as CT would have increased our detection rate of occult ALI and altered our findings, but the clinical significance of isolated lung opacification by CT remains to be demonstrated. Finally, any implications for practice change must be considered in the context of all risks and benefits of treatment. ALI was associated with a longer hospital stay after BPA, thereby impacting service provision. Our analysis does not consider other procedure related ALI complications of BPA, such as hemorrhage, or all the potential benefits.
CONCLUSION
ALI is predicted at a patient and procedure level by baseline haemodynamic severity and the number of sessions of BPA performed as well as treating occlusions. Patients undergoing BPA complicated by ALI in this study achieved an equivalent haemodynamic improvement, measured by change in indices from baseline, but appear to have superior biomarker and symptomatic responses, compared to those without ALI.
AUTHOR CONTRIBUTIONS
Stephen P. Hoole conceived the study. The data were collected by Matthew S. Rodgers, Louise C. Kirkby, and Liliana Amaral-Almeida. Matthew S. Rodgers performed the analysis with the assistance of Stephen P. Hoole. All authors have reviewed and agree with the published manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge their patients and the care provided by the catheter laboratory and ward staff at Royal Papworth Hospital. The work was supported by the Cambridge BRC. S. P. H. acts as the guarantor.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.