Commercial human pulmonary artery endothelial cells have in-vitro behavior that varies by sex
Abstract
It is unknown whether biological sex influences phenotypes of commercially available human pulmonary artery endothelial cells (HPAECs). Ten lots of commercial HPAECs were used (Lonza Biologics; PromoCell). Five (50%) were confirmed to be genotypically male (SRY+) and five (50%) were confirmed to be female (SRY−). Experiments were conducted between passages five and eight. HPAEC phenotype was confirmed with a panel of cell expression markers. Standard assays for proliferation, migration and tube formation were performed in triplicate with technical replicates, under three treatment conditions (EndoGRO; Sigma-Aldrich). Apoptosis was assessed by exposing cells treated with complete media or low serum media to hypoxic (1% oxygen) or normoxic (20% oxygen) conditions. Laboratory staff was blinded. The median (range) age of male and female donors from whom the HPAECs were derived was 58 (48–60) and 56 (33–67), respectively. Our results suggest decreased proliferation in genotypically female cells compared with male cells (p = 0.09). With increasing donor age, female cells were less proliferative and male cells were more proliferative (p = 0.001). Female cells were significantly more apoptotic than male cells by condition (p = 0.001). Female cells were significantly more migratory than male cells in complete media but less migratory than male cells under vascular endothelial growth factor enriched conditions (p = 0.001). There are subtle sex-based differences in the behavior of HPAECs that depend on donor sex and, less so, age. These differences may undermine rigor and reproducibility. Future studies should define whether biological sex is an important regulator of HPAEC function in health and disease.
INTRODUCTION
Sex plays an important and complex role in biological function, both in health and disease.1, 2 Humans are sexually dimorphic due to a combination of genetic and hormonal factors that start early in development and change throughout life.3 Sex chromosomes have the potential to directly influence biochemical pathways and cellular physiology apart from the hormonal milieu.4 Acknowledgment of genomic sex as a biological variable is therefore essential in preclinical research, as observed experimental differences may be explained in part or entirely by sex.
Sex-stratified experimental approaches and their influence on results have historically been both under-recognized and under-studied. A survey of all studies published in AJP-Cell Physiology in 2013 revealed that 75% did not report the sex of cell lines or animals.4 In an effort to ensure routine integration of sex in biomedical research, the National Institutes of Health now requires that all research proposals utilizing humans or vertebrate animals consider sex as a biological variable in design, analysis, and reporting unless investigators provide strong justification for single-sex studies.5 Despite this requirement, reporting of biological sex in cell culture studies remains low. In a recent systematic review including articles from 16 peer reviewed cardiovascular journals using cultured cells, sex was reported in only 39% of all studies and 55% of those studies exclusively used cells derived from males.6
Sex-based phenotypes have been observed in a variety of cardiopulmonary diseases, but in many, whether or not intrinsic sex differences exist at the cellular level remains unknown. Endothelial cell dysfunction is a hallmark of pulmonary vascular disease, as is sexual dimorphism.7 Sex-specific differences in cellular phenotypes have been described in human umbilical vein endothelial cells (HUVECs), mouse lung endothelial cells as well as in bone marrow-derived circulating endothelial progenitor cells.8-12 Whether sexual dimorphism exists in normal human pulmonary artery endothelial cells (HPAEC) remains unknown. We sought to determine whether sex-based differences exist between commercially acquired HPAECs in vitro at baseline, independent of exogenous sex steroids. Here, we quantified proliferation, apoptosis, migration, and tube formation in secondary cultures of HPAECs from 10 commercial cell lines and hypothesized that female cells would demonstrate increased apoptosis, proliferation, migration, and tube formation. We also examined whether donor age moderated the relationships between biological sex and HPAEC phenotype.
METHODS
Commercially available HPAECs were purchased from Lonza Biologics, Inc or PromoCell. We obtained all available demographic, anthropometric and clinical information from vendors. These cells were grown in primary culture (Complete Media, EndoGRO) to confluence and then seeded until passaged cells reached confluence as previously described.13 All studies were done on cells between passages 5–8 to avoid phenotypic drift. All experiments were performed in triplicate per lot with technical replicates per experiment. Pulmonary artery endothelial cell (PAEC) origin was confirmed by use of a routine screening panel including von Willebrand factor (vWF)(Dako), vascular endothelial (VE)-cadherin (Santa Cruz Biotechnologies) staining and acetylated low-density lipoprotein (AcLDL)(Thermo Fisher Scientific) uptake. Alpha-smooth muscle cell actin (α-SMA)(Abcam) was used as a negative control. Cells were required to express vWF, VE-cadherin, and AcLDL and show negative staining of α–SMA. Previously validated assays were employed to characterize behavior and function,13-15 as follows:
Functional assays
Proliferation: HPAECs were seeded at a density of 25,000 cells per well on eight chamber glass slides coated with 0.2% gelatin (Millicell EZ SLIDE eight well glass slide). Cells were incubated for 24 h at 37°C after which, media was aspirated and 0.4% (400 µl) of low serum media was added. Cells were incubated at 37°C for an additional 15 h. On the second day, media was aspirated and cells were treated with 400 µl of either complete, 0.4% low serum, or vascular endothelial growth factor (VEGF) enhanced low serum media in duplicates. Cells were incubated at 37°C for 48 h and fixed (4% paraformaldehyde) then stained with Ki-67 (Abcam ab16667, 1:100 dilution), a nonhistone nuclear protein expressed during the active phase of the cell cycle. The cell nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole, Invitrogen Hoescht 3342, 1:2000 dilution). Cells were imaged using a BioTek Cytation 5 Imager and counted for total nuclei and Ki-67 positive nuclei.14
Apoptosis
HPAECs were seeded on two chamber slides (Millicell EZ SLIDE eight well glass slide) at a density of 35,000 cells per well. Media was changed to 0.4% low serum and complete media with one slide incubated under normoxic conditions (37°C, 5% carbon dioxide, 20% oxygen) and one slide under hypoxic conditions (37°C, 5% carbon dioxide, 1% oxygen). Cells were subsequently fixed (4% paraformaldehyde) and stained with Ki-67 and DAPI. Total nuclei were counted utilizing ImageJ software (NIH) with manual determination of apoptosis by examining nuclear morphology and membrane integrity.16-19
Migration
HPAECs were seeded at a density of 50,000 cells per well in a 96-well dish. Once confluent monolayers were formed, a scratch was made using the BioTek Autoscratch mechanism, which performs reproducible scratch wounds in monolayers of cells. Cells were then washed three times with low serum media and incubated under low-serum (0.4%), complete, or VEGF enriched conditions. Cell migration was assessed at 2 h intervals and images were taken using the BioTek Cytation 5. Migration was assessed using MiToBo analyzer software in ImageJ and expressed as average pixels lost per hour.14
Tube formation
HPAECs were plated at a density of 50,000 cells per well in a 24-well dish coated with growth factor reduced Matrigel® in low serum, complete media, or VEGF conditions for 6 h. Phase contrast images were obtained at 2, 4, and 6 h and tube formation was quantified using the AngioTool plugin for Fiji.14
Karyotypic sex
Sex is biologically defined based on genetics, anatomic features, and hormones.20 For the purposes of this study, due to the limited scope of information available about donors, sex was defined in a binary fashion as male or female in reference to a donor's sex chromosomes (extrapolated by the presence or absence of the SRY gene).
DNA isolation was performed using the Invitrogen PureLink Genomic DNA Mini Kit purchased from Thermo Fisher Scientific. DNA quality was assessed with Nanodrop One (Thermo Fisher Scientific). Standard PCR was performed using Applied Biosystems Veriti Thermocycler. Primers were designed and obtained from Integrated DNA Technologies (human SRY Forward primer: 5′-TCCAGAAGTGAGCCTGCCTA-3′ Reverse primer: 5′-TCGGCGTTCTCAACATTCCT-3′) with Human β-actin (Forward primer: 5′-ACGTTGCTATCCAGGCTGTG-3′ Reverse primer 5′- GAGGGCATACCCCTCGTAGA-3′) as a housekeeping gene. Agarose was prepared (2%) and gel electrophoresis was performed. Gels were stained with ethidium bromide and were imaged using ChemiDoc Imaging System (Bio-Rad). Karyotypic sex was defined as the absence (female) or presence of the SRY gene (male).4, 21
Blinding
Laboratory staff was blinded to the sex of the cell line for assessment of apoptosis, migration, and analysis. Staff was not blinded for tube formation as the analysis was automated. Staff was not initially blinded for the analysis of proliferation but we conducted a blinded post-hoc assessment with two external reviewers and found results were consistent (varied by <10%). Each assay was given a unique identifier using a random number generator and only those not involved in the analysis had access to the key.
Statistical methods
Data are reported as median (range) or n (%) as appropriate. Generalized linear mixed modeling with a Poisson distribution was used for variables that used counts (e.g., components of tube formation), otherwise, a lognormal distribution was used to examine functional assay results as a function of sex. Two- and three-way interactions with condition (low serum, complete media, VEGF and hypoxia, respectively) and age as a continuous variable for all models were also considered. Alpha was established a priori at the 0.05 level, and all interval estimates were calculated for 95% confidence interval. All analyses were conducted with SAS Software 9.4 with the GLIMMIX procedure.
RESULTS
Donor characteristics
Ten commercial HPAEC lots were obtained, five (50%) derived from females and five (50%) derived from males. Biological sex of the donors was determined based upon their medical record information. Genomic sex was confirmed to be concordant with vendor descriptions for all lots by the presence or absence of the SRY gene (Supporting Information: Figure E1). Available donor characteristics are listed in Table 1, Supporting Information: Tables E1 and E2. The median age among females was 56 years (range 33–67) and among males was 58 years (range 48–60). All donors were white. Female donors had lower body mass index than males (25.7, range 21.4–30 vs. 35, range 29.2–35.9, respectively). No donors were known to be diabetic; one female was a known smoker.
| Male (n = 5) | Female (n = 5) | |
|---|---|---|
| Age, yrs | 58 (48–60) | 56 (33–67) |
| Race | ||
| White | 5 (100) | 5 (100) |
| Other | 0 (0) | 0 (0) |
| Smoking Status | ||
| Smoker | 0 (0) | 1 (20) |
| Nonsmoker | 3 (60) | 1 (20) |
| Unknown | 2 (40) | 3 (60) |
| Diabetes history | ||
| Diabetic | 0 (0) | 0 (0) |
| Nondiabetic | 3 (60) | 2 (40) |
| Unknown | 2 (40) | 1 (20) |
| BMI, kg/m2 | 35 (29.2–35.9)a | 25.7 (21.4–30)b |
- Note: Distinction between sex and gender not made in materials provided by vendor.
- Results reported as median (range) or n (%).
- Abbreviation: BMI, body mass index.
- a Available for n = 3.
- b Available for n = 2.
Functional assays
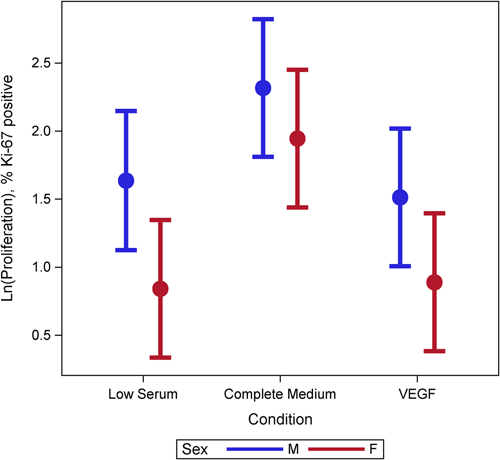
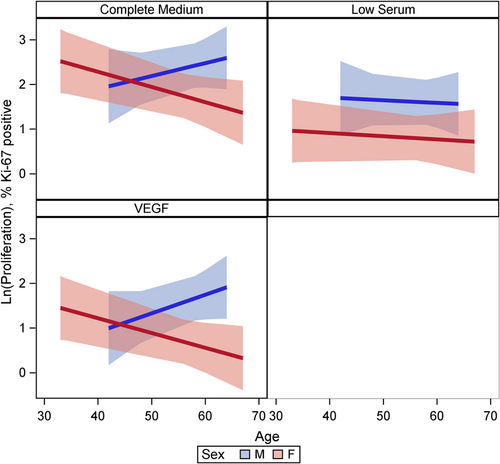
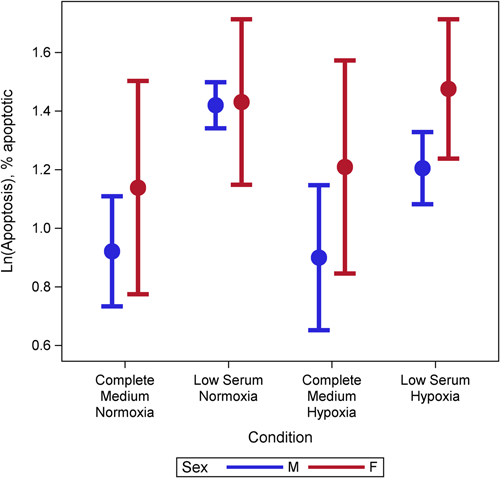
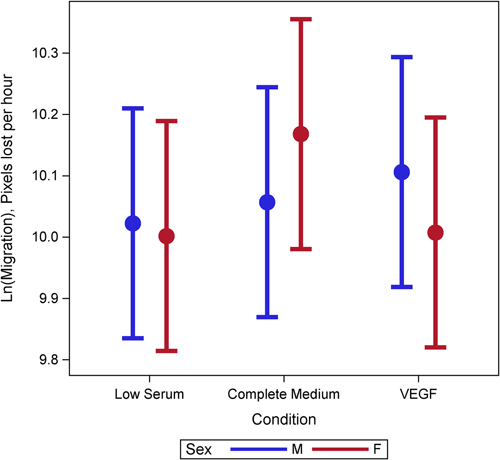
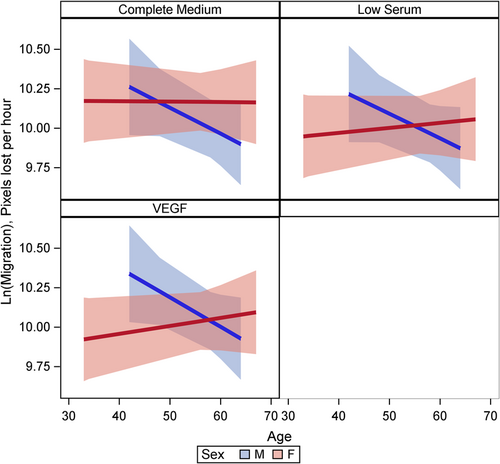
Female cells tended to have lower rates of proliferation (p = 0.09) than male cells (Figure 1). This may have been moderated by media condition, such that the differences were largest under low serum conditions (p = 0.18) but did not reach statistical significance (Figure 1). There was evidence of a three-way interaction between age, sex, and condition (p = 0.001). Female cells were less proliferative and male cells were more proliferative with increasing age under complete and VEGF enriched conditions (Figure 2). Female cells tended to be more apoptotic, and while there was no evidence of a main effect (p = 0.22), this was moderated by condition (p = 0.001), with significant differences in complete media, under both normoxic and hypoxic conditions as well as low serum hypoxic conditions (Figure 3). Age did not modify the relationship between sex and condition for apoptosis (p = 0.63) (Supporting Information: Figure E2). There was no evidence of a main effect between sex and migration (p = 0.98), however, there was a sex-based interaction by condition (p < 0.001), where female cells were more migratory in complete media and less migratory under VEGF enriched conditions. This relationship was not seen in low-serum conditions (Figure 4). Age did not modify the relationship between sex, migration, and condition (p = 0.22) (Figure 5). Overall, there was no evidence of a consistent difference in tube formation by sex, interaction of sex by condition, or modification of the effect by age (Supporting Information: Figures E3 and E4).
DISCUSSION
We have demonstrated that there are subtle differences in commercial HPAEC behavior, which differs between donors and by genomic sex. We observed substantial heterogeneity across individual commercial cell lines despite identical treatment conditions. The age of the individuals from whom the cells were harvested may further influence phenotype. To our knowledge, this is the first time that sexual dimorphism has been described in commercially acquired HPAECs. These findings corroborate that sex specific differences at the cellular level should be considered in experimental design.
The study of sex-based differences in cellular biology is essential to ensure rigor and reproducibility in preclinical research and may help us to understand clinical observations in human health and disease. There are few studies from outside of the central pulmonary circulation that have specifically addressed sex at the endothelial cell level. In one study, HUVECs derived from healthy neonates born to healthy mothers demonstrated diverse characteristics between males and females. These differences included increased cell proliferation, migratory properties, and nitric oxide synthase 3 (NOS3) mRNA and protein expression, which led to synthesis of nitric oxide in female HUVECs and increased markers of autophagy in male HUVECs at baseline.22 Female HUVECs exposed to hyperoxic stress demonstrate more robust cellular viability and tube formation as compared to male HUVECs.10 Further, HUVECs from male and female donors have divergent transcriptional signatures in response to shear stress, with greater expression of immune related genes in females.21 In neonatal human pulmonary microvascular endothelial cells, sexual dimorphism has been demonstrated at baseline in migration, angiogenesis and in response to hyperoxia.23 Taken together, these studies suggest that in neonatal vascular endothelial cells, those derived from females tend to have higher proliferation and migratory potential but are less apoptotic when compared to those from males. Contrary to the existing literature, we found that HPAECs derived from adult female donors were less proliferative, and were more apoptotic when compared with cells derived from males.
Our findings may contradict those from the aforementioned literature for several reasons. First, cellular phenotypes vary by vascular bed.12 Observations in animal models and HUVECs are unlikely to be applicable to the pulmonary vasculature. Within the pulmonary circulation, heterogeneity from the central (PAEC) to peripheral vasculature is being increasingly recognized.13, 24 Second, cells from prenatal life may not accurately recapitulate differences seen in adulthood due to the complexities of aging, hormonal shifts and exposures over the life cycle. Neonatal cell phenotype may be influenced by the maternal environment and hormonal exposures during gestation.25, 26 We found evidence of a significant interaction between sex, age, and condition on proliferation, with female HPAECs becoming less proliferative and males becoming more proliferative with age. We speculate that this observation could be due to age-related hormonal shifts, though this is purely conjecture at this stage. To our knowledge, it is unknown whether the hormonal status of the organism impacts functional activity in vitro at the tissue level. In HUVECs, androgen treatment augments proliferation and migration in male but not female cell lines and this effect is mediated by the androgen receptor,27 which could explain sex-based differences in cardiovascular disease with aging. In murine lungs, there are age- and sex-based differences in endothelial cell function, angiogenesis, and gene transcription regulating these pathways,28 which may also influence human cellular phenotypes. Finally, we noticed substantial variation in behavior and function across cell lines supporting the concept that additional confounders (comorbid illness, BMI, etc.) may account for phenotypic variability.
Our study has limitations. The sample size is relatively low as we were limited by vendor availability. There is extraordinarily limited clinical information available about the individuals from whom commercially acquired cells are derived, a gap that should be systematically addressed. It is broadly accepted that commercially available cells are from “non-diseased” individuals, however this is an assumption. We strongly advocate that vendors should provide more transparent information about the medical history of the donors from who cells are harvested. Sample size (and missing vendor data) also limited our ability to examine additional interactions with BMI. While we completed a number of functional assays that are well established, each has relative strengths and weaknesses and we acknowledge that using multiple confirmatory approaches (e.g., annexin V/PI and TUNEL assays to assess apoptosis) would have increased the strength of our results. Our findings likely oversimplify the differences between males and females, as the in vitro environment does not recapitulate the complex cellular interactions and hormonal signaling that exists in vivo. We suspect that in vivo phenotypic differences may be even greater than those detected here. We studied HPAECs but acknowledge that pulmonary artery smooth muscle cells (PASMCs) are also frequently used in pulmonary vascular disease preclinical experimentation. There is premise for sex-based differences in this cell type. For example, it has been demonstrated that baseline hypoxia-inducible factor-1-alpha signaling is higher in female PASMCs and miRNA-96 expression is reduced in BMPR-II mutated female PASMCs associated with increased proliferation.29, 30 Examining baseline sex differences in PASMCs is an area of future interest. Finally, we did not control for potential sex hormone exposure from serum components in media, though all cells were treated with identical conditions.
In summary, we observed subtle sex-based differences in the behavior of commercially acquired HPAECs. Cell culture research often involves the use of a low numbers of cells and characteristics of the donors from whom the cells are derived is rarely described. Using cells derived from an organism of one sex is unlikely to yield accurate results that represent both sexes. As commercial HPAECs are used as the control condition in experimental pulmonary vascular disease models, future studies should define the sex of the organism from whom cells are obtained to ensure balance in experimental groups and rigor.
AUTHOR CONTRIBUTIONS
Katherine Cox-Flaherty: Conceptual design; data collection and analysis; data interpretation; drafting the manuscript. Julie Braza: Data collection; data analysis; critical review of the manuscript; final approval of manuscript. Amy Princiotto: Data collection; data analysis; critical review of the manuscript; final approval of manuscript. Brianna D. Guarino: Data collection and interpretation; critical review of the manuscript; final approval of manuscript. Grayson L. Baird: Statistical analysis; critical review of the manuscript; final approval of manuscript. Corey E. Ventetuolo: Conceptual design; data interpretation; critical review of the manuscript; final approval of manuscript. Elizabeth O. Harrington: Conceptual design; data interpretation; critical review of the manuscript and final approval of the manuscript.
ACKNOWLEDGMENT
This work was completed with support from the National Institutes of Health 5P20GM103652 (E. O. H.), R01-HL141268 (C. E. V.) and T32-HL134625 (K. C. F., B. D. G., and E. O. H.).
CONFLICT OF INTEREST
Corey E. Ventetuolo has served on advisory boards for United Therapeutics, Acceleron/Merck, and Altavant Sciences, outside the submitted work. Grant funding to institution (United Therapeutics); funds to institution as clinical trial site (Altavant Sciences).
ETHICS STATEMENT
Not applicable.