Dichotomous role of integrin-β5 in lung endothelial cells
Neil Blanchard, Patrick A. Link, and Daniela Farkas contributed equally to this study.
Abstract
Pulmonary arterial hypertension (PAH) is a progressive, devastating disease, and its main histological manifestation is an occlusive pulmonary arteriopathy. One important functional component of PAH is aberrant endothelial cell (EC) function including apoptosis-resistance, unchecked proliferation, and impaired migration. The mechanisms leading to and maintaining physiologic and aberrant EC function are not fully understood. Here, we tested the hypothesis that in PAH, ECs have increased expression of the transmembrane protein integrin-β5, which contributes to migration and survival under physiologic and pathological conditions, but also to endothelial-to-mesenchymal transition (EnMT). We found that elevated integrin-β5 expression in pulmonary artery lesions and lung tissue from PAH patients and rats with PH induced by chronic hypoxia and injection of CD117+ rat lung EC clones. These EC clones exhibited elevated expression of integrin-β5 and its heterodimerization partner integrin-αν and showed accelerated barrier formation. Inhibition of integrin-ανβ5 in vitro partially blocked transforming growth factor (TGF)-β1-induced EnMT gene expression in rat lung control ECs and less in rat lung EC clones and human lung microvascular ECs. Inhibition of integrin-ανβ5 promoted endothelial dysfunction as shown by reduced migration in a scratch assay and increased apoptosis in synergism with TGF-β1. In vivo, blocking of integrin-ανβ5 exaggerated PH induced by chronic hypoxia and CD117+ EC clones in rats. In summary, we found a role for integrin-ανβ5 in lung endothelial survival and migration, but also a partial contribution to TGF-β1-induced EnMT gene expression. Our results suggest that integrin-ανβ5 is required for physiologic function of ECs and lung vascular homeostasis.
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a progressive, severe disease of the pulmonary arterial bed without definitive cure.1 The main histological feature is occlusive pulmonary arteriopathy, characterized by endothelial cell (EC) apoptosis and subsequent switch to an apoptosis-resistant, hyperproliferative, but dysfunctional EC phenotype, potentially through expansion of primitive stem-like ECs.1-4 Components of the dysfunctional endothelial phenotype are a pro-inflammatory state, altered cellular metabolic activity and endothelial-to-mesenchymal transition (EnMT).2, 5-7 In addition, other pulmonary artery (PA) mural cells show functional changes, including PA smooth muscle cells and adventitia fibroblasts.8-10
Many factors influence EC function in the pulmonary circulation, and recent data suggest that the interaction of ECs with the extracellular matrix (ECM) may be crucial for maintaining physiologic EC function.11, 12 PAH lung tissue shows evidence for altered ECM turnover with increased collagen deposition and cross-linkage, augmented breakdown of elastin and accumulation of tenascin and fibronectin in the PAs of PAH patients.13 These changes affect the mechanical features of the PAs and cause increased stiffness and loss of elastic properties.14, 15 In addition, such fundamental remodeling of the ECM in PAs contributes to the aberrant function of ECs and other PA mural cells.16, 17
Endothelial cells use multiple ways to interact with the ECM, and integrins represent one of the most important ones. Integrins are heterodimeric transmembrane molecules and contain α and β subunits in various combinations.18 Assembled integrins function as cell adhesion molecules and every cell type expresses them, albeit the subtypes and their combinations differ between cell types.18 The main function of integrins is to mediate cell-cell and cell-ECM interactions.19, 20 In ECs, the integrin heterodimers ανβ1 and ανβ3 have received particular attention, because these integrins have a critical role in vascular development, EC migration, angiogenesis, and endothelial-to-mesenchymal transition (EnMT).21-27 EnMT is the process of ECs undergoing a phenotypic switch from cobblestone-like ECs to spindle-shaped mesenchymal cells akin to smooth muscle cell (SMC)- or myofibroblast-like cells.6, 7 EnMT occurs by downregulation of endothelial markers, including CD31 and vascular endothelial cadherin (VE-cadherin), and upregulation of mesenchymal/SMC marker, such as α-smooth muscle actin (α-SMA, ACTA2), calponin-1 (CNN1) or SM22α (Transgelin, TAGLN).6, 7 Further, several transcription factors regulate the cell marker switch, including Snail (SNAI1), Slug (SNAI2) and TWIST1.28-30 EnMT contributes to endothelial dysfunction and PA remodeling in PAH.6, 7 One key driver of EnMT is transforming growth factor-β (TGF-β) and in PAH, impaired bone morphogenetic protein receptor 2 (BMPR2) signaling promotes EnMT by shifting the signaling balance towards TGF-β and activation of High Mobility Group AT-hook 1 and Slug1.28, 29, 31-33 In addition, α3-integrin protects from programmed cell death, or apoptosis, in lung ECs.34
Integrin-β5 forms an integrin-ανβ5 heterodimer and binds to the ECM glycoprotein vitronectin, mediating cell adhesion.35 The knowledge of the role of integrin-β5 in ECs is limited so far to cell migration, cell growth, survival, and lung EC barrier function.36-40 Integrin-β5 contributes to TGF-β-induced mesenchymal transition in epithelial cells.41 However, there are no studies that investigate the function of integrin-β5 in pulmonary hypertension (PH). To ensure consistency with nomenclature, we will use the term “integrin-β5” when referring to the protein, the term “integrin-ανβ5” when referring to the heterodimer of integrin-αν and integrin-β5, the term “ITGB5” when referring to the human integrin-β5 gene, and the term “Itgb5” when referring to the rat integrin-β5 gene.
Clonal expansion of primitive, rat lung CD117+ ECs yields hyperproliferative, EnMT-prone EC clones, which induce occlusive PA remodeling and more severe PH after injection and chronic hypoxia exposure.4
We hypothesized that PA lesions from PAH patients and PH animal models show increased expression of integrin-β5 in ECs and that the augmented integrin-β5 expression contributes to survival and EnMT in ECs. In our experiments, we found increased expression of integrin-β5 in lung tissue from PAH patients and in PA lesions of the chronic hypoxia and CD117+ EC clone model, as well as in cultured CD117+ EC clones. A neutralizing antibody targeting integrin-ανβ5 induced EC dysfunction as shown by reduced migration and enhanced apoptosis and partially affected TGF-β1-induced EnMT gene expression in vitro. In vivo, integrin-ανβ5 inhibition exaggerated PH induced by chronic hypoxia and CD117+ EC clones.
MATERIALS AND METHODS
Human tissue samples
Deidentified human lung tissue samples originated from the Department of Pathology, University of Colorado, Denver. These sources prepared 5 μm sections of formalin-fixed and paraffin-embedded lung tissue samples. The local institutional research ethics board approved the collection of human tissue samples at the University of Colorado Denver in conformity with ethical guidelines of the Declaration of Helsinki of 1975, as revised in 1983. The review board waived informed consent. The Office of Research Subjects Protection at Virginia Commonwealth University and The Ohio State University approved using deidentified tissue samples as nonhuman subjects research.
Animal experiments
The Ohio State University Institutional animal care and utilization committee approved all animal experiments (protocol #2019A00000092). Performance of the experiments complied with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, 2011). Chronic hypoxia (cHx), and cHx + CD117+ EC clone rat PH models and normoxia controls and normoxia + CD117+ EC clone control groups were employed as published previously and lung tissue was used from previously published animals.4 In brief, the chronic hypoxia + EC clone rat PH model was induced by intravenous (tail vein) administration of 1 × 106 CD117+ rat lung quaternary EC clones, followed by exposure to 21 days of chronic hypoxia. We have provided a detailed characterization of the animals whose tissue we used in this study in.4 For the integrin-ανβ5 inhibition experiment, cHx + CD117+ EC clone rat model was induced as described above and reported in.4 For integrin-ανβ5 inhibition, rats were administered a neutralizing anti-integrin-ανβ5 antibody (MAB2528, R&D Systems) or an isotype control antibody (MAB002) at a dose of 100 μg/kg three times a week by intraperitoneal injection during the 21 day chronic hypoxia exposure. Lung tissue was harvested after euthanasia by exsanguination at the indicated time points under anesthesia with Ketamine (100 mg/kg) and Xylazine (15 mg/kg) (Henry Schein) following invasive hemodynamic measurements using a 1.4 F Millar catheter and Powerlab acquisition system (AD Instruments) and echocardiography using a GE Vivid IQ Premium system (GE Healthcare) similar to.4, 42 We have described previously the preparation of histology.4, 42, 43
Histology
Paraffin-embedded and formalin-fixed rat lung tissue was sectioned at a thickness of 3 μm. Serial sections of human lung tissue were obtained from the Department of Pathology, University of Colorado Denver and the PHBI. We previously published the details of immunohistochemistry (IHC) using heat-induced antigen retrieval in citrate buffer at pH 6.0.4, 42, 44 The following primary antibody was used: Integrin β5 (sc-390186, Santa Cruz Biotechnology). A secondary antibody conjugated to biotin, followed by horseradish peroxidase conjugated streptavidin and 3,3'-diaminobenzidine staining reaction, were used for IHC detection. Controls with unspecific Immunoglobin G were run in parallel with each staining batch. Images were taken with an AXIO imager.A1 microscope, Axiocam HRc camera and Axiovision software (all Zeiss). Slides for anti-integrin-ανβ5 antibody treated rats were sectioned, stained and scanned by Histowiz.
Quantitative real-time PCR (real-time polymerase chain reaction)
Messenger RNA extraction followed previously published protocols using the miRNeasy Mini Kit according to manufacturer's instructions, followed by DNAse I treatment and random hexamer primer-based reverse transcriptase reaction.4, 42, 43
For quantitative RT-PCR, the following QuantiTect Primer Assays (Qiagen) or KiCqStart SYBR green qPCR primer assays (Sigma-Aldrich) were used: Rattus norvegicus: Itgb5 (forward: FR1_Itgb5_1, reverse: RR_Itgb5_1), B2m (QT00176295), Vwf (QT01588713), Cnn1 (QT0181115), Tagln (QT00188769), Twist1 (QT01290149), Snai1 (QT00380331), Serpine1 (QT00189994); Homo sapiens: ITGB5 (forward: FH1_ITGB5, reverse: RH1_ITGB5), VCAM1 (QT00018347), PECAM1 (forward: FH1_PECAM1, reverse: RH1_PECAM1), ACTA2 (forward: FH1_ACTA2, reverse: RH1_ACTA2), SNAI1 I (forward: FH1_SNAI1, reverse: RH1_SNAI1), B2M (QT00088935). The amplification was performed using BioRad CFX 384 (BioRad) using the SYBR green master mix (BioRad). The cycling conditions were previously published4, 42, 43 and used as follows: Preincubation for 15 min at 95°C, then Amplification (45 cycles with 15 s at 94°C, 30 s at 55°C and 30 s at 72°C each). The values were calculated according to the mathematical model published by Pfaffl M by normalization against B2m (rat) and B2M (human) as housekeeping gene.45 Values were expressed as n-fold of control samples.
Protein isolation and Western blot
Protein lysates from human lung microvascular endothelial cells (HLMVECs) were prepared in radioimmunoprecipitation assay (RIPA) buffer (Millipore Sigma) as previously published.4, 42 In brief, cells were scraped off in RIPA buffer on ice, then incubated on shaker for 30 min. at 4°C, followed by centrifugration at 13,000g for 15 min. Supernatants were aliquoted and protein quantification was performed using BioRad Protein Assay DC-2 kit (5000112). Gel electrophoresis and Western blot were prepared as published previously4, 42 and the following antibodies were used for analysis: β-actin (loading control, Millipore Sigma; A5441), vascular cell adhesion molecule-1 (VCAM1, Abcam; ab1067777), α-smooth muscle antigen (α-SMA, Agilent; M085129), SM22α (Cell Signaling Technologies; #4047) and SNAIL1 (Cell Signaling Technologies, #3879). Data were analyzed using band intensity with background subtraction in Image Studio (BioRad). Data were normalized versus β-actin (loading control) and expressed as n-fold of control antibody.
Isolation of rat lung endothelial cells (ECs)
Rat lung CD117- control ECs and rat lung CD117+ ECs were isolated as published previously.42, 43 Four generation of clonal expansion of CD117+ ECs was published previously.4 Rat lung EC lines used in this study were early passage2-5 and their characterization was previously published.4 The experiments shown in this study were performed at the same time and with the same cell lines as the previously published study.
Culture of HLMVECs
As published previously,43 HLMVECs were obtained from Lonza clonetics (CC-2527) and cultured in EGM-2MV (Lonza). HLMVECs were used in passages 3–7.
Inhibition of integrin ανβ5 and stimulation with TGF-β1
The ECs were treated with 5 ng/ml recombinant human (rh) TGF-β1 (10 ng/ml, 240-B, R&D Systems) and/or a neutralizing 5–10 μg/ml anti-integrin ανβ5 antibody (MAB2528; R&D Systems) for 72 h. Controls were vehicle for TGF-β1 (PBS with 4 mM HCl and 1 mg/ml bovine serum albumin) and a control isotype antibody (MAB002; R&D Systems).
Gene array analysis
For the gene array data, we have used previous data derived from CD117- ECs and fourth generation CD117+ EC clones which is available online at NCBI Gene Expression Omnibus (GEO), accession number GSE198151. RNA isolation and microarray preparation were described in publication.4
In vitro functional assays
Apoptosis assay
ECs were treated with 10 ng/ml rhTGF-β1 (240-B; R&D Systems)/vehicle and/or 5–10 μg/ml anti-integrin ανβ5 antibody (MAB2528; R&D Systems)46 or control isotype antibody (MAB002; R&D Systems) for 24 h. Apoptosis was detected by Annexin V staining and labeling with 7-Amino-Actinomycin (7-AAD) as published previously.42, 43 The staining was analyzed using flow cytometry in FACS Canto flow cytometer located in the VCU flow cytometry core and FlowJo software.
Migration assay
We measured cell migration using a scratch wound assay like previously published protocols.43 In brief, we grew HLMVECs to confluence in 96 well plates. For the scratch assay, we applied a linear wound using a sterile 20 μl pipet tip. The cells were treated with rhTGF-β1 or vehicle, and/or anti-integrin ανβ5 antibody or isotype control (see apoptosis assay). Differential interference contrast (DIC) Images of the scratch injury were taken at 0, 4, 8, and 24 h with an inverted microscope (Olympus IX70 microscope and Olympus XM10 camera with cellSens Dimension software [all Olympus]). Measurements were performed using Fiji software.47 Analysis was done according to protocol modified from Yue et al.47
Measuring barrier function of EC clones using transendothelial electrical resistance (TEER)
We coated the 96W10idf Polyethylene terephthalate (PET) plates (Applied Biophysics) with 0.05 mg/ml rat tail collagen overnight at 4°C. We plated 200,000 cells/well for confluency. Cell attachment and cell-cell junction resistances were quantified using electric cell-substrate impedance sensing according to manufacturer instructions (ECIS, Applied Biophysics). Resistance is reported at low frequencies (250 Hz) to quantify cell-cell junctions.
Statistical analysis
Data are presented as mean + SEM. We compared two Groups with t-test or Mann–Whitney test (non-parametric data). For multiple groups, we used ANOVA or Kruskal–Wallis (non-parametric) test followed by multiple comparison according to Dunn, Holm-Šidák or Benjamin, Krieger and Yekutieli (>2 groups). Normal distribution of data was evaluated with the Shapiro–Wilk test. Statistical analysis of microarray data has been described previously.4 Statistical analysis and graphs were done with GraphPad Prism 8.0 (GraphPad Software). p < 0.05 was considered significant.
RESULTS
Expression of Integrin-β5 in human and experimental PH
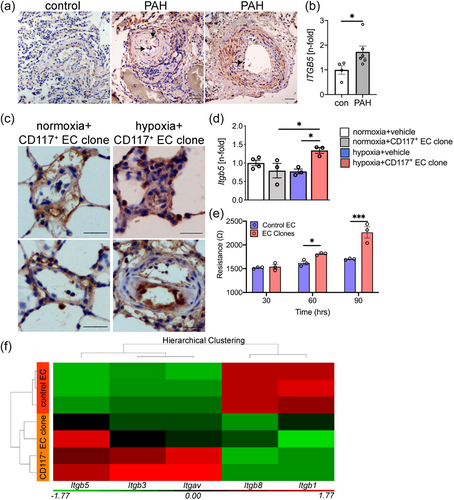
To determine the expression pattern of integrin-β5 in the pulmonary vasculature from patients with PAH, we used immunohistochemistry to identify integrin-β5 expression. First, we studied integrin-β5 expression in lung tissue sections from controls and PAH patients. We found increased integrin-β5 staining in PAs from PAH patients and elevated mRNA expression of ITGB5 (integrin-β5) in lung tissue from patients with PAH (Figure 1). Then, we tested the expression of integrin-β5 in the cHx + CD117+ EC clone model and found that expression of integrin-β5 was elevated in lumen-obliterating and endothelial cells in PA lesions from cHx + CD117+ EC clone rats (Figure 1). In addition, we found that Itgb5 mRNA expression was elevated in cHx + CD117+ EC clone rats, but not in normoxia, normoxia + CD117+ EC clone, and cHx alone rats (Figure 1). To identify a potential relationship between increased Itgb5 expression in CD117+ EC clones and EC function, we tested if CD117+ EC clones and control ECs exhibit differences in barrier function using TEER in vitro. We found increased resistance in CD117+ EC clones, which indicates accelerated development of the endothelial barrier (Figure 1). We then tested using an existing gene array data set [published in4], if rat lung CD117+ EC clones have changes in mRNA expression of integrins and found elevated expression of Itgb3, Itgb5, and Itgav, while the expression of Itgb1 and Itgb8 were reduced. However, the most consistently upregulated integrin was Itgb5 (Figure 1).
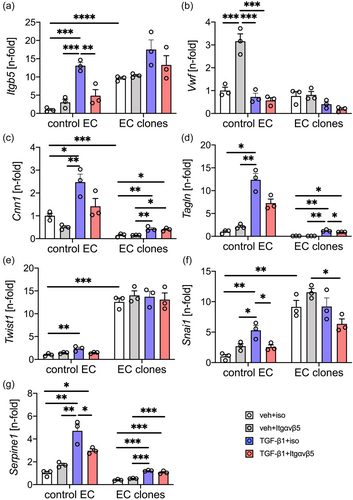
Inhibition of integrin-ανβ5 partially protects from TGF-β1 induced changes in mRNA expression, but not in protein expression
To identify if integrin-β5 contributes to TGF-β1 induced EnMT-like gene expression, we treated control ECs and CD117+ EC clones with TGF-β1 and/or a neutralizing antibody targeting integrin ανβ5. First, we tested if inhibition of integrin-ανβ5 induces changes in mRNA expression in control ECs and CD117+ EC clones. Blocking integrin-ανβ5 only causes nonsignificant changes in gene expression, except for a several-fold induction of Vwf expression in control ECs (Figure 2). In control ECs, TGF-β induced the expression of Itgb5, the mesenchymal markers Cnn1 and Tagln, the EnMT transcription factors Twist1 and Snai1, and the TGF-β1 downstream target Serpine1 (Figure 2). Similarly, in CD117+ EC clones, TGF-β1 induced mRNA expression of Cnn1, Tagln and Serpine1, but left the expression of Itgb5, Vwf, Twist1 and Snai1 unchanged (Figure 2). Further, we found a higher level of Itgb5, Twist1 and Snai1 in vehicle + isotype antibody treated CD117+ EC clones compared to vehicle + isotype antibody treated control ECs (Figure 2). Neutralization of integrin-ανβ5 induced expression of Vwf in control ECs without TGF-β1 (Figure 2). Blocking integrin-ανβ5 reduced TGF-β1 induced Itgb5, Snai1 and Serpine1 expression in control ECs, showing a trend towards reduced TGF-β1-induced expression of Cnn1, Tagln and Twist1. In CD117+ EC clones, TGF-β1-induced expression of Tagln, but not of Cnn1 and Serpine1 was decreased by inhibition of integrin-ανβ5.
In another set of experiments, we found in HLMVECs that TGF-β1 treatment reduced mRNA expression of VCAM1 and the EC marker PECAM1, and increased the expression of the mesenchymal marker ACTA2 (Figure 3). Inhibition of integrin-ανβ5 increased mRNA expression of ITGB5 and SNAI1 in the presence of TGF-β1, but reduced expression of ACTA2 compared to TGF-β1 + control antibody (Figure 3). Using immunoblots, we found a similar trend in α-SMA protein expression when comparing TGF-β1 + control antibody treatment with TGF-β1 + anti-integrin-ανβ5 antibody treatment (Figure 3). Immunoblots further confirmed the increase in SNAIL1 protein expression with TGF-β1 and TGF-β1 + anti-integrin-ανβ5 antibody treatment (Figure 3). The SM22α protein level (corresponding protein to TAGLN gene) was increased by TGF-β1 treatment and remained elevated following TGF-β1 + anti-integrin-ανβ5 treatment (Figure 3). We found no effect of blocking integrin-ανβ5 on VCAM1 and PECAM1 mRNA expression and on VCAM1, SNAIL1, and SM22α protein expression (Figure 3).
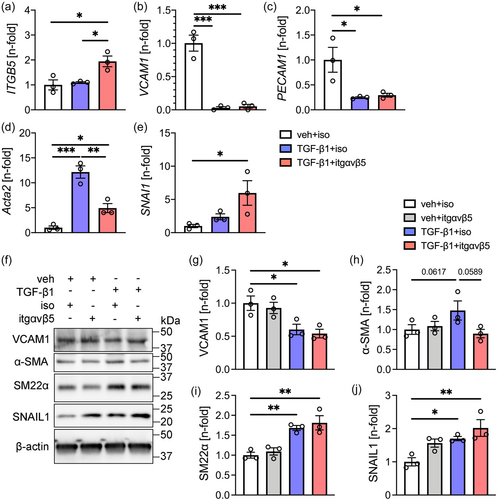
Overall, inhibition of integrin-ανβ5 partially prevented TGF-β1-induced upregulation of mesenchymal marker mRNA expression but had no effect on the downregulation of EC markers in the three cell lines studied.
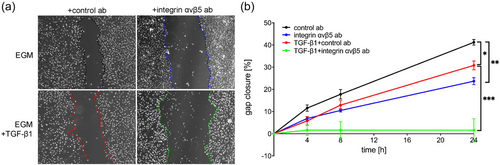
Integrin-ανβ5 is required for migration and survival of ECs
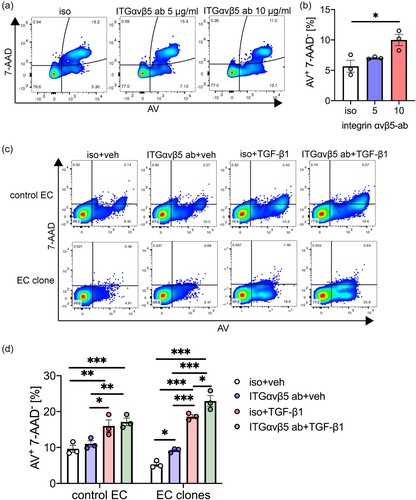
To determine the functional consequences of inhibition of integrin-ανβ5 in ECs, we tested the role of integrin-ανβ5 in EC migration using a scratch gap closure assay. In HLMVECs, we found that blocking integrin-ανβ5 reduced migration as measured by scratch wound assay (Figure 4). Further, recombinant TGF-β1 reduced gap closure and inhibition of integrin-ανβ5 almost completely abolished gap closure when combined with TGF-β1 (Figure 4). Then we tested if integrin-ανβ5 is required for EC survival. Blocking integrin-ανβ5 induced apoptosis as measured by the fraction of Annexin V+ 7-AAD− cells in HLMVECs (Figure 5). In control rat lung ECs, blocking integrin-ανβ5 did not induce apoptosis even in combination with TGF-β1. In CD117+ EC clones, which express higher levels of integrin-αν and integrin-β5 (Figure 1), baseline apoptosis was reduced compared to control ECs. Integrin-ανβ5 inhibition alone induced apoptosis in HLMVECs and CD117+ EC clones and exaggerated apoptosis in synergism with TGF-β1 in CD117+ EC clones.
Integrin-ανβ5 inhibition exaggerates PH
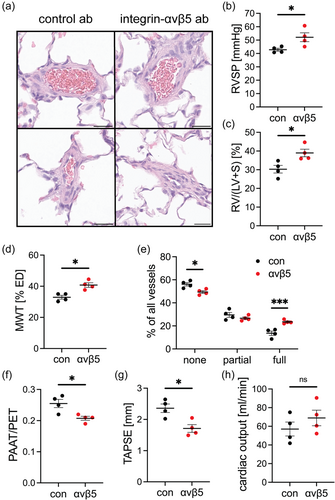
To test the role of integrin-ανβ5 in the development of PH in vivo, we treated rats following induction of PH by exposure to chronic hypoxia + EC clone with the neutralizing anti-integrin-ανβ5 antibody. We found that treatment with the anti-integrin-ανβ5 antibody during PH development exaggerated PH, Fulton index, pulmonary artery media wall thickness and pulmonary artery occlusion and reduced tricuspid annular plane systolic excursion and the ratio of pulmonary artery acceleration time versus pulmonary ejection time (PAAT/PET) (Figure 6). There was no change in the right ventricular cardiac output as estimated by echocardiography (Figure 6). These data indicate that treatment with anti-integrin-ανβ5 antibody amplified PA remodeling, RV hypertrophy and PH compared to control antibody treated PH rats.
DISCUSSION
PAH is a progressive devastating disease, and its main histological manifestation is an occlusive pulmonary arteriopathy.1 One important component is aberrant EC function, including apoptosis-resistance, unchecked proliferation, impaired migration, and metabolic dysregulation.1, 48 Further investigation is required to understand the mechanisms underlying both physiologic EC function and aberrant EC function in PAH. Here, we tested the hypothesis that the transmembrane protein integrin-β5 is required in ECs for migration and survival but also contributes to TGF-β1-induced EnMT.
Our main findings are: (1) integrin-β5 is increased in PA lesions and lung tissue from PAH patients and rats with cHx + CD117+ EC clone-induced PH; (2) Clonally expanded rat lung CD117+ ECs have higher expression of integrin-β5 and its heterodimerization partner integrin-αν; (3) Neutralization of integrin-ανβ5 had a partial effect on TGF-β1-induced EnMT mRNA expression; (4) Blocking integrin-ανβ5 reduces migration and promotes apoptosis; (5) Inhibition of integrin-ανβ5 in vivo exaggerates PH induced by cHx + CD117+ EC clones.
In PAH, many studies have shown the alteration of ECM components and their turnover and these studies indicate that ECM remodeling is an integral part of the PA remodeling.14, 49 One important question remains how changes in ECM composition affect vascular cell function and how altered vascular cell function leads to changes in ECM remodeling in PAH. Despite substantial progress, various aspects of the “outside-in” and “inside-out” signaling remain to be investigated. Integrins provide one central aspect to understand both “outside-in” and “inside-out” signaling, because integrins mediate such bidirectional signaling. Integrins not only show specificity for different ECM components, including fibronectin (β1, β3, β5) or vitronectin (β3, β5), but also activate downstream signaling via various growth factor receptors regulating cell function, including vascular endothelial growth factor receptor 2 (VEGFR2) for ανβ3, VEGFR3 for ανβ1, platelet derived growth factor receptor (PDGFR) for ανβ3, fibroblast growth factor receptor 3 (FGFR3) for ανβ1 and ανβ3 and TIE2 for ανβ1.23, 50 In endothelial biology, most studies have focused on the heterodimeric integrin-ανβ1 and integrin-ανβ3. The knowledge of the role of integrin-ανβ5 in EC biology is far more limited as it pertains to lung ECs and lung vascular disease. Integrin-ανβ1 has important functions in ECs for endothelial stabilization, barrier function, angiogenesis, mechanosensing and vascular patterning.51-53 Integrin-ανβ3 is the best studied integrin heterodimer in ECs and regulates central processes in ECs, including angiogenesis, cell survival, leukocyte adhesion and migration.24, 25, 34, 37, 39, 54, 55 In addition, we have shown that integrin-ανβ3 serves as a receptor for interleukin-32, which promotes aberrant angiogenesis in PAH.56
In this study, we focused on the role of integrin β5 and the integrin-ανβ5 heterodimer for lung EC function and PAH pathobiology. First, we studied the histological localization of integrin-β5 in lung tissue sections from human PAH patients and from rats with PH. We found upregulation of integrin-β5 expression in lung tissue, ECs and, to a lesser degree, in pulmonary artery smooth muscle cells in human PAH. Our group has recently shown that repeated clonal selection of primitive CD117+ ECs (derived from rat lungs) yields ECs with enhanced proliferative potential and that injection of these EC clones induces an occlusive pulmonary arteriopathy and more severe PH in chronic hypoxic rats.4 We found elevated integrin-β5 mRNA expression in the lungs of cHx + CD117+ EC clone rats. We confirmed increased integrin-β5 expression in occluding cells in PAs. Further, in our previously published gene array experiments, integrin-β5 was most consistently increased in CD117+ EC clones compared to control ECs. One potential explanation is that clonal selection of stem cell-like ECs upregulates integrin-β5 because enhanced integrin-β5 expression provides a survival advantage over ECs with lower integrin-β5 expression. Our new data support this concept because blocking integrin-ανβ5 using a neutralizing antibody promotes apoptosis in HLMVECs and rat lung CD117+ EC clones. Interestingly, rat lung control ECs have lower baseline integrin-β5 expression and in these ECs, inhibition of integrin-ανβ5 fails to induce apoptosis. In addition, blocking integrin-ανβ5 reduced migration and stimulation with TGF-β1 and inhibition of integrin-ανβ5 synergistically blocked endothelial migration. Previously published work has indeed shown that integrin-β5 protects ECs from apoptosis outside of the lung, such as in brain microvascular ECs, and that integrin-β5 mediates lung endothelial barrier permeability in animal models of acute lung injury.37, 39, 40 Integrin-ανβ5 and focal adhesion kinase form a signaling complex that mediates vascular permeability downstream of vascular endothelial growth factor.36 Interestingly, we found that CD117+ EC clones show accelerated endothelial barrier formation, which seems to imply a potential connection between endothelial barrier function and higher integrin-β5 expression. However, we can also explain this accelerated EC barrier development by the higher proliferation rate of CD117+ EC clones, as recently shown by us.4 Integrin-ανβ5 further promotes endothelial migration and angiogenic tube formation.57 Hence, integrin-ανβ5 supports survival and migration, and protects from apoptosis in lung ECs.
Besides apoptosis, EnMT contributes to pathologic PA remodeling and endothelial dysfunction.6, 7 We have previously shown that clonal expansion of rat lung CD117+ ECs gives rise to ECs that are prone to undergo EnMT in vitro and in vivo after injection to rats and exposure to cHx.4 We found in our current studies that inhibition of integrin-ανβ5 had a limited effect on TGF-β-induced EnMT, mainly by preventing the increase in mRNA expression of mesenchymal markers. Further, clonally selected rat lung CD117+ lung ECs had elevated baseline expression of Snai1 and Twist1, and inhibition of integrin-ανβ5 reduced Snai1, but not Twist1. These data provide one explanation for our previous findings that these clonal ECs are prone to undergoing EnMT.4 In contrast to integrin-ανβ1 or integrin-ανβ3, there is no knowledge about a potential role of integrin-ανβ5 in the mesenchymal transition of ECs. In mammary epithelial cells, integrin-ανβ5 knockdown reduces TGF-β-induced mesenchymal transition by reducing cell adhesion and integrin signaling without affecting downregulation of epithelial cadherin expression and upregulation of TGF-β-inducible genes.41 In addition, integrin-ανβ5 and -ανβ3 promote activation of latent TGF-β1 by contraction of fibroblasts in the heart and thereby contribute to differentiation of cardiac myofibroblasts.58
While the increased expression of integrin-β5 in lung tissue from PAH patients suggest targeting integrin-ανβ5 function in PAH patients as a potential therapeutic target, our in vivo data demonstrate that blocking integrin-ανβ5 exaggerates PH substantially. Our in vitro data indicate that integrin-ανβ5 inhibition promotes apoptosis and impairs migration in ECs, hence inhibition of integrin-ανβ5 cripples physiologic endothelial function. Crippled EC function is a known driver of pulmonary vascular remodeling and PH.48 Based on our data it also appears that integrin-ανβ5 has a limited role in EnMT but an important homeostatic role in physiologic EC function. Indeed, integrin-β5 has been shown to support pro-survival and proliferative pathways including signal transducer and activator of transcription 3 (STAT3) and Src.59 In addition, integrin-β5 mediates some of the downstream effects of VEGF and inhibition of VEGF signaling is known to induce severe PH and occlusive arteriopathy when combined with hypoxia.36, 60 The elevated expression of integrin-β5 in human PAH and the cHx + CD117+ EC clone model could be interpreted as endogenous activation of pro-survival mechanisms in an injured and dysfunction pulmonary vasculature. Hence, inhibition of integrin-ανβ5 during PH development could trigger EC apoptosis and dysfunction and hence worsens PH, which is a potential explanation for our in vivo findings. Multiple drugs are available for inhibiting other integrins, such as β1 and β3,61, 62 yet the use of such inhibitory drugs may not be useful for PAH, because the expression of integrin-β1 and integrin-β3 is already reduced in the pulmonary arteries of cHx- and monocrotaline-treated rats.63
There are however limitations to our current study. First, we have not shown the role of integrin-β5 using gene manipulation in ECs. Because a neutralizing anti-integrin-ανβ5 antibody was available, we focused on inhibiting integrin-ανβ5 function rather than silencing integrin-ανβ5 expression due to the potential translational relevance. Second, we have evaluated the potential implications of blocking integrin-ανβ5 in our cHx + CD117+ EC clone animal model of PH. Because of the lack of elevation of Itgb5 without hypoxia and the need to limit animal experimentation, treatment with anti-integrin-ανβ5 and isotype antibody was only performed in the chronic hypoxia + CD117+ EC clone model. Lastly, because the cHx + CD117+ EC clone model is reversible after cessation of hypoxia as reported by us in,4 we were not able to identify if inhibition of integrin-ανβ5 exaggerates or reduces established PH.
In summary, we found a role for integrin-ανβ5 in lung endothelial survival and migration, but also a partial contribution to TGF-β1-induced EnMT gene expression. Our results of increased integrin-β5 expression in ECs in PA lesions from PAH patients and a rat PH model and amplified PH development following inhibition of integrin-ανβ5 suggest that integrin-ανβ5 is required for physiologic function of ECs and lung vascular homeostasis.
AUTHOR CONTRIBUTIONS
Neil Blanchard, Daniela Farkas, Carlyne D. Cool, Robert Freishtat, and Laszlo Farkas conceived and designed the study. Neil Blanchard, Patrick A. Link, Daniela Farkas, Brennan Harmon, Jaylen Hudson, Srimathi Bogamuwa, Bryce Piper, Kayla Authelet, Carlyne D. Cool, Rebecca L. Heise, Robert Freishtat, Laszlo Farkas performed experiments, data analysis, and interpretation. Neil Blanchard, Patrick A. Link, Daniela Farkas, Brennan Harmon, Kayla Authelet, Carlyne D. Cool, Robert Freishtat, and Laszlo Farkas generated figures and wrote the manuscript. All authors edited and approved the final manuscript.
ACKNOWLEDGMENT
The study was funded by grants from the National Institutes of Health to L.F. (HL123044, HL139881). Services and products in support of the research project were generated by the VCU Massey Cancer Center Flow Cytometry Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. The opinions expressed in this manuscript do not necessarily represent the opinion of the NIH.
CONFLICT OF INTEREST
The authors declares no conflict of interest.
ETHICS STATEMENT
The local institutional research ethics board approved the collection of human tissue samples at the University of Colorado Denver in conformity with ethical guidelines of the Declaration of Helsinki of 1975, as revised in 1983. The review board waived informed consent. The Office of Research Subjects Protection at Virginia Commonwealth University and The Ohio State University approved using deidentified tissue samples as nonhuman subjects research. The Ohio State University Institutional animal care and utilization committee approved all animal experiments (protocol #2019A00000092). Performance of the experiments complied with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, 2011).
Open Research
DATA AVAILABILITY STATEMENT
Data files are available at Open Science Foundation https://osf.io/t9cvd/?view_only=5ba36fe963584256bc8aea9180a9233f
Gene array data have been deposited to NCBI GEO accession number GSE198151.