Clinical characteristics and outcomes in pulmonary manifestations of systemic sclerosis: Contribution from pulmonary hypertension and interstitial lung disease severity
Ruchika A. Sangani and Justin K. Lui contributed equally to this study.
Abstract
Patients with systemic sclerosis complicated by both pulmonary hypertension (SSc-PH) and interstitial lung disease (SSc-PH-ILD) have poor prognosis compared to those with SSc-PH or SSc-ILD alone. Little is known of how ILD severity affects outcomes in those with SSc-PH, or how PH severity affects outcomes in those with SSc-ILD. Herein, we aimed to delineate clinical features of patients with SSc-PH and SSc-ILD and determine to what degree PH and ILD severity contribute to mortality in patients with SSc. We conducted parallel retrospective studies in cohorts of patients with SSc-PH and SSc-ILD. We categorized ILD severity by pulmonary function testing and PH severity by cardiopulmonary hemodynamics. Our primary outcome was all-cause mortality from time of PH or ILD diagnosis for the SSc-PH and SSc-ILD cohorts, respectively. We calculated adjusted risks of time to all-cause mortality using Cox proportional hazards models. In patients with SSc-PH, severe ILD (HR: 3.54; 95% CI: 1.05, 11.99) was associated with increased hazards for all-cause mortality. By contrast, mild and moderate ILD were not associated with increased mortality risk. In patients with SSc-ILD, both moderate (HR: 2.65; 95% CI: 1.12, 6.31) and severe PH (HR: 6.60; 95% CI: 2.98, 14.61) were associated with increased hazards for all-cause mortality, while mild PH was not. Through our parallel study design, the risk of all-cause mortality increases as severity of concomitant ILD or PH worsens. Therapies that target slowing disease progression earlier in the disease course may be beneficial.
INTRODUCTION
Systemic sclerosis (SSc) is a multisystem, autoimmune disease characterized by three principal features: (1) excessive collagen production and deposition causing fibrosis of the skin and internal organs; (2) endothelial dysfunction that promotes vascular damage; and (3) immunologic aberrations.1 While SSc can affect any organ, pulmonary manifestations including interstitial lung disease (ILD) and pulmonary hypertension (PH) comprise the leading causes of death, accounting for 33% and 28% of mortality, respectively.2 SSc-related PH (SSc-PH), in particular, is highly heterogeneous due to the diversity of clinical phenotypes.3 Patients with SSc are at risk for developing Group 1 pulmonary arterial hypertension (PAH),4 Group 2 PH typically due to left ventricular fibrosis and diastolic dysfunction,5 Group 3 PH due to ILD and chronic hypoxia,6 and potentially, Group 4 PH secondary to chronic thromboembolic disease from the observed increased risk of venous thromboembolism.7 Delineating the contribution of each PH phenotype is often challenging. As ILD is the most common pulmonary manifestation in SSc, occurring in nearly 40% of patients, there is often significant overlap with PH.2
Prior studies have shown that PH severity is not linked to the presence of ILD in SSc,8 although prognosis in patients with SSc and concomitant ILD and PH is far worse than in those with ILD or PH alone.9, 10 Furthermore, in other forms of ILD, such as idiopathic pulmonary fibrosis, PH has been recognized as a prognostic indicator for mortality.11-13 However, in SSc, it is unclear the degree to which PH severity affects outcomes in those with SSc-ILD or the degree to which ILD severity affects outcomes in those with SSc-PH. The goals of this observational study were to: (1) delineate the clinical characteristics of patients with SSc and its pulmonary manifestations; (2) determine to what degree PH severity contributes to mortality in patients with SSc-ILD; and (3) determine to what degree ILD severity contributes to mortality in patients with SSc-PH. We hypothesized that for patients with SSc-ILD, worsening PH severity increases mortality risk with those with severe PH having the highest risk, and, similarly, for patients with SSc-PH, worsening ILD severity increases mortality risk with severe ILD having the highest risk.
METHODS
Study population
- (1)
Pre-capillary PH: mean pulmonary artery pressure (mPAP) > 20 mmHg, pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg, pulmonary vascular resistance (PVR) ≥ 3 Wood units.
- (2)
Isolated post-capillary PH: mPAP > 20 mmHg, PAWP > 15 mmHg, PVR < 3 Wood units.
- (3)
Combined pre- and post-capillary PH: mPAP > 20 mmHg, PAWP > 15 mmHg, PVR ≥ 3 Wood units.
PH severity was categorized by mPAP as mild (mPAP > 20 and <25 mmHg), moderate (mPAP ≥ 25 and <35mmHg), and severe (mPAP ≥ 35 mmHg).14 Patients with isolated post-capillary PH were excluded from the present study. ILD was defined by the following criteria at any point during the patient's clinical course:
- (1)
Any of the following chest CT findings:
- (a)
Ground-glass/reticular opacities
- (b)
Bronchiectasis
- (c)
Interlobular septal/subpleural thickening
- (d)
Honeycombing15
- (a)
- (2)
Confirmation of ILD diagnosis by primary rheumatologist/pulmonologist.
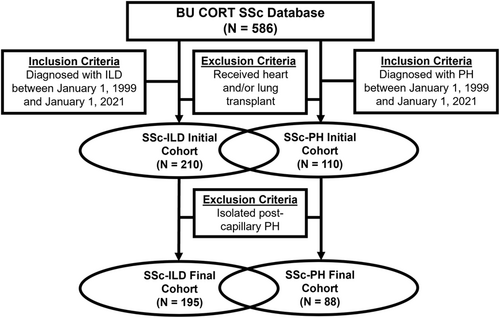
ILD severity was sub-classified by forced vital capacity (FVC) on pulmonary function testing as mild (FVC ≥ 85% predicted), moderate (FVC ≥ 45% predicted and <85% predicted), or severe (FVC < 45% predicted).16 We excluded patients with a history of heart and/or lung transplantation as this may have impacted interpretability of the results. Herein, we utilized a parallel study design that examined two overlapping cohorts of (1) patients with SSc-ILD and (2) patients with SSc-PH (Figure 1).
Clinical data and outcomes
The primary outcome was all-cause mortality from the time of ILD or PH diagnosis for the SSc-ILD and SSc-PH cohorts, respectively. In all patients with SSc, the following data were collected: (1) demographics; (2) overlapping autoimmune disease; (3) comorbid conditions; (4) autoantibody positivity (i.e., antinuclear antibody, anti-topoisomerase I antibody [Scl-70]); (5) degree of skin disease (modified Rodnan skin score [mRSS]); and (6) pulmonary function testing (i.e., FVC and diffusing capacity for carbon monoxide [DLCO]). Additionally, for patients in the SSc-ILD cohort, radiologic findings on chest CT and initial ILD therapy were compiled. For patients in the SSc-PH cohort, the following data were collected: (1) serum brain natriuretic peptide (BNP) levels; (2) New York Heart Association (NYHA) functional classification; (3) initial PAH-specific therapy; and (4) cardiopulmonary hemodynamics including right atrial pressure (RAP), mPAP, PAWP, PVR and cardiac output (CO) from RHC at the time of PH diagnosis (or the most proximal RHC to the time of PH diagnosis if the RHC at diagnosis was unavailable).
Statistical analysis
Continuous variables were described by the mean (± standard deviation), and categorical variables were described by their frequencies and percentages for each group. Univariable and multivariable Cox proportional hazard regression models were applied to determine associations with all-cause mortality in the SSc-ILD and SSc-PH cohorts. Within the SSc-ILD cohort, we ascertained the additive effect of PH severity to all-cause mortality as a time-varying covariate while adjusting for FVC and DLCO. Within the SSc-PH cohort, we ascertained the additive effect of ILD severity to all-cause mortality as a time-varying covariate while adjusting for mPAP, PVR, and PAWP. Additionally, both models were adjusted for the following fixed variables, defined at the time of ILD/PH diagnosis: (1) age; (2) sex; and (3) diffuse cutaneous SSc. Patients with missing data for any covariate used in the model were excluded from the Cox analysis. All covariates were assessed for proportional hazards assumption by Schoenfeld residuals. To verify the reliability of hazard ratio estimates and their confidence intervals considering the small sample size, estimates were also generated through a bootstrapped Cox proportional hazards model that resampled the data 1000 times with replacement. Finally, a sensitivity analysis was conducted using a Cox proportional hazard regression model excluding patients who were lost to follow-up to determine whether this was informative about mortality. Statistical analyses were conducted in RStudio® (Boston, MA) using the survival package.
RESULTS
Contribution of PH severity on SSc-ILD outcomes
Study population and clinical characteristics
Within the SSc-ILD cohort (n = 195) (Figure 1), there were 51 (26.2%) patients who had concomitant PH. Of these, 5 (9.8%) had mild PH, 26 (51.0%) had moderate PH, and 20 (39.2%) had severe PH. Patient demographics and baseline clinical characteristics are summarized in Table 1. Compared to those without PH, there was a greater frequency of males amongst those with SSc-ILD and concomitant PH (31.4% vs. 18.1%). Furthermore, patients with SSc-ILD and concomitant PH developed ILD at a later age in each of the mild, moderate, and severe PH groups compared to those without PH. The frequency of diffuse cutaneous SSc (35.3% vs. 44.4%) and anti-Scl-70 positivity (9.8% vs. 50.0%) was lower in patients with SSc-ILD and concomitant PH compared to those without PH. Chest CT findings were similar regardless of the presence of PH with ground-glass and/or reticular opacities observed most commonly, followed by bronchiectasis, interlobular septal and/or subpleural thickening, and honeycombing. The most frequently prescribed initial ILD therapy was cyclophosphamide (40.5%), followed by mycophenolate (30.3%).
| No PH (N = 144) | Mild PH (N = 5) | Moderate PH (N = 26) | Severe PH (N = 20) | |
|---|---|---|---|---|
| Demographics | ||||
| Age at ILD diagnosis, years (mean ± SD) | 50.9 ± 12.2 | 57.8 ± 12.6 | 54.0 ± 12.1 | 56.2 ± 11.9 |
| Male sex, n (%) | 26 (18.1%) | 2 (40.0%) | 8 (30.8%) | 6 (30.0%) |
| Diffuse cutaneous SSc, n (%) | 64 (44.4%) | 2 (40.0%) | 8 (30.8%) | 8 (40.0%) |
| Former/current smoker, n (%) | 68 (47.2%) | 3 (60.0%) | 12 (46.2%) | 12 (60.0%) |
| Overlapping autoimmune disease | ||||
| Sjogren's syndrome, n (%) | 15 (10.4%) | 0 | 4 (15.4%) | 1 (5.0%) |
| Systemic lupus erythematosus, n (%) | 4 (2.8%) | 0 | 3 (11.5%) | 0 |
| Dermatomyositis/polymyositis, n (%) | 4 (2.8%) | 0 | 2 (7.7%) | 0 |
| Rheumatoid arthritis, n (%) | 7 (4.9%) | 0 | 1 (3.8%) | 1 (5.0%) |
| Comorbid conditions | ||||
| Obstructive lung disease, n (%) | 11 (7.6%) | 1 (20.0%) | 3 (11.5%) | 1 (5.0%) |
| Coronary artery disease, n (%) | 7 (4.9%) | 1 (20.0%) | 2 (7.7%) | 2 (10.0%) |
| Atrial fibrillation/atrial flutter, n (%) | 16 (11.1%) | 0 | 3 (11.5%) | 3 (15.0%) |
| Venous thromboembolism, n (%) | 12 (8.3%) | 0 | 5 (19.2%) | 1 (5.0%) |
| Hypertension, n (%) | 32 (22.2%) | 2 (40.0%) | 11 (42.3%) | 10 (50.0%) |
| Chronic kidney disease, n (%) | 4 (2.8%) | 0 | 0 | 2 (10.0%) |
| Antinuclear antibody | ||||
| ANA, number (%)a | 109 (91.6%) | 2 (100%) | 14 (87.5%) | 15 (88.2%) |
| Antitopoisomerase/antibody | ||||
| Anti-Scl-70 antibody, number (%)b | 51 (50.0%) | 1 (50.0%) | 2 (15.4%) | 2 (16.7%) |
| Degree of skin disease | ||||
| Modified Rodnan skin score (mean ± SD)c | 12.7 ± 12.0 | 15.3 ± 11.6 | 6.7 ± 5.6 | 14.9 ± 15.9 |
| Pulmonary function testing | ||||
| FVC, % predicted (mean ± SD) | 76.5 ± 17.1 | 70.8 ± 17.3 | 70.3 ± 18.8 | 70.1 ± 18.7 |
| DLCO, % predicted (mean ± SD) | 58.7 ± 18.6 | 45.8 ± 17.0 | 43.7 ± 17.1 | 40.7 ± 16.7 |
| Chest CT findings | ||||
| Ground-glass and/or reticular opacities, n (%) | 111 (77.1%) | 4 (80.0%) | 17 (65.4%) | 16 (80.0%) |
| Bronchiectasis, n (%) | 60 (41.7%) | 1 (20.0%) | 14 (53.8%) | 9 (45.0%) |
| Interlobular septal and/or subpleural thickening, n (%) | 44 (30.6%) | 0 | 10 (38.5%) | 10 (50.0%) |
| Honeycombing, n (%) | 34 (23.6%) | 0 | 12 (46.2%) | 4 (20.0%) |
| Initial ILD therapy | ||||
| Cyclophosphamide, n (%) | 57 (39.6%) | 5 (100%) | 11 (42.3%) | 6 (30.0%) |
| Mycophenolate, n (%) | 44 (30.6%) | 0 | 6 (23.1%) | 9 (45.0%) |
| Azathioprine, n (%) | 3 (2.1%) | 0 | 2 (7.7%) | 0 |
| Pirfenidone, n (%) | 1 (0.7%) | 0 | 0 | 0 |
| Nintedanib, n (%) | 0 | 0 | 1 (3.8%) | 0 |
- Abbreviations: DLCO, diffusing capacity for carbon monoxide; FVC, forced vital capacity; ILD, interstitial lung disease; PH, pulmonary hypertension; SSc, systemic sclerosis.
- a No PH: N = 119, mild PH: N = 2, moderate PH: N = 16, severe PH: N = 17.
- b No PH: N = 102, mild PH: N = 2, moderate PH: N = 13, severe PH: N = 12.
- c No PH: N = 118, mild PH: N = 4, moderate PH: N = 17, severe PH: N = 14.
Associations with all-cause mortality
In both the univariable and multivariable Cox models, male sex was significantly associated with increased hazards for mortality. When adjusted for age at ILD diagnosis, male sex, and pulmonary function testing, both moderate (HR: 2.65; 95% CI: 1.12, 6.31) and severe PH (HR: 6.60; 95% CI: 2.98, 14.61) were significantly associated with an increased hazard ratio for all-cause mortality (Table 2). The adjusted bootstrapped HRs for moderate and severe PH were 2.96 (95% CI: 1.32, 6.98) and 8.33 (95% CI: 3.60, 20.70), respectively. A sensitivity analysis in which those who were lost to follow-up were excluded resulted in an adjusted HR of 3.02 (95% CI: 1.21, 7.52) for moderate PH and 6.12 (95% CI: 2.74, 13.68) for severe PH. The median duration of follow-up was 42.1 months with 43 events. The median of the maximum time between clinical variables for each patient was 18.5 months.
| Univariable | Multivariable | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Age at ILD diagnosis, years | 1.02 (1.00, 1.05) | 1.01 (0.98, 1.04) |
| Male sex | 3.01 (1.62, 5.61) | 2.28 (1.16, 4.50) |
| Diffuse cutaneous SSc | 1.51 (0.83, 2.73) | 1.36 (0.72, 2.56) |
| FVC, % predicted | 0.98 (0.96, 0.99) | 0.99 (0.97, 1.02) |
| DLCO, % predicted | 0.96 (0.94, 0.98) | 0.98 (0.96, 1.01) |
| PH severity | ||
| Mild PH | 4.29 (0.57, 32.07) | 2.67 (0.35, 20.39) |
| Moderate PH | 4.04 (1.79, 9.16) | 2.65 (1.12, 6.31) |
| Severe PH | 10.76 (5.39, 21.50) | 6.60 (2.98, 14.61) |
- Abbreviations: DLCO, diffusing capacity for carbon monoxide; FVC, forced vital capacity; ILD, interstitial lung disease; PH, pulmonary hypertension; SSc, systemic sclerosis.
Contribution of ILD severity on SSc-PH outcomes
Study population and clinical characteristics
Within the SSc-PH cohort (n = 88) (Figure 1), there were 51 (58.0%) patients who had concomitant ILD. Of these, 9 (17.6%) had mild ILD, 35 (68.6%) had moderate ILD, and 7 (13.7%) had severe ILD. Patient demographics and baseline clinical characteristics are summarized in Table 3. Compared to those without ILD, there was a greater frequency of males in patients with SSc-PH and concomitant ILD. With increasing ILD severity, there was a trend toward younger age at the time of PH diagnosis. Additionally, those with more severe ILD demonstrated an increased frequency of diffuse cutaneous SSc and anti-Scl-70 antibody positivity. Patients without ILD demonstrated more severe PH with higher mPAP and PVR compared to those with any severity of ILD. Compared to those without ILD, patients with ILD had a greater frequency of NYHA class III/IV dyspnea (73.5% vs. 62.2%). Most patients with SSc-PH (86.4%) were initiated on PAH-specific therapy.
| No ILD (N = 37) | Mild ILD (N = 9) | Moderate ILD (N = 35) | Severe ILD (N = 7) | |
|---|---|---|---|---|
| Demographics | ||||
| Age at PH diagnosis, years (mean ± SD) | 63.6 ± 11.3 | 66.4 ± 11.7 | 56.9 ± 9.3 | 51.7 ± 13.3 |
| Male sex, n (%) | 4 (10.8%) | 2 (22.2%) | 12 (34.3%) | 2 (28.6%) |
| Diffuse cutaneous SSc, n (%) | 4 (10.8%) | 1 (11.1%) | 13 (37.1%) | 4 (57.1%) |
| Former/current smoker, n (%) | 18 (48.6%) | 9 (100%) | 17 (48.6%) | 1 (14.3%) |
| Overlapping autoimmune disease | ||||
| Sjogren's syndrome, n (%) | 3 (8.1%) | 2 (22.2%) | 2 (5.7%) | 1 (14.3%) |
| Systemic lupus erythematosus, n (%) | 1 (5.4%) | 1 (11.1%) | 2 (5.7%) | 0 |
| Dermatomyositis/polymyositis, n (%) | 1 (2.7%) | 0 | 2 (5.7%) | 0 |
| Rheumatoid arthritis, n (%) | 0 | 1 (11.1%) | 1 (2.9%) | 0 |
| Comorbid conditions | ||||
| Obstructive lung disease, n (%) | 7 (18.9%) | 2 (22.2%) | 3 (8.6%) | 0 |
| Coronary artery disease, n (%) | 6 (16.2%) | 1 (11.1%) | 4 (11.4%) | 0 |
| Atrial fibrillation/atrial flutter, n (%) | 8 (21.6%) | 1 (11.1%) | 4 (11.4%) | 1 (14.3%) |
| Venous thromboembolism, n (%) | 8 (21.6%) | 0 | 5 (14.3%) | 1 (14.3%) |
| Hypertension, n (%) | 21 (56.8%) | 4 (44.4%) | 16 (45.7%) | 3 (42.9%) |
| Chronic kidney disease, n (%) | 2 (5.4%) | 0 | 2 (5.7%) | 0 |
| Antinuclear antibody | ||||
| ANA, number (%)a | 29 (100%) | 5 (83.3%) | 20 (87.0%) | 6 (100%) |
| Antitopoisomerase/antibody | ||||
| Anti-Scl-70 antibody, number (%)b | 2 (8.7%) | 0 | 3 (15.8%) | 2 (50.0%) |
| Degree of skin disease | ||||
| Modified Rodnan skin score (mean ± SD)c | 7.5 ± 7.3 | 4.0 ± 3.4 | 13.2 ± 13.0 | 5.2 ± 4.3 |
| Cardiopulmonary hemodynamics | ||||
| RAP, mmHg (mean ± SD) | 7.8 ± 4.7 | 9.3 ± 7.5 | 7.1 ± 6.5 | 4.7 ± 9.1 |
| mPAP, mmHg (mean ± SD) | 43.3 ± 12.2 | 38.7 ± 11.6 | 34.9 ± 9.0 | 32.7 ± 7.8 |
| PAWP, mmHg (mean ± SD) | 9.7 ± 3.5 | 12.2 ± 4.0 | 11.2 ± 6.6 | 5.1 ± 4.6 |
| CO, L/min (mean ± SD) | 4.6 ± 1.8 | 4.6 ± 1.1 | 4.9 ± 1.2 | 4.9 ± 0.6 |
| PVR, Wood units (mean ± SD) | 8.8 ± 5.2 | 5.2 ± 6.0 | 5.1 ± 1.9 | 5.5 ± 1.3 |
| BNP measurementsd | ||||
| BNP < 100 pg/ml, n (%) | 14 (42.4%) | 3 (37.5%) | 17 (50.0%) | 4 (57.1%) |
| BNP ≥ 100 pg/mL and <400 pg/ml, n (%) | 9 (27.3%) | 3 (37.5%) | 8 (23.5%) | 1 (14.3%) |
| BNP ≥ 400 pg/ml, n (%) | 10 (30.3%) | 2 (25.0%) | 9 (26.5%) | 2 (28.6%) |
| NYHA functional classificatione | ||||
| NYHA Class I, n (%) | 2 (5.9%) | 1 (12.5%) | 1 (3.2%) | 0 |
| NYHA Class II, n (%) | 7 (20.6%) | 3 (37.5%) | 9 (29.0%) | 3 (50.0%) |
| NYHA Class III, n (%) | 24 (70.6%) | 3 (37.5%) | 21 (67.7%) | 3 (50.0%) |
| NYHA Class IV, n (%) | 1 (2.9%) | 1 (12.5%) | 0 | 0 |
| Initial PAH-specific therapy | ||||
| Oral/systemic therapy, n (%) | 34 (91.9%) | 7 (77.8%) | 29 (82.9%) | 6 (85.7%) |
| Oral therapy, n (%) | 24 (64.9%) | 5 (55.6%) | 24 (68.6%) | 4 (57.1%) |
| Endothelin receptor antagonist, n (%) | 12 (32.4%) | 2 (22.2%) | 14 (40.0%) | 2 (28.6%) |
| Phosphodiesterase-5 inhibitor, n (%) | 17 (45.9%) | 4 (44.4%) | 13 (37.1%) | 3 (42.9%) |
| Soluble guanylate cyclase stimulator, n (%) | 0 | 0 | 1 (2.9%) | 0 |
| Systemic prostacyclin therapy, n (%) | 10 (27.0%) | 2 (22.2%) | 5 (14.3%) | 2 (28.6%) |
- Abbreviations: BNP, brain natriuretic peptide; CO, cardiac output; ILD, interstitial lung disease; mPAP, mean pulmonary artery pressure; NYHA, New York Heart Association; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SSc, systemic sclerosis.
- a No ILD: N = 29, mild ILD: N = 6, moderate ILD: N = 23, severe ILD: N = 6.
- b No ILD: N = 23, mild ILD: N = 4, moderate ILD: N = 19, severe ILD: N = 4.
- c No ILD: N = 19, mild ILD: N = 4, moderate ILD: N = 26, severe ILD: N = 5.
- d No ILD: N = 33, mild ILD: N = 8, moderate ILD: N = 34, severe ILD: N = 7.
- e Not ILD: 34, mild ILD: N = 8, moderate ILD: N = 31, severe ILD: N = 6.
Associations with all-cause mortality
In the univariable Cox model, age at PH diagnosis, male sex, and diffuse cutaneous SSc were associated with a nonsignificant increased hazards ratio for all-cause mortality from the time of PH diagnosis. Compared to those without concomitant ILD, patients with severe ILD had an increased hazards for all-cause mortality with an HR of 1.81 (95% CI: 0.67, 4.89). When adjusted for age at PH diagnosis, male sex, diffuse cutaneous SSc, and cardiopulmonary hemodynamics, patients with SSc-PH and severe ILD had a significantly increased HR of 3.54 (95% CI: 1.05, 11.99) for all-cause mortality compared to those with SSc-PH without concomitant ILD (Table 4). This effect was not seen for mild or moderate ILD. The adjusted bootstrapped HR for all-cause mortality in patients with SSc-PH and severe ILD was 4.28 (95% CI: 1.10, 17.79). A sensitivity analysis in which those who were lost to follow-up were excluded resulted in an adjusted HR of 5.37 (95% CI: 1.55, 18.53) for severe ILD. The median duration of follow-up was 90.1 months with 44 events. The median of the maximum time between clinical variables was 3 months.
| Univariable | Multivariable | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Age at PH diagnosis, years | 1.02 (0.99, 1.05) | 1.03 (1.00, 1.06) |
| Male sex | 1.45 (0.71, 2.98) | 1.68 (0.74, 3.79) |
| Diffuse cutaneous SSc | 1.60 (0.79, 3.24) | 1.70 (0.71, 4.05) |
| mPAP, mm Hg | 1.04 (1.01, 1.06) | 1.00 (0.95, 1.06) |
| PAWP, mmHg | 1.00 (0.94, 1.07) | 1.04 (0.95, 1.13) |
| PVR, Wood units | 1.12 (1.05, 1.19) | 1.16 (0.99, 1.34) |
| ILD severity | ||
| Mild ILD | 0.54 (0.13, 2.36) | 0.57 (0.12, 2.64) |
| Moderate ILD | 0.95 (0.48, 1.86) | 1.30 (0.55, 3.08) |
| Severe ILD | 1.81 (0.67, 4.89) | 3.54 (1.05, 11.99) |
- Abbreviations: ILD, interstitial lung disease; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SSc, systemic sclerosis.
DISCUSSION
While it is understood that patients with SSc and concomitant ILD and PH experience worse outcomes compared to those limited to ILD or PH, alone,6, 9, 10, 17, 18 it has been unclear to what degree PH and ILD severity contribute to mortality in each of these respective groups. This is important as it may aid in risk stratifying patients with SSc and informing management decisions, including early intensification of ILD/PAH-specific therapy and referral for lung transplantation. In patients with SSc-ILD, we found that severe PH was associated with a >6-fold increased hazard for mortality, and moderate PH was associated with a >2-fold increased hazard for mortality compared to those without concomitant PH. In patients with SSc-PH, we found that severe ILD was associated with a > 3-fold increased hazard for mortality compared to those without concomitant ILD.
In contrast to a prior study that suggested that the presence of moderate to severe PH was similar in patients with and without concomitant ILD,8 we found that indeed there was greater PH severity by cardiopulmonary hemodynamics in patients without ILD compared to those with ILD. This and the lack of diffuse cutaneous SSc in this group is congruent with the prior literature which suggested that SSc-PH was observed primarily in limited SSc.3, 19 One big difference between these two studies is the reliance on pulmonary arterial systolic pressures estimated by echocardiography in the prior work, inaccurate in 50.6% of patients as opposed to RHC.20 In contrast, ILD was associated with increased frequency of diffuse cutaneous SSc and anti-Scl-70 antibody positivity. Moreover, with increasing ILD severity by FVC, there were increasing frequencies of diffuse cutaneous SSc and anti-Scl-70 antibody positivity. This was consistent with prior findings from a large SSc registry in which ILD was observed in 53% of patients with diffuse cutaneous SSc compared to 35% of those with limited cutaneous SSc.21
Interestingly, in patients with SSc-ILD, the distribution of chest CT findings appeared similar between patients with and without concomitant PH, and the differences in FVC and DLCO were small. This implies that the ILD findings were not solely due to PH. Yet, there were significantly increased HRs for all-cause mortality in patients with moderate/severe PH compared to those without. This suggests that concomitant PH may be the primary driver of mortality in these patients. Furthermore, among patients with SSc-PH, the distributions of NYHA functional classification and BNP were similar across patients with and without ILD. This indicates that PH was also the primary factor influencing symptomatology and, as one would suspect, the presence of ILD had no impact on BNP. In fact, as highlighted previously, patients with no ILD had worse cardiopulmonary hemodynamics.
Our study has limitations. First, only seven patients were identified as having severe ILD, introducing the possibility of random error. However, our use of bootstrapped models, which demonstrated findings consistent with our primary models, strengthened our confidence in the results. Second, as the study was retrospective, collection of data was dictated by clinical practice, leading to inconsistencies in data availability and timing. Furthermore, given the longitudinal nature of the database with a study period between January 1, 1999 and January 1, 2021, standards of care likely changed. However, more than half of patients in our cohort were diagnosed with ILD and/or PH on/after 2010 with more than 75% of patients diagnosed on/after 2006, the time of Scleroderma Lung Study I which established cyclophosphamide therapy for SSc-ILD.22 Additionally, as an SSc and PH referral center, many of our patients did not have their radiologic films available in our database. This required our reliance on the radiologic interpretation of the films and that of the primary physicians caring for the patient with an ILD diagnosis. This is less than ideal. However, in the majority of patients, CT and physician diagnosis was associated with at least moderate restriction by pulmonary function testing, suggesting that this was a persistent finding and correlated with a physiologic abnormality. Furthermore, while FVC was not included as a diagnostic criterion for ILD, it was used to stratify patients. Third, there were missing data from the 54 (23.3%) patients who were lost to follow-up. As our sensitivity analysis excluding those lost to follow-up was consistent with our primary results, any bias that this may have introduced was likely minimal. Finally, the large study time encompassed different approaches to clinical practice which may have impacted our findings. This included the newer definition of PH (mPAP > 20 mmHg)11 which may have had implications in terms of initiation of PAH-specific therapy based on whether there were significant symptoms.
CONCLUSION
Among patients with SSc-ILD or SSc-PH, concomitant PH, or ILD disease, respectively, may increase risk of all-cause mortality in a severity-response relationship. These findings emphasize the importance of therapies to target slowing of disease progression earlier in the disease course. This suggests the need to assess the impact of both ILD-specific and PAH-specific therapy in randomized controlled trials in patients with SSc-PH and co-existent ILD, but our findings should be confirmed in a larger cohort.
AUTHOR CONTRIBUTIONS
Ruchika A. Sangani made substantial contributions to the acquisition of data, drafted the article, and revised it critically for important intellectual content. Justin K. Lui made substantial contributions to study conception, design, acquisition of data, analysis, and interpretation of data, drafted the article, and revised it critically for important intellectual content. Kari R. Gillmeyer made substantial contributions to analysis and interpretation of data and revised the article critically for important intellectual content. Marcin A. Trojanowski made substantial contributions to the acquisition of data and revised the article for important intellectual content. Andreea M. Bujor made substantial contributions to the acquisition of data and revised the article for important intellectual content. Michael P. LaValley made substantial contributions to analysis and interpretation of data and revised the article critically for important intellectual content. Elizabeth S. Klings made substantial contributions to study conception, design, analysis, and interpretation, and revised the article critically for important intellectual content. All authors gave final approval of the version of the article.
ACKNOWLEDGMENTS
Justin K. Lui is supported by NIH/NHLBI 1F32HL156614-01. Kari R. Gillmeyer is supported by NIH/NHLBI 5F32HL149236-02 and a Parker B. Francis Fellowship Award. Andreea M. Bujor is supported by Tobé and Stephen E. Malawista, MD, Endowment in Academic Rheumatology, Rheumatology Research Foundation. Michael P. LaValley is supported by NIAMS CCCR P30 AR072571. Elizabeth S. Klings is supported by NIH/NHLBI 1UG3 HL143192-01A1, NCATS 2UL1TR001430-05A1, and HRSA U1EMC27864-08-00. Elizabeth S. Klings accepts official responsibility for the overall integrity of this study.
CONFLICTS OF INTEREST
Elizabeth S. Klings received research support from Bayer, Novartis, FORMA Therapeutics, and United Therapeutics. She received royalties for three topic cards in UpToDate. She is a consultant for Bluebird Bio and CSL Behring for sickle cell disease-related clinical trials (no conflict with the present work).
ETHICS STATEMENT
The study was approved by the Institutional Review Board at Boston University School of Medicine.