Prognostic factors of overall and prostate-specific antigen-progression-free survival in metastatic castration-resistant prostate cancer patients treated with 177Lu-PSMA-617. A single-center prospective observational study
Abstract
Background
Metastatic castration-resistant prostate cancer (mCRPC) is characterized by heterogeneity among patients as well as therapy responses due to diverse genetic, epigenetic differences, and resistance mechanisms. At this stage of the disease, therapy modalities should be individualized in light of the patients' clinical state, symptoms, and genetic characteristics. In this prospective study, we aimed to evaluate the outcome of patients with mCRPC treated with 177Lutetium labeled PSMA-617 therapy (PSMA-RLT), as well as baseline and therapy-related parameters associated with survival.
Methods
This prospective study included 52 patients who received two to six cycles of PSMA-RLT. Primary endpoints were overall survival (OS) and prostate-specific antigen (PSA)-progression-free survival (PFS). 18F-Fluorodeoxyglucose (FDG) and 68Ga-PSMA (PSMA) Positron Emission Tomography/Computer Tomography (PET/CT) scans were performed for a comprehensive assessment of tumor burden and heterogeneity. Biochemical, imaging, clinical, and therapy-related parameters were analyzed with the Kaplan–Meier, log-rank, and Cox regression analyses to predict OS and PFS.
Results
Median OS and PSA-PFS were 17.7 (95% confidence interval [CI]: 15.2–20.2) and 6.6 months (95% CI: 4.5–8.8), respectively. Primary resistance to PSMA-RLT (hazard ratio [HR]: 12.57, 95% CI: 2.4–65.2, p: 0.003), <30% PSA response rate after first cycle of PSMA-RLT (HR: 1.016, 95% CI: 1.006–1.03, p: 0.003), FDG > PSMA disease (HR: 4.9, 95% CI: 1.19–20.62, p: 0.03), PSA doubling time (PSA DT) of ≤2.4 months (HR: 15.7, 95% CI: 3.7–66.4, p: <0.0001), and low hemoglobin levels (HR: 0.59, 95% CI: 0.41–0.83, p: 0.003) were correlated with poor OS in the multivariate analysis. Bone scintigraphy > PSMA disease (HR: 5.6; 95% CI: 1.8–17, p: 0.002) and high C-reactive protein (HR: 1.4, 95% CI: 1.1–1.7, p: 0.001) were significant predictive biomarkers for PFS in the multivariate analysis.
Conclusion
PSA response rate and pattern to PSMA-RLT are the most important predictors of survival in patients receiving PSMA-RLT. Being a strong predictive biomarker, combined FDG and PSMA PET can be helpful for the decision of PSMA-RLT eligibility.
1 INTRODUCTION
Prostate cancer is the second most common cancer in men and the fifth leading cause of cancer death.1 Although patients with localized prostate cancer have an excellent outcome, 10%–20% of the patients become castration-resistant (CRPC) within 5 years after diagnosis.2 In this phase of the disease, the mean survival significantly declines to 14 months.2 Docetaxel as first-line treatment for metastatic CRPC (mCRPC), as well as abiraterone, cabazitaxel, enzalutamide, and 223Radium-dichloride have shown an additional overall survival (OS) benefit ranging between 3 and 5 months.3-6
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein that is overexpressed in prostate cancer cells and associated with early recurrence, castration resistance, and poor prognosis.7, 8 It can be labeled with 177Lutetium (177Lu) which delivers beta radiation to metastatic sites. Recently, in a prospective phase 2 LuPSMA trial, Hofman et al. demonstrated a median OS of 13.5 months and a median progression-free survival (PFS) of 7.6 months in patients treated with 177Lu-PSMA radioligand therapy (PSMA-RLT).9 Randomized prospective phase 2 TheraP trial showed that the prostate-specific antigen (PSA) response rate at 3 months (66% vs. 37%) and PFS at 12 months (19% vs. 3%) were significantly higher in patients who received PSMA-RLT compared to cabazitaxel.10 The recent phase 3 VISION trial also found that PSMA-RLT in addition to standard of care prolonged imaging-based PFS (median, 8.7 vs. 3.4 months, p < 0.001) and OS (median, 15.3 vs. 11.3 months, p < 0.001).11, 12 Although numerous studies have shown clinical benefit,13-17 there is a lack of precise biomarkers to predict outcomes of PSMA-RLT which would refine the patient selection criteria. The aim of this prospective observational study is to assess the efficacy of PSMA-RLT in patients with mCRPC and to correlate the clinical, laboratory, and imaging parameters with therapy outcomes.
2 MATERIALS AND METHODS
2.1 Patients
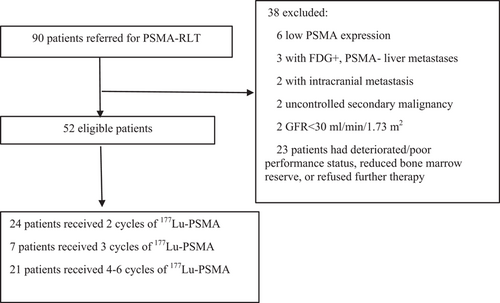
Ninety mCRPC patients with progressive disease after docetaxel and/or second generation anti-androgen treatment were referred to our department for PSMA-RLT between January 2017 and January 2019. Fifty-two patients who were eligible and included in this study, received a minimum of two cycles of 177Lu-PSMA-617, and followed up until March 2021 (Figure 1). The inclusion/exclusion criteria and therapy algorithm are summarized in Supporting Information: Table S1. The study was approved by the local ethics committee (Permission no: 17-AKD-157, 2019/04/23 KA-17123) and performed in accordance with the Declaration of Helsinki.
2.2 Baseline work-up, treatment, and follow-up
All patients underwent 68Ga-PSMA Positron Emission Tomography/Computer Tomography (PET/CT),18F-Fluorodeoxyglucose (FDG) PET/CT, and bone scintigraphy (BS) with 99m Technetium Hydroxydiphosphonate (99mTc-HDP) within 4 weeks of therapy initiation. Patients eligible for PSMA-RLT underwent further imaging with 99mTc-mercaptoacetyltriglycine (MAG-3) renal scintigraphy to exclude renal outlet obstruction (see details in Supporting Information: Methods). Clinical (performance status, pain severity, analgesic use) and laboratory assessment including tumor markers (PSA, Carcinoembryonic Antigen [CEA], Neuron-Specific Enolase [NSE]), blood tests (full blood count, urea and electrolytes, liver function tests) were repeated every 2 weeks (Supporting Information: Table S2).
2.3 Molecular imaging analysis
Tumor characteristics including total lesion number, maximum standardized uptake value (SUVmax) of the dominant lesion, tumor volume on PSMA PET (TV-PSMA), total lesion PSMA uptake (TL-PSMA), metabolic tumor volume (MTV), total lesion glycolysis (TLG) were calculated by drawing regions of interest around metastatic lesions using a semi-automated tool on PSMA and FDG PET/CT. Advantage Workstation 4.7 (GE Healthcare) was used for analysis.18
All scans were also visually evaluated for the number, size, and intensity of lesion uptake. PSMA ≥ FDG disease was defined for the patients with metastases showing equal or higher PSMA uptake compared to FDG. FDG > PSMA disease was defined when FDG uptake in the majority of lesions was greater than 68Ga-PSMA. BS > PSMA disease was defined as when the majority of bony lesions had higher 99mTc-HDP uptake than PSMA. Metastases showing higher or similar to PSMA uptake compared to 99mTc-HDP were defined as PSMA ≥ BS disease. All categories according to visual assessment are summarized in Supporting Information: Figures S1 and S2.
2.4 Therapy
177Lu-PSMA-617 was administered intravenously with a median of 7.3 GBq (5.9–7.4 GBq) every 6–10 weeks. Concomitant androgen-deprivation therapy (ADT), second generation anti-androgens, bisphosphonate/denosumab, and palliative radiation therapy to bone were allowed. Imaging and therapy protocols are detailed in Supporting Information: Methods.
2.5 PSA response evaluation
PSA responses during the therapy cycles and at 3 months after completion of therapy were recorded. The percentage of change in PSA from the baseline to the end of therapy was calculated. PSA response 6–8 weeks after the first cycle, the maximum decline in PSA that occurred at any point after the treatment, and PSA response at 3 months after completion of therapy were determined for each patient. The PSA response was defined as a durable decline of ≥50% from the baseline.
Additionally, PSA response patterns were evaluated on the basis of the PSA changes during the PSMA-RLT cycles according to the Prostate Cancer Working Group 3 (PCWG3) recommendations.19 Briefly, PSA response patterns were categorized into 3 groups at the end of PSMA-RLT; (1) Substantial and durable decline more than 50% consistent with response to treatment (response; RE), (2) initial PSA decline more than 50% in first 6–8 weeks, followed by a slow rise (acquired resistance; AR), and (3) PSA progression without any significant (>25%) response (primary resistance; PR). The OS in different PSA response patterns was compared.
2.6 Therapy outcomes
The primary endpoints of the study were OS and PSA-PFS. OS was defined as the time (in months) from the first cycle of therapy to the date of death from any cause or the last follow-up visit of a patient is last known alive. PFS was defined as the time (in months) between the date of first cycle of therapy and the date of a rise in PSA 25% or greater or an absolute increase of 2 ng/mL or more from the nadir which is confirmed by a second value obtained 3 or more weeks later. PCWG3 criteria were used to define PSA progression.19
2.7 Statistical analysis
IBM SPSS Statistics Version 23 (IBM Inc.) was used for statistical analyses. The compliance of variables to normal distribution was determined by Kolmogorov–Smirnov test. Normally distributed variables were reported as mean and standard deviation (SD) and skewed variables as median and interquartile range (IQR) or range. A p value of <0.05 was considered statistically significant. OS and PSA-PFS were analyzed with the Kaplan–Meier method. The impact of the parameters on OS and PFS was evaluated with Kaplan–Meier, log-rank, and stepwise Cox regression analyses. Upper normal values were used for certain baseline parameters (Alkaline Phosphatase [ALP], CEA, and NSE), while medians of our cohort were used for the remaining parameters for binarization and determination of cutoff values for the survival analysis.
3 RESULTS
3.1 Patient characteristics and therapy
Before PSMA-RLT, 10 (19.2%), 30 (57.7%), and 12 (23.1%) of the patients had progressed with one, two, and three previous lines of therapies, respectively. All patients had castrated levels of testosterone (<50 ng/dL). Detailed patient characteristics are outlined in Table 1 and Supporting Information: Table S3.
| Clinical parameters | Number (%), median (range), or mean ± SD |
|---|---|
| Age | 70.3 ± 1.2 |
| Gleason Score | 9 (5–10) |
| ISUP grade | 5 (1–5) |
| Initial Pca TNM stage (AJCC 8th edition) | |
| ≤3b | 10 (20%) |
| 3c | 7 (14%) |
| 4a | 5 (10%) |
| 4b | 28 (56%) |
| Time to castration resistance from initial diagnosis (months) | 36 (0–228) |
| Prior therapies after castration resistance | |
| Docetaxel | 47 (90.4%) |
| Cabazitaxel | 12 (23.1%) |
| Abiraterone or/and enzalutamide | 46 (88.4%) |
| Status of metastases | |
| Lymph node dominant | 7 (13.5%) |
| Bone dominant | 33 (63.5%) |
| Patients with visceral metastases | 12 (23.1%) |
| Presence of additional liver metastases | 6 (11.5%) |
| Initial PSA (ng/mL) | 41.5 (0.1–6812) |
| Initial PSA DT (months) | 2.4 (−11.5 to 23.2) |
| Initial LDH (U/L) | 262.5 (153–922) |
- Abbreviations: AJCC, American Joint Committee on Cancer; ISUP, International Society of Urological Pathology; LDH, lactate dehydrogenase; PCa, prostate cancer; PSA, prostate-specific antigen; PSA DT, prostate-specific antigen doubling time.
Majority (n = 43, 87.2%) of the patients had multiple metastases (>10 lesions) on PSMA PET/CT. The median SUVmax of the dominant lesion was 26.2 (range, 5.5–120.6). Seven (15.9%) patients had FDG > PSMA disease and eight (18.6%) of the patients had BS > PSMA disease. Molecular imaging characteristics derived from FDG and PSMA PET/CT are summarized in Table 2.
| Parameters | Number (%), median (range) |
|---|---|
| PSMA PET | |
| Total number of lesions on PSMA PET | |
| 1–5 | 4 (7.7%) |
| 6–10 | 5 (9.6%) |
| >10 | 43 (82.7%) |
| SUVmax of the dominant lesion on PSMA PET | 26.2 (5.5–120.6) |
| TV-PSMA (mm3) | 129.8 (6–3506) |
| TL-PSMA on PSMA PET | 1031.1 (8.1–20,314.4) |
| FDG PET | |
| SUVmax of the dominant lesion on FDG PET | 6.3 (0–27.2) |
| MTV (mm3) | 73.6 (0–996.6) |
| TLG | 212.9 (0–22,052.4) |
| MTV-FDG/TV-PMSA | 0.4 (0–11.2) |
- Abbreviations: FDG, 18F-Fluorodeoxyglucose; MTV, metabolic tumor volume; PSMA, prostate-specific membrane antigen; PET, positron emission tomography; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis; TL-PSMA, total lesion PSMA uptake; TV-PSMA, tumor volume on PSMA PET.
3.1.1 177Lu-PSMA-617 therapy
Fifty-two patients in our cohort received a total of 163 cycles of 177Lu-PSMA. Two, three, four, five, and six cycles of 177Lu-PSMA-617 therapy were administered to 24 (46.2%), 7 (13.5%), 15 (28.8%), 2 (3.8%), and 4 (7.7%) patients, respectively. The median cumulative activity was 21.1 GBq (range: 13.5–44.4 GBq) and the median administered activity per cycle was 7.3 GBq. Twenty-nine (55.8%) were on concomitant zoledronate or denosumab treatment.
3.1.2 Survival
The median OS and PSA-PFS were 17.7 months (95% CI: 15.2–20.2) and 6.6 months (95% CI: 4.5–8.8), respectively (Supporting Information: Figure S3). Fifty (96.2%) of 52 patients showed biochemical progression and 35 (67.3%) patients died during a median follow-up period of 17 months (IQR: 10.2–20.7 months).
3.2 Biomarkers to predict therapy outcome
3.2.1 Predictive factors for OS
Univariate analysis showed that biochemical response and response on PSMA PET/CT were significantly associated with better OS (Supporting Information: Table S4). Presence of the liver metastases, high number of bone lesions, poor performance status, baseline moderate-severe pain, short PSA DT, high LDH, ESR, CRP/albumin ratio, and low hemoglobin were associated with poor OS (Supporting Information: Table S4). In addition, certain molecular imaging findings (FDG > PSMA and BS > PSMA disease, high MTV, TLG/TL-PSMA ratio) were associated with poor OS (Supporting Information: Table S4).
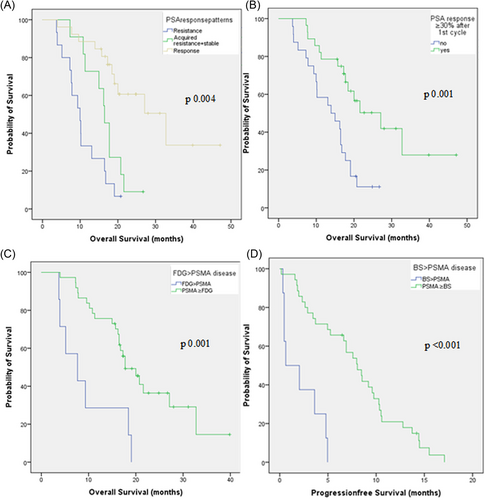
In the multivariate analysis, PSA DT of ≤2.4 months (hazard ratio [HR]: 15.7, 95% confidence interval [CI]: 3.7–66.4, p: <0.0001) and primary resistance (HR: 12.57, 95% CI: 2.4–65.2, p: 0.003) were the most significant parameters associated with poor OS. Initial FDG > PSMA disease (HR: 4.9, 95% CI: 1.19–20.62, p: 0.03), lower hemoglobin (HR: 0.59, 95% CI: 0.41–0.83, p: 0.003), <30% PSA response rate after first cycle of therapy (HR: 1.016, 95% CI: 1.006–1.03, p: 0.003), baseline moderate-severe pain (HR: 10.88, 95% CI: 2.9–40.8, p: <0.001) also retained their significance as unfavorable prognostic biomarkers of OS (Table 3, Figure 2A–C).
| Parameter | Patients (n) | Death (n, %) | mOS (months)a | Multivariate analysisb | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | ||||
| PSA response curve patterns | ||||||
| Response | 26 | 14 (53.8%) | 32.7 | Reference | ||
| Acquired Resistance | 11 | 10 (90.9%) | 16.5 | Not significant | ||
| Primary Resistance | 15 | 11 (73.3%) | 9.9 | 12.57 | 2.4–65.2 | 0.003 |
| PSA decline ≥ 30% after 1st Cycle of 177Lu-PSMA | ||||||
| Yes | 28 | 14 (50%) | 27.1 | Reference | ||
| No | 24 | 21 (87.5%) | 13.9 | 1.016 | 1.006–1.03 | 0.003 |
| Initial PSMA-FDG discordance | ||||||
| PSMA ≥ FDG disease | 37 | 23 (62.2%) | 17.7 | Reference | ||
| FDG > PSMA disease | 7 | 7 (100%) | 7.6 | 4.9 | 1.19–20.62 | 0.03 |
| Baseline PSA DT | ||||||
| >2.4 months | 26 | 13 (50%) | 27.1 | Reference | ||
| ≤2.4 months | 26 | 22 (84.6%) | 14.9 | 15.7 | 3.7–66.4 | <0.0001 |
| Baseline pain severity | ||||||
| No pain/mild | 32 | 19 (59.3%) | 20.8 | Reference | ||
| Moderate/severe | 19 | 16 (84.2%) | 10.8 | 10.88 | 2.9–40.8 | <0.0001 |
- Note: Only statistically significant parameters in the multivariate analysis were shown.
- Abbreviations: CI, confidence interval; HR, hazard ratio; FDG18, F-Fluorodeoxyglucose; mOS, median estimated overall survival; PET, positron emission tomography; PSA, prostate-specific antigen; PSA DT, PSA doubling time, PSMA, prostate-specific membrane antigen.
- a Log-rank analysis.
- b Cox regression.
3.2.2 Predictive factors for PFS
Univariate analysis showed that presence of the liver metastases, FDG > PSMA, and BS > PSMA disease (Figure 2D), short PSA DT, high LDH, ESR, CRP, low hemoglobin, high TLG/TL-PSMA ratio at baseline were associated with shorter PFS (Supporting Information: Table S5).
In the multivariate analysis, BS > PSMA disease (HR: 5.6; 95% CI: 1.8–17, p: 0.002) and high CRP (HR: 1.4, 95% CI: 1.1–1.7, p: 0.001) retained their significance as predictive biomarkers of PFS (Supporting Information: Table S5).
4 DISCUSSION
In this prospective observational study, we aimed to determine prognostic factors to predict OS and PFS in 52 patients with mCRPC treated with PSMA-RLT.
The median OS of patients treated with 177Lu-PSMA RLT was 17.7 months in our cohort. This finding is slightly better than the VISION trial results, which reported a median OS of 15.3 months.11, 12 Longer OS in our study may be related to study cohorts, such as differences in inclusion criteria and prior therapies. In the VISION trial, the percentage of patients previously treated with docetaxel (90.4% vs. 97.9%) and cabazitaxel (23.1% vs. 41.8%) were higher than our cohorts.11, 12 Furthermore, contrary to the VISION trial, the addition of FDG PET/CT to our patient work-up might have resulted in a better outcome, since nine patients with FDG+/PSMA− or low PSMA expressing lesions were excluded.
This study reveals that PSA response patterns to PSMA-RLT were correlated with OS. Patients with therapy response had a longer OS (32.7 months) compared to those with acquired resistance (16.5 months) and primary resistance (9.9 months). Primary and acquired resistance despite high PSMA expression may be due to sublethal radiation dose delivered to tumor as well as the presence of radioresistant tumor cells and tissue hypoxia. Apart from its prognostic importance, PSA response patterns may aid in decision-making. Combining more cytotoxic alpha emitters in adjusted doses as an augmentation to PSMA-RLT with beta emitters (tandem therapy) may increase the therapeutic efficacy in comparison to monotherapy using beta emitters alone and reduce the adverse effects in comparison to monotherapy using alpha emitters alone.20 Khreish et al. treated patients with primary or acquired resistance to 177Lu-PSMA with tandem 225Actinium-labeled PSMA and 177Lu-PSMA.21 Those who had acquired resistance to 177Lu-PSMA RLT had a better PSA response compared to the primary resistance group (≥50% best PSA response rates were 83.3% vs. 50%, respectively).21
As another predictor, we found that patients who had ≥30% of PSA decline rate after first PSMA-RLT had a better OS (27.1 vs. 13.9 months). This was also supported by Gafita et al. reported that ≥30% of PSA response rate 6ixth week after the first cycle of 177Lu-PSMA therapy predicted longer OS (16.7 months) in comparison to stable PSA (11.8 months) or PSA progression (6.5 months).22 On the other hand, Ahmadzadehfar et al. reported that ≥50% PSA response assessed at 12 weeks was predictive of OS (18.4 vs. 8.7 months).17 In their study, the initial PSA decline assessed 2 months after the first cycle was not significant predictor. Likewise, Rahbar et al. reported that 29% of the biochemical non-responders after first cycle of therapy showed PSA response to further cycles.23 Therefore, delayed PSA measurement after second cycle may be a better predictor of OS.
High tumor heterogeneity and burden expressed as TLG/TL-PSMA ratio and presence of discordant disease (17.6 vs. 7.7 months) predicted OS in the univariate analysis. In the multivariate analysis, FDG > PSMA disease was associated with poorer OS. This is likely due to the presence of aggressive tumor cells, heterogeneity, and clonal diversity of metastases. Although the intensity of PSMA expression on the cell surface is correlated with aggressiveness and resistance to standard therapies, tumor cells may lose their PSMA expression, while they increase their glucose metabolism and GLUT1 expression as a way of coping mechanism to heavy treatment.24-27 These tumors with more aggressive behavior cannot be targeted with PSMA-RLT may cause poor outcome. Well in line with our study, patients with discordant FDG disease and excluded from the therapy associated with poorer survival.28 In our study, although patients who had lesions with low or no PSMA uptake and high FDG uptake were excluded, those with FDG > PSMA disease with high PSMA uptake patients were given PSMA-RLT. The median OS of this group was 7.6 months in our study. This is higher than the patients excluded from LuPSMA trial due to low PSMA uptake (2.3 months) or discordant FDG-avid disease (3.9 months).28 These results suggest that PSMA-RLT may even have a survival benefit for a limited group of patients with FDG > PSMA disease with high PSMA uptake.
Among the biochemical parameters, PSA DT < 2.4 months was associated with poor OS in the multivariate analysis. Short PSA DT represents a rapid tumor proliferation rate and aggressive behavior.29 Similar to other studies, lower baseline hemoglobin values were also associated with poorer OS in our study.30, 31 Anemia is a common finding among the mCRPC patients which can be due to marrow infiltration, inhibition of erythropoiesis, poor nutritional status, and long-term anti-androgenic treatment or caused by the pro-inflammatory cytokines.32 It is well known that anemia results in intra-tumoral hypoxia, which may cause radioresistance and poor outcome.33, 34
In our cohort, the median PSA-PFS was 6.6 months which is well in line with both TheraP (5.8 months) and LuPSMA trials (7.6 months).9, 10 We found that PFS was shorter in patients with BS > PSMA disease category. Correlative imaging with FDG PET showed that bone metastases seen on BS were also FDG-avid; therefore, bone healing or flare response was ruled out. The presence of osteoblastic lesions with low PSMA uptake may represent bone metastases that cannot be targeted with PSMA-RLT. Performing a bone scan before PSMA-RLT may allow nuclear medicine physicians to consider alpha-emitting 223Radium-dichloride therapy in patients with low PSMA uptake.
Patients with elevated CRP had a shorter PFS. CRP is a nonspecific marker of inflammation that can induce tumorigenesis, invasion, and metastasis.35 Inflammatory cells produce an attractive environment for tumor growth, induce DNA damage, promote angiogenesis, favor metastasis,36 and may affect prognosis.37 Per contra, increased CRP may also identify the patients with impaired T-lymphocytic response to tumor cells and poor outcomes.38 On the other hand, Buresova et al. also found a significant correlation between serum CRP and LDH levels and circulating DNA levels in advanced non-small cell lung carcinoma.39 The relationship between the prognosis of mCRPC patients receiving PSMA-RLT and selected oncomarkers has been investigated by Rathke et al.40 They found that the strongest value for response prediction was baseline LDH, which is an unspecific surrogate for aggressiveness. Since LDH is a marker of tissue breakdown, elevated levels may reflect a higher tumor turnover rate. Moreover, the combination of Chromogranin A and NSE may help to detect neuroendocrine trans-differentiation of the mCRPC patients who had progressive disease.
Although we found a significant correlation with outcome and semi-quantitative parameters such as MTV, TLG/TL-PSMA ratio in the univariate analysis, only visual assessment of FDG > PSMA and BS > PSMA disease retained their significance in the multivariate analysis. However, in a very recent study, a nomogram including tumor SUVmean on PSMA PET was found to be predictive of therapy-related outcomes in addition to time since diagnosis, previous chemotherapy status, baseline hemoglobin, and metastatic involvement was created to predict therapy-related outcomes.31
Albeit its prospective design, our cohort is a small and single-center study without a control arm. Moreover, differing management of the patients during and post-PSMA-RLT may have affected the OS.
5 CONCLUSIONS
PSA response patterns, PSA decline rate after the first RLT and pretherapy PSA doubling time are the most important predictors of OS in patients receiving Lu-177 PSMA RLT. FDG PET/CT can be utilized as additional decision-making for PSMA-RLT eligibility since high tumor heterogeneity and the presence of FDG > PSMA disease is associated with poor OS.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by the local ethics committee (Permission no: 17-AKD-157, 2019/04/23 KA-17123) and performed in accordance with the Declaration of Helsinki. All patients signed written informed consent.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.