The role of lymph node dissection in salvage radical prostatectomy for patients with radiation recurrent prostate cancer
Abstract
Purpose
To examine the effect of lymph node dissection on the outcomes of patients who underwent salvage radical prostatectomy (SRP).
Material and Methods
We retrospectively reviewed data from radiation-recurrent patients with prostate cancer (PCa) who underwent SRP from 2000–2016. None of the patients had clinical lymph node involvement before SRP. The effect of the number of removed lymph nodes (RLNs) and the number of positive lymph nodes (PLNs) on biochemical recurrence (BCR)-free survival, metastases free survival, and overall survival (OS) was tested in multivariable Cox regression analyses.
Results
About 334 patients underwent SRP and pelvic lymph node dissection (PLND). Lymph node involvement was associated with increased risk of BCR (p < .001), metastasis (p < .001), and overall mortality (p = .006). In a multivariable Cox regression analysis, an increased number of RLNs significantly lowered the risk of BCR (hazard ratio [HR] 0.96, p = .01). In patients with positive lymph nodes, a higher number of RLNs and a lower number of PLNs were associated with improved freedom from BCR (HR 0.89, p = .001 and HR 1.34, p = .008, respectively). At a median follow-up of 23.9 months (interquartile range, 4.7–37.7), neither the number of RLNs nor the number of PLNs were associated with OS (p = .69 and p = .34, respectively).
Conclusion
Pathologic lymph node involvement increased the risk of BCR, metastasis and overall mortality in radiation-recurrent PCa patients undergoing SRP. The risk of BCR decreased steadily with a higher number of RLNs during SRP. Further research is needed to support this conclusion and develop a precise therapeutic adjuvant strategy based on the number of RLNs and PLNs.
1 INTRODUCTION
Prostate cancer (PCa) is estimated to be the most commonly diagnosed cancer in men and the second leading cause of cancer-related deaths in the United States in 2020.1 It is estimated that approximately one-third of patients with clinically nonmetastatic PCa undergo primary radiation therapy, with 30%–60% of patients eventually experiencing biochemical recurrence (BCR).2-7 If not treated, approximately half of these patients will experience distant metastasis.5, 7, 8 Salvage radical prostatectomy (SRP) can offer durable disease control and the possibility of a cure for nonmetastatic radiation-recurrent patients with PCa.
Several studies have reported 10-year cancer-specific survival of up to 70%–83% for patients undergoing SRP.9-11 However, despite the potential survival benefit, SRP is still underutilized in clinical practice. According to the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database, only 2% of patients with radiation-recurrent prostate cancer undergo SRP.12 This is likely due to the fear of the associated high rate of complications that were described in earlier reports.13, 14 However, modern series have demonstrated a significantly reduced SRP morbidity, mainly due to improved radiation therapy and surgical techniques.9, 10
The role of pelvic lymph node dissection (PLND) in SRP is poorly investigated and its benefits are still inconclusive. Therefore, in this study, we sought to evaluate the impact of the number of removed lymph nodes (RLNs) and the number of positive lymph nodes (PLNs) in a large multicentric cohort of patients with radiation-recurrent clinically nonmetastatic PCa treated with SRP and PLND.
2 MATERIAL AND METHODS
2.1 Patient population and treatment
Six participating centers provided information on men treated with SRP. We retrospectively reviewed 334 patients with radiation-recurrent PCa treated with SRP between 2007 and 2016. Clinical staging was performed using conventional imaging and none of the patients had clinical lymph node involvement before SRP. All patients underwent SRP with concomitant PLND. The radiotherapy modalities included brachytherapy, external beam radiation therapy (EBRT), or between distinct radiotherapy techniques (EBRT and brachytherapy, EBRT and intensity-modulated radiation therapy, or EBRT and three-dimensional conformal radiation therapy). BCR after radiotherapy was defined as prostatic specific antigen (PSA) increase of ≥ 2 ng/ml greater than the nadir, according to the Radiation Therapy Oncology Group-American Society for Radiation Oncology-Phoenix criteria.15 A pre-SRP biopsy was performed in all patients to confirm the diagnosis of locally recurrent PCa. All patients underwent open surgical SRP with PLND.
All prostate specimens were staged according to the 2007 American Joint Committee on Cancer TNM staging system by experienced genitourinary pathologists.16, 17
2.2 Follow-up
The postoperative follow-up was performed according to institutional protocols. In general, the patients were followed every three months within the first two years and every six months thereafter. BCR after SRP was defined as a total PSA value of ≥ 0.2 ng/ml. No patients received adjuvant androgen deprivation therapy before the diagnosis of BCR. Distant metastases were identified using radiologic imaging.18 All study time intervals and follow-up durations were calculated from the date of surgery to the analyzed event.19
2.3 Statistical analysis
Univariable and multivariable Cox regression analyses were performed to determine the association of the number of RLNs and the number of PLNs with BCR free survival, metastases-free survival (MFS), and overall survival (OS). The association between the number of RLNs and the probability of lymph node involvement was assessed by logistic regression analysis. Results were considered significant if the two-sided p value was less than .05. Data analyses were performed using STATA 16 (Stata Corp.).
3 RESULTS
3.1 Clinical and pathologic characteristics
The clinical and pathologic features of the 334 men with radiation-recurrent PCa treated with SRP with PLND are summarized in Table 1. The median patients' age at SRP was 68 years (interquartile range [IQR]: 63–72). The median pre-SRP PSA was 3.55 ng/ml (IQR: 2.2–6.2) and Gleason Score (GS) ≥ 8 was found in 119 (35.8%) patients on pre-SRP biopsy. Pathologic lymph node involvement was found in 19.8% of the patients. The median number of removed lymph nodes was 13 (IQR: 10–17). The median follow-up duration was 23.9 months (IQR: 4.7–34.7).
| Patients number | 344 |
|---|---|
| Age at SRP, year, median (IQR) | 68 (63–72) |
| Total PSA before radiotherapy, median (IQR) | 6.2 (4.1–10) |
| Gleason score before radiotherapy, n (%) | |
| 6 | 130 (48.5) |
| 7 | 107 (39.9) |
| ≥8 | 31 (11.57) |
| Total PSA before SRP, median (IQR) | 3.55 (2.2–6.2) |
| Gleason score before SRP, n (%) | |
| 6 | 68 (19.8) |
| 7 | 145 (43.7) |
| ≥8 | 119 (35.8) |
| Pathological Gleason score, n (%) | |
| G6 | 28 (8.4) |
| G7 | 161 (48.5) |
| G ≥ 8 | 143 (43.1) |
| Positive surgical margins, n (%) | 87 (26.1) |
| Extracapsular extension, n (%) | 48 (14.4) |
| Seminal vesicle invasion, n (%) | 113 (33.8) |
| Lymph node metastasis, n (%) | 66 (19.8) |
| Number of removed lymph nodes, median (IQR) | 13 (10–17) |
| Number of positive lymph nodes, median (IQR) | 1 (1–2) |
- Abbreviations: IQR, interquartile range; PSA, prostatic specific antigen; SRP, salvage radical prostatectomy.
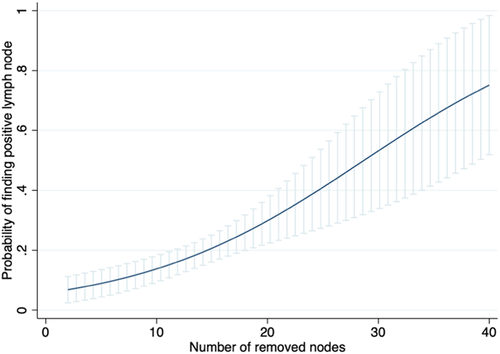
In a multivariable logistic regression analysis, a higher number of RLNs was associated with an increased probability of finding positive lymph nodes (odds ratio [OR] 1.11, 95% confidence interval [CI]: 1.05–1.17, p < .001) (Figure 1).
3.2 Association with biochemical recurrence (BCR)
During follow-up, 137 patients (41.1%) developed BCR. On multivariabke cox regression analysis, lymph node involvement was significantly associated with a higher risk of BCR (HR 1.75, 95% CI: 1.12–2.74, p = .014). On multivariable Cox regression analysis, the risk of BCR decreased with increased number of RLNs after adjustments for the effect of established pathologic confounders (HR 0.96, 95% CI: 0.93–0.99, p = .01) (Table 2).
| Biochemical recurrence | Metastasis | Overall mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Seminal vesicle invasion | 1.81 | 1.21–2.7 | .004 | 1.76 | 0.91–3.41 | .09 | 1.58 | 0.71–3.49 | .26 |
| Extracapsular extension | 2.24 | 1.35–3.74 | .002 | 2.21 | 0.92–5.3 | .074 | 0.79 | 0.17–3.63 | .76 |
| Positive surgical margins | 1.27 | 0.88–1.85 | .19 | 1.63 | 0.92–2.87 | .09 | 1.28 | 0.59–2.77 | .52 |
| Pathological Gleason score | |||||||||
| 7 | 1.43 | 0.61–3.39 | .41 | 1.79 | 0.22–14.19 | .58 | 1.02 | 0.21–4.88 | .98 |
| ≥8 | 3.02 | 1.27–7.18 | .012 | 9.69 | 1.27–73.93 | .029 | 4.07 | 0.86–19.21 | .08 |
| Number of removed lymph nodes (cont.) | 0.96 | 0.93–0.99 | .01 | 1.01 | 0.96–1.07 | .58 | 0.93 | 0.86–1.004 | .064 |
- Abbreviations: CI, confidence interval; HR, hazard ratio.
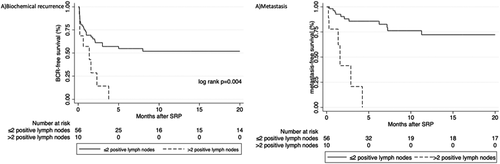
In a subgroup of 66 patients with positive lymph node involvement (Table 3), multivariable Cox regression analysis revealed that a higher number of RLNs and a lower number of PLNs were both associated with improved freedom from BCR (HR 0.89, 95% CI: 0.83–0.96, p = .001 and HR 1.34, 95% CI: 1.08–1.66, p = .008, respectively). Patients with at least two positive lymph nodes had a significantly higher risk of developing BCR (log-rank p < .001) (Figure 2A).
| Biochemical recurrence | Metastasis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Seminal vesicle invasion | 1.52 | 0.59–3.92 | 0.39 | 0.69 | 0.19-2.46 | 0.57 |
| Extracapsular extension | 1.99 | 0.64–6.22 | 0.24 | 0.99 | 0.19-5.14 | 0.99 |
| Positive surgical margins | 0.45 | 0.19–1.06 | 0.07 | 1.56 | 0.56-4.31 | 0.39 |
| Pathological Gleason score | 0.88 | 0.60–1.29 | 0.52 | 1.93 | 1.11-3.33 | 0.019 |
| Number of removed lymph nodes (cont.) | 0.89 | 0.84–0.96 | 0.001 | 0.98 | 0.91-1.06 | 0.67 |
| Number of positive nodes (cont.) | 1.37 | 1.11–1.69 | 0.003 | 1.31 | 1.02-1.67 | 0.033 |
- Abbreviations: CI, confidence interval; HR, hazard ratio.
3.3 Association with metastasis
During follow-up, 53 patients (15.9%) developed metastasis. Lymph node involvement was associated with an increased risk of developing metastasis (HR 3.93, 95% CI: 2.26–6.85, p < .001). On a multivariable Cox regression analysis, the number of RLNs did not influence the risk of developing metastasis after adjustments for the effects of established pathologic confounders (HR 1.01, 95% CI: 0.96–1.07, p = .58) (Table 2).
In a subgroup of 66 patients with positive lymph node involvement, a multivariable Cox regression analysis revealed no statistically significant association between the number of RLNs and the risk of metastasis (HR 0.98, 95% CI: 0.91–1.06, p = .67). A higher number of PLNs showed a statistically significant increase risk of metastasis (HR 1.31, 95% CI: 1.02–1.67, p = .033) (Table 3). Patients with more than or equal to two positive lymph nodes had a significantly higher risk of experiencing metastasis (log-rank p = .004) (Figure 2B).
3.4 Association with overall survival (OS)
During follow-up, 32 patients (9.6%) died. Lymph node involvement was associated with an increased risk of overall mortality (HR 2.88, 95% CI: 1.36–6.13, p = .006). On a multivariable Cox regression analysis, the number of RLNs was not associated with OS (HR 0.93, 95% CI: 0.86–1.004, p = .064)) (Table 2).
In a subgroup of 66 patients with positive lymph node involvement, a multivariable Cox regression analysis showed no statistically significant association between the number of RLNs and the number of PLNs with OS (HR 0.94, 95% CI:0.82–1.06, p = .31 and HR 1.08, 95% CI: 0.61–1.91, p = .78, respectively).
4 DISCUSSION
In this large multicentric study, we tested the impact of the number of RLNs and the number of PLNs in patients with radiation-recurrent clinically nonmetastatic PCa treated with SRP and PLND.
Patients with pathologic lymph node involvement were at significantly increased risk of BCR, metastasis, and overall mortality. Although non of the patients in our study had clinical lymph node involvement, 19.8% of patients were found to have pathologic lymph node involvement, which is comparable to previous SRP reports. For example, Heidenreich et al.10 reported a 20% rate of positive lymph nodes in patients undergoing SRP with PLND, highlighting the important staging role of PLND in these patients.
One of the key findings of our study was that a higher number of RLNs leads to lower risk of BCR. Several studies have investigated the prognostic effect of the number of RLNs in primary radical prostatectomy yielding contrasting results.20-22 However, due to the different natural history of radiation-recurrent PCa undergoing SRP, data from primary radical prostatectomy cannot be extrapolated to radiation-recurrent PCa patients planned for SRP.16, 17, 23 In a report from the Surveillance, Epidemiology, End Results registry (SEER) evaluating 364 patients who underwent SRP between 1988 and 2010, Pokala et al.24 did not find a significant association between the number of RLNs and cancer-specific survival (HR 0.5, 95% CI: 0.2–1.4, p = .2).24 However, a more recent analysis of the SEER database which included patients who were treated between 2004 and 2016, Wenzel et al.25 did demonstrate a significantly lower cancer-specific mortality with a higher number of removed nodes (HR: 0.61, CI: 0.40–0.91; p = .02). Both studies did not report on the association with BCR. In our study of 334 SRPs performed at tertiary referral centers, we demonstrated that the number of RLNs was associated with the risk of BCR. Although the clinical relevance of BCR and its impact on survival have been debated in general, in this cohort of salvage patients, BCR is certainly a more reliable surrogate endpoint for OS.26 Moreover, it is possible that the adverse events associated with androgen depreviation therapy (ADT), which is used in patients who develop BCR, can contribute to the worse OS.27 In general, we believe that further studies with longer follow-up are needed to determine the prognostic value of BCR after SRP.
The extent of lymph node dissection is an important factor in the likelihood of finding positive lymph nodes. In our study, there was a significantly higher probability of detecting positive lymph nodes with an increased number of RLNs. The relationship between increased node yield and the probability of finding lymph node involvement has not been previously evaluated in SRP patients. However, this association was demonstrated in patients undergoing primary RP. Briganti et al.28 evaluated 858 patients treated with primary RP and extended PLND. In their study, they demonstrated a 90% ability to detect positive nodes in patients with more than or equal to 28 RLNs as compared to less than 10% in patients in patients with less than or equal to 10 RLNs.28 In the case of primary radical prostatectomy, patients with lymph node involvement would be informed about the risks, benefits, and alternatives to adjuvant therapy. For SRP, there is currently no optimal management, with irradiation of the lymph nodes area if not done previously or androgen deprivation therapy being possible options. Nevertheless, our results indicate that patients with radiation-recurrent PCa, treated with SRP should undergo extensive PLND, which provides a better staging of the disease and may even result in better short-term oncologic outcomes. Despite concerns of the associated high rate of complications that were described in old reports,13, 14 modern series have demonstrated a reduced SRP morbidity, mainly due to improved radiation therapy and surgical techniques.9, 10
Our study has limitations, including its retrospective study design. Furthermore, no central pathological review was performed. We were not able to report on other clinically meaningful events such as cancer-specific survival because of the limited follow-up duration.29, 30 Moreover, all patients in our series were staged using conventional imaging, due to the limited availability of prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT) at the time of study enrollment.31-33 Indeed, several studies have demonstrated the superiority of PSMA PET/CT over conventional imaging in staging patients with radiation recurrent PCa.34, 35 Despite these limitations, we were able to present the first study to comprehensively analyze the role of lymph node yield and the number of positive lymph nodes in patients with radiation recurrent PCa treated with SRP.
5 CONCLUSION
Lymph node involvement increased the risk of BCR, metastasis, and overall mortality in radiation-recurrent PCa patients undergoing SRP. A higher number of removed lymph nodes improved staging leading to a higher probability of detecting lymph node metastasis. Furthermore, an increased number of removed nodes during SRP reduced the risk of BCR, hinting at a possible therapeutic benefit. Moreover, the poor prognosis of patients with more than two positive lymph nodes highlights the need for further adjuvant treatments and closer follow-up of these patients. We believe the findings in our study should support performing extended PLND in patients undergoing SRP. Further research with standardized lymph node templates is still needed to support this conclusion and develop a precise therapeutic adjuvant strategy based on the number of RLNs and the number of PLNs.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Fahad Quhal: Protocol/project development, Data analysis, Manuscript writing/editing. Pawel Rajwa: Data analysis, Manuscript writing/editing. Keiichiro Mori: Data analysis. Ekaterina Laukhtina: Data analysis, Manuscript writing/editing. Nico C. Grossmann: Data analysis, Manuscript writing/editing. Victor M. Schuettfort: Manuscript writing/editing. Frederik König: Data analysis, Manuscript writing/editing. Abdulmajeed Aydh: Data analysis, Manuscript writing/editing. Reza Sari Motlagh: Manuscript writing/editing. Satoshi Katayama: Data analysis, Manuscript writing/editing. Hadi Mostafai: Manuscript writing/editing. Benjamin Pradere: Data analysis, Manuscript writing/editing. Giancarlo Marra: Manuscript writing/editing. Paolo Gontero: Manuscript writing/editing. Romain Mathieu: Manuscript writing/editing. Pierre I. Karakiewicz: Manuscript writing/editing. Alberto Briganti: Manuscript writing/editing. Shahrokh F. Shariat: Protocol/project development, Data collection or management, Manuscript writing/editing. Axel Heidenreich: Protocol/project development, Data collection or management, Manuscript writing/editing.
RESEARCH INVOLVING HUMAN PARTICIPANTS AND/OR ANIMALS
This article does not contain any studies with animals performed by any of the authors.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




