Enhancing schistosomiasis drug discovery approaches with optimized proteasome substrates
Elany B. Silva, Zhenze Jiang, and Chenxi Liu are co-first authors.
Review Editor: John Kuriyan
Abstract
Schistosomiasis, a neglected tropical disease infecting over 200 million people globally, has limited therapeutic options. The 20S proteasome is a validated drug target for many parasitic infections, including those caused by Plasmodium and Leishmania, and we have previously demonstrated antischistosomal activity with inhibitors targeting Schistosoma mansoni 20S proteasome (Sm20S). Here, we developed optimized subunit-specific substrates for Sm20S based on data generated by Multiplex Substrate Profiling by Mass Spectrometry (MSP-MS). These substrates exhibit 9-fold or more improved activity compared to traditional human constitutive 20S proteasome (c20S) substrates. The optimized substrates also eliminated the need for extensive Sm20S purification, as robust enzyme activity could be detected in parasite extracts following an ammonium sulfate precipitation step. Finally, we show that the substrate and inhibition profiles for the 20S proteasome from the three medically important schistosome species are similar. This suggests that Sm20S-focused inhibitor development can be efficiently extrapolated to the other schistosome species, leading to significant time and resource savings.
1 INTRODUCTION
Schistosomiasis, also known as bilharzia, is a neglected tropical disease caused by blood flukes of the Schistosoma genus. This debilitating illness thrives in areas lacking safe drinking water and sanitation, and the World Health Organization estimates 251.4 million people are in need of treatment (WHO 2023). Three main species, Schistosoma mansoni, Schistosoma haematobium and Schistosoma japonicum, are responsible for human schistosomiasis. Adult worms in the bloodstream release eggs that become lodged in organs like the spleen, liver, intestine, and/or bladder. These eggs trigger chronic inflammation and fibrosis, leading to fatigue, pain, and disability. The resulting lost school days for children and reduced worker productivity create a vicious cycle, whereby schistosomiasis both perpetuates and stems from poverty (Colley et al. 2014; McManus et al. 2018; Verjee 2019).
Praziquantel (PZQ) has been the cornerstone of schistosomiasis treatment and control since the early 1980s (Andrews et al. 1983; Caffrey 2015; Caffrey et al. 2019), and is primarily delivered via mass drug administration programs to reduce disease burden at the community level (Mwanga et al. 2020). Although reasonably effective at the standard single 40 mg kg−1 oral dose (Cioli et al. 2014; Fukushige et al. 2021; Hailegebriel et al. 2021; Zwang and Olliaro 2017), PZQ has several pharmacological and pharmaceutical limitations. These include incomplete efficacy across all parasite growth stages, a racemic composition that renders half the dose inactive (contributing to large, unpleasant-tasting tablets), and rapid metabolism to inactive components such that exposure of the parasite to the parent active ingredient is brief (Badoco et al. 2022; Caffrey 2015; Caffrey et al. 2019; Cioli et al. 2014; Danso-Appiah et al. 2022; Gryseels et al. 2001; Sabah et al. 1986; Wolfe et al. 2018). Furthermore, the threat of drug resistance is ever-present (Melman et al. 2009; Summers et al. 2022). Consequently, these PZQ-related drawbacks have spurred the search for new drugs.
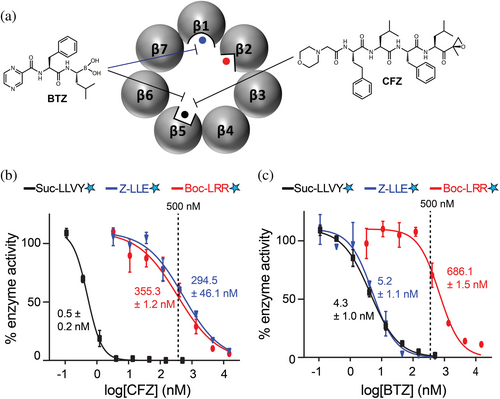
Among the potential molecular drug targets discussed over the years for chemotherapy of schistosomiasis, a more recent addition has been the proteasome (Bibo-Verdugo et al. 2017; Bibo-Verdugo et al. 2019; do Patrocinio et al. 2020) which is an evolutionarily conserved, multi-subunit, ATP-dependent proteolytic complex that is vital to cellular proteostasis (Bard et al. 2018; Sahu and Glickman 2021). Each 20S proteasome core comprises two stacked rings of seven β subunits between two rings of seven α subunits. Among the seven β subunits in each ring, three (β1, β2, and β5) are proteolytically active (Figure 1a), and, based on their peptidyl cleavage specificities, are described as having caspase-, trypsin-, and chymotrypsin-like specificities, respectively. The 20S proteasome is often associated with 19S regulatory particles at one or both ends, forming the 26S or 30S proteasome, respectively. Proteins identified for degradation are ubiquitinated and then recognized by the 19S regulatory particle. The proteins are unfolded in an ATP-driven process and then threaded into the 20S proteolytic core for degradation into short peptides. The human constitutive 20S proteasome (c20S) is a well-established target for various cancers, and the US Food and Drug Administration has approved three small-molecule inhibitors, bortezomib (BTZ) (Chen et al. 2011), carfilzomib (CFZ) (Groen et al. 2019), and ixazomib (IXZ) (Ramirez 2017). These drugs preferentially target β5; however, at higher concentrations, BTZ and IXZ also inhibit β1 while CFZ inhibits β2 (Altun et al. 2005; Augello et al. 2018; Schots et al. 2012). In addition, the marine natural product, marizomib (MZB), is a brain-penetrable proteasome inhibitor that preferentially targets the β5 subunit but also inhibits β1 and β2 at higher concentrations (Potts et al. 2011). MZB is in phase 3 clinical trials for the treatment of glioblastoma (Bota et al. 2021).
Despite being evolutionarily conserved, key differences between human and parasite proteasomes have been identified and exploited to develop selective inhibitors (Bennett et al. 2023; Bibo-Verdugo et al. 2019; LaMonte et al. 2017; Lawong et al. 2024; Li et al. 2016; Liu et al. 2024a; Nagle et al. 2020; O'Donoghue et al. 2019; Robbertse et al. 2024; Wyllie et al. 2019), the most prominent of which are two drug candidates (GSK245 and LXE408) currently undergoing clinical trials for leishmaniasis (Nagle et al. 2020; Wyllie et al. 2019). Potent inhibitors of the Plasmodium falciparum proteasome (Pf20S) have been identified using various approaches, such as analyzing how the proteasome processes specific substrates (cleavage profiling) (Li et al. 2016) and the screening of potential inhibitor libraries (Kirkman et al. 2018; Li et al. 2012; Xie et al. 2018). These efforts have yielded covalent inhibitors incorporating different chemical reactive groups (epoxyketone (Almaliti et al. 2023; LaMonte et al. 2017), vinyl sulfone (Yoo et al. 2018), or boronic acid (Xie et al. 2018)) with selectivity indices of up to 2640 between parasite and human cells. Our own research has focused on synthesizing analogs of carmaphycin B, a marine natural product containing the epoxyketone group, resulting in compounds that are effective in a mouse model of malaria (Almaliti et al. 2023; LaMonte et al. 2017).
We previously demonstrated that targeting the S. mansoni 20S (Sm20S) in vitro with proteasome inhibitor drugs approved for cancer treatment leads to immobility and, ultimately, fatal degenerative changes in the adult worm (Bibo-Verdugo et al. 2017). In addition, using a small collection of carmaphycin B analogs, we identified carmaphycin-17 as a hit compound that decreased worm mobility by >95% after 24 h at 1 μM while also having a 27-fold lower cytotoxicity to HepG2 cells relative to carmaphycin B (Bibo-Verdugo et al. 2019). This suggests that a path forward exists to build carmaphycin B-based inhibitors with greater specificity for the Sm20S compared to the c20S anti-target.
Here, we describe the purification of Sm20S and define its substrate cleavage specificities using Multiplex Substrate Profiling by Mass Spectrometry (MSP-MS) (Small et al. 2013). MSP-MS is a rapid, simple, and versatile mass spectrometry-based peptide digestion assay used to detect and quantify proteolytic activity (Lapek et al. 2019; O'Donoghue et al. 2012; Rohweder et al. 2023). It consists of a substrate library of 228 synthetic 14-mer peptides with high sequence diversity and a total of 2964 cleavable bonds. This assay has been used to uncover the substrate specificity of numerous endoproteases and exopeptidases from bacteria, fungi, insects, and mammalian cells (Beekman et al. 2018; Jiang et al. 2021; Lysyk et al. 2021; Maffioli et al. 2020; O'Donoghue et al. 2015; O'Donoghue et al. 2025; Small et al. 2013). MSP-MS has also been used to uncover the proteolytic activity of c20S, in addition to the human immunoproteasome and the Trichomonas vaginalis proteasome (Fajtova et al. 2024; Winter et al. 2017). In this study, we used MSP-MS to reveal that each of the Sm20S catalytic subunits cleaves at distinct sites within the peptide library, therefore revealing that Sm20S has three distinct substrate recognition sites. The data arising from this study were then used to design fluorogenic peptidyl substrates that are each specific for the three proteolytically active subunits. We show that each new substrate is more efficiently cleaved by Sm20S compared to the c20S substrates that were previously used (Bibo-Verdugo et al. 2019). Part of this process involved developing a method to measure Sm20S proteolysis in extracts. Last, we demonstrate that the rate of cleavage for the new substrates is similar between the three schistosome species most responsible for human disease. This suggests that a single campaign of inhibitor development focusing on Sm20S may be sufficient to develop a pan-schistosomicide, consistent with the target product profile for schistosomiasis (Caffrey 2007; Caldwell et al. 2023).
2 METHODS
2.1 Preparation of schistosomes
The acquisition and preparation of mixed-sex adult S. mansoni have been described (Abdulla et al. 2007; Abdulla et al. 2009). The Naval Medical Research Institute (NMRI) isolate of S. mansoni was cycled between Biomphalaria glabrata snails and male Golden Syrian hamsters (infected at 4–6 weeks of age) as intermediate and definitive hosts, respectively. In brief, adult schistosomes were harvested from hamsters 42 days post-infection in RPMI or DMEM and washed five times prior to maintenance overnight at 37°C and 5% CO2 in Basch medium (Basch 1981) containing 4% heat-inactivated FBS, 100 μg mL−1 streptomycin, and 100 U mL−1 penicillin. Worms were then washed five times in RPMI or DMEM prior to freezing at −80°C. The use of small vertebrate animals was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California San Diego. UCSD-IACUC derives its authority for these activities from the United States Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, and the Animal Welfare Act and Regulations (AWAR). Frozen, mixed-sex adult S. haematobium and S. japonicum adult worms were provided to us by the Schistosomiasis Resource Center at the Biomedical Research Institute, Rockville, MD, USA (www.beiresources.org).
2.2 Purification of the S. mansoni 20S proteasome (Sm20S)
Adult, mixed-sex S. mansoni worms were washed and homogenized using a motorized Teflon pestle in 1.5 mL tubes containing ice-cold 100 mM Tris–HCl, 100 μM E-64, pH 7.5. Protein extracts were centrifuged for 15 min at 14,000 g and 4°C, and the supernatant was subjected to two ammonium sulfate precipitation steps on ice at 30% and 60% saturation, respectively, each for 60 min. After centrifugation for 15 min at 14,000g and 4°C, the supernatant was discarded, and the precipitated proteins were resuspended in ice cold 100 mM Tris–HCl, pH 7.5, 100 μM E-64. The samples were enriched for Sm20S when concentrated using 100 kDa centrifugal filter units (Amicon). Protein concentration was quantified using the Pierce BCA kit.
Samples were either used for enzyme assays (defined as Sm20S-enriched) or subjected to column chromatography for Sm20S purification. For the latter, samples were loaded onto a Superose 6 10/300 gel filtration column under the control of an ÄKTA Pure instrument (GE Healthcare Life Sciences). Proteins were eluted using 50 mM HEPES, 10% glycerol, 0.125M NaCl, pH 7.5. Fractions of 0.5 mL were collected and assayed for proteasome activity with 25 μM Succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-amc) in assay buffer (50 mM Tris, 0.02% SDS, pH 7.5). The assay was performed at 24°C using a Synergy HTX multi-mode reader (ex/em = 360 nm/460 nm; BioTek Instruments). Fractions containing proteasome activity were pooled and loaded onto a 5-mL anion exchange HiTrap DEAE FF column (Sigma). Proteins were eluted using 50 mM HEPES, 10% glycerol, pH 7.5, and a linear gradient of 0.125 to 0.6M NaCl. Fractions (1.5 mL) were collected and assayed with Suc-LLVY-amc as described above. Fractions containing proteasome activity were pooled, concentrated using a 100 kDa centrifugal filter unit (Amicon), and stored at −80°C.
2.3 Concentration-response assays
CFZ and BTZ were dissolved in 100% DMSO to a final concentration of 10 mM. Each compound was then 3-fold serially diluted 10 times in 100% DMSO such that the concentration ranged from 3.33 mM to 169 μM. Each dilution was then further diluted 250-fold in 20 mM Tris–HCl pH 7.5, 0.02% SDS containing 20 nM Sm20S. The enzyme and inhibitor were preincubated for 15 min, and then 15 μL was added into 9 wells on a 384-well plate containing either 15 μL 20 μM Suc-LLVY-amc, 20 μM Boc-LRR-amc, or 20 μM Z-LLE-amc in 20 mM Tris–HCl pH 7.5, 0.02% SDS. The final concentration of enzyme and substrate in each well was 10 nM and 10 μM, respectively, while the final inhibitor concentration ranged from 740 to 0.3 nM for the most potent inhibitors and 20 μM to 9 nM for the less potent inhibitors. A control reaction consisted of 0.% DMSO in place of CFZ and BTZ. Assays were performed at room temperature for 2 h, with readings recorded at 2-min intervals. The maximum velocity over eight sequential readings was calculated and reported as relative fluorescent units per sec (RFU/s). Activity was normalized to the control reaction (0.1% DMSO) and concentration-response curves were generated using GraphPad Prism (version 10.0.0) with a four parameter logistic curve fitting.
2.4 Proteasome MSP-MS
MSP-MS was performed with a library of 228 synthetic tetradecapeptides that were designed to contain all neighbor and near-neighbor cleavage sites (Lapek et al. 2019; O'Donoghue et al. 2012). Sm20S was first incubated with 500 nM BTZ, CFZ, or 0.1% DMSO (vehicle control). Assays were conducted in quadruplicate by incubating Sm20S with the peptide library at a final concentration of 0.5 μM for each peptide in 50 mM Tris–HCl, pH 7.5. Assays were incubated at 25°C for 15, 60, and 240 min. At each time point, 20 μL of the reaction mixture was removed and quenched by the addition of 80 μL 8M GuHCl. Samples were immediately stored at −80°C. Control reactions consisted of Sm20S pre-incubated with GuHCl to inactivate the enzyme prior to the addition of the peptide library. All samples were desalted using custom-made C18 spin tips and dried in a vacuum centrifuge.
Samples were resuspended in 40 μL 0.1% formic acid, and ~0.4 μg peptides were injected into a Q-Exactive Mass Spectrometer equipped with an Ultimate 3000 HPLC (Thermo). Peptides were separated by reverse phase chromatography on a C18 column (1.7 μm bead size; 75 μm × 25 cm; 65°C) at a flow rate of 300 nL min−1 using a 60-min linear gradient of 5%–30% solvent B (0.1% formic acid in acetonitrile), with solvent A being 0.1% formic acid in water. Survey scans were recorded over a 150–2000 m/z range (70,000 resolutions at 200 m/z, AGC target 3 × 106, 100 ms maximum). MS/MS was performed in a data-dependent acquisition mode with HCD fragmentation (28 normalized collision energy) on the 12 most intense precursor ions (17,500 resolutions at 200 m/z, AGC target 1 × 105, 50 ms maximum, dynamic exclusion 20 s). Data were processed using PEAKS 8.5 (Bioinformatics Solutions, Inc.). MS2 data were searched against the tetradecapeptide library sequences with decoy sequences in reverse order. A precursor tolerance of 20 ppm and 0.01 Da for MS2 fragments were defined. No protease digestion was specified. Data were filtered to a 1% peptide level false discovery rate with the target-decoy strategy. Peptides were quantified with label-free quantification, and data were normalized by median and then filtered by 0.3 peptide quality. Missing and zero values were imputed with random normally distributed numbers in the range of the average of the smallest 5% of the data ± SD. All mass spectrometry data generated in this study can be accessed on the MASSive repository at this link: ftp://massive.ucsd.edu/v07/MSV000096255/.
Cleaved peptides were identified in each dataset as having an 8-fold (q < 0.05) or more increase in intensity between 0 and 3 h of incubation. Only cleavage sites located between the 4th and 13th amino acids in the 14-mer peptides were considered. The P4 to P4′ amino acids were inputted into the iceLogo software (Colaert et al. 2009) and compared to all possible P4 to P4′ sequences in the library. Icelogo plots were generated to illustrate the frequency of amino acids present around the cleavage site.
2.5 Proteasome activity and inhibition assays
Subunit-specific fluorogenic substrates were custom synthesized and purified by HPLC to >95% by GenScript (New Jersey). Substrates contained either an N-terminal acetylation group and a C-terminal amc group, or an N-terminal 7-methoxycoumarin (mca) and a C-terminal lysine 2,4-dinitrophenyl (K(dnp)). Fluorogenic activity assays were performed in black, round-bottomed 96-well plates. Protein extracts from Schistosoma sp. were incubated with inhibitors for 1 h at room temperature, and activity was assayed in a 50-μL total volume containing 7.5 μg of protein and 25 μM Suc-LLVY-amc in 20 mM Tris–HCl, 10 μM E-64, 0.02% SDS, pH 7.5. For purified Sm20S assays, 10 nM of the enzyme was incubated with a 2-fold serial dilution of the substrate. Controls contained 0.0001% DMSO. Fluorescence was monitored at 24°C in a Synergy HTX multi-mode reader (BioTek Instruments, Winooski, VT). Excitation and emission wavelengths for the amc and mca substrates were 360 and 460 nm, and 320 and 400 nm, respectively. Protease activity was quantified as RFU min−1 μg−1 protein and normalized to DMSO control reactions.
2.6 Detection of 20S proteasome activity in three Schistosoma species using a fluorescent probe
Schistosoma mansoni, S. haematobium and S. japonicum protein extracts were subjected to two ammonium sulfate precipitation steps on ice at 30% and 60% saturation for 60 min. The proteasome-containing fractions were concentrated and buffer exchanged into 100 mM Tris–HCl, 50 μM E-64, 2 mM AEBSF, 2 μM pepstatin, pH 7.5, using a 100 kDa centrifugal filter unit (Amicon). Protein was quantified using the bicinchoninic acid assay, and 2.5 and 5 μg protein were incubated with 2 μM of the activity-based probe, Me4BodipyFL-Ahx3Leu3VS (R&D Systems, #I-190) (Berkers et al. 2007) at 37°C for 3 h. Samples were mixed with 4X NuPage lithium dodecyl sulfate loading buffer (Invitrogen), heated to 90°C for 5 min, and then loaded onto 12% NuPAGE Bis-Tris Gels (Invitrogen). Proteins were separated using NuPAGE MOPS (3-(N-morpholino)propanesulfonic acid) as the running buffer. Twenty nanomolar of human constitutive 20S proteasome (c20S) was used as a positive control. Direct in-gel visualization of Me4BodipyFL-Ahx3Leu3VS-labeled proteasome subunits was performed using a ChemiDoc MP fluorescence scanner (Biorad) with a 530 nm emission filter.
2.7 Proteasome activity and inhibition assays in three Schistosoma species
Extracts of S. haematobium (0.625 μg μL−1), or S. japonicum and S. mansoni (each 1.25 μg μL−1) were enriched for proteasome by ammonium sulfate precipitation and pre-incubated for 1 h with 500 nM BTZ, CFZ, MZB, or the carmaphycin B analog, CP-17. DMSO (0.125%) was used as a vehicle control. Proteolytic activity was quantified following the addition of an equal volume of 20 μM mca-VDQMDGW-K(dnp)-NH2 (β1-substrate), mca-FnKRR-K(dnp)-NH2 (β2-substrate), or 40 μM Ac-FNKL-amc (β5-substrate). Assays were performed in 100 mM Tris–HCl, 50 μM E-64, 1 mM AEBSF, 2 μM pepstatin, 0.03% SDS, pH 7.5, in a total volume of 8 μL in a 384-well plate (Greiner Bio-One #784900). The release of the fluorophore was recorded at 24°C in a Synergy HTX plate reader with excitation/emission set to 360/460 nm for the β5 substrate, and 320/400 nm for the β1 and β2 substrates. Experiments were performed in triplicate wells, and DMSO was used as a vehicle control.
2.8 Protein and structure alignment
Protein sequences were obtained from UniProt that included P28072 (human β1), G4V926 (S. mansoni β1), C1L5H5 (S. japonicum β1), A0A922LVA0 (S. haematobium β1), Q99436 (human β2), G4VSW3 (S. mansoni β2), Q5DEZ9 (S. japonicum β2), A0A922LT82 (S. haematobium β2), P28074 (human β5), A0A5K4F079 (S. mansoni β5), Q5DHC0 (S. japonicum β5), and A0A094ZZD4 (S. haematobium β5). Sequence alignments were performed using MUSCLE (Madeira et al. 2024), and the results were visualized with Jalview (Waterhouse et al. 2009). A structural analysis of the active site was performed using human proteasome structures available in the Protein Data Bank and the Schistosoma proteasome structures predicted by AlphaFold and available on UniProt. For the analysis of the human β1 and β5 sites, the PDB ID: 6RGQ (Toste Rêgo and da Fonseca 2019) structure was used, while PDB ID: 4R3O (Harshbarger et al. 2015) was used for the β2 site as 6RGQ lacked the complete side-chain structure of certain residues. The β1 and β2 subunit structures of S. haematobium were unavailable on UniProt; therefore, these proteins were modeled using ColabFold (Mirdita et al. 2022) (v1.5.5) with default parameters in a Google Colab notebook.
3 RESULTS
3.1 Revealing the substrate specificities of the Sm20S β catalytic subunits
We previously isolated Sm20S and identified inhibitors that target the catalytic β2 and β5 subunits (Bibo-Verdugo et al. 2019). To generate those data, we repurposed three human proteasome substrates, Z-LLE-amc, Boc-LRR-amc, and Suc-LLVY-amc, that had been designed to detect the activity of the c20S β1, β2, and β5 subunits, respectively. Based on the sequence similarity of the c20S β1, β2, and β5 subunits with their equivalent subunits in Sm20S, we predicted that each of the three classical c20S subunit-specific substrates would also be cleaved by the respective β subunits of Sm20S. However, when comparing the turnover rate of these substrates by c20S and Sm20S, it was evident that they were more efficiently cleaved by c20S (Bibo-Verdugo et al. 2019). This encouraged us to develop Sm20S subunit-specific substrates that would be cleaved with higher efficiencies and serve as valuable tools to screen for new inhibitors of Sm20S, to uncover the importance of each catalytic subunit for the growth and survival of S. mansoni and to provide a starting point for rational inhibitor design. Accordingly, we utilized MSP-MS to define the cleavage specificities of each of the three catalytic β subunits.
We have previously shown that c20S, the human 20S immunoproteasome (i20S), and Pf20S each cleave at more than 200 sites within the MSP-MS peptide library (Li et al. 2016; Winter et al. 2017). In this study, we chromatographically isolated Sm20S and incubated it with the same peptide library for 1 and 3 h, which resulted in the detection of 164 sites and 252 cleavage sites, respectively. The frequency of each amino acid found in the P4 to P4′ sites surrounding the cleavage site was evaluated using iceLogo, which revealed that Sm20S has a strong preference for cleavage of peptides with Lys in the P2 position, Arg in P3, P1′ and P2′, and Trp in P4 (Figure S1, Supporting Information).
It is not clear which of the three catalytic β subunits was responsible for cleaving each of these peptides, and their physical separation is not possible without inactivation of the whole complex. Accordingly, we used inhibitors to selectively inactivate one or more of the Sm20S catalytic β subunits such that the activity of the remaining non-inhibited subunits could be measured. To do this, we generated a concentration-response curve with CFZ and determined that it inhibits the β5 subunit more potently than either β1 or β2. In the presence of 500 nM CFZ, the β5 subunit activity measured using Suc-LLVY-amc was strongly inhibited, whereas the activities of β1 (Z-LLE-amc) and β2 (Boc-LRR-amc) were only reduced by ~40% (Figure 1b). Therefore, 500 nM CFZ was used for follow-up MSP-MS studies to distinguish β5 activity from β1 and β2.
Next, we performed a concentration-response study with BTZ and showed that the β5 and β1 subunits were preferentially inhibited over β2. Using 500 nM BTZ, β5 and β1 are strongly inhibited, whereas β2 activity was reduced by only ~35% (Figure 1c). Thus, the data reveal that BTZ distinguishes β1 and β5 activities from β2. The cleavage specificity of β1 can then be determined as enzyme activity that is preferentially inhibited by BTZ relative to CFZ, whereas the β2 activity is weakly inhibited by both. By understanding the inhibitor conditions needed to isolate one or the other β catalytic activity, we then performed MSP-MS in the presence of either 500 nM CFZ or BTZ to determine which subunit was responsible for cleaving which peptides.
3.2 Development of a Sm20S β5 substrate
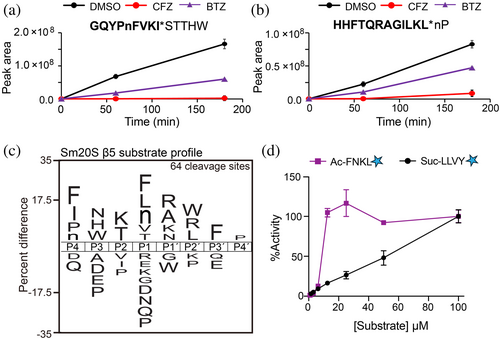
MSP-MS cleavage products generated in the absence (DMSO) and presence of CFZ were compared. The intensity of 64 of those cleavage products was reduced by 2.5-fold or more in the presence of CFZ (Data S1). Two example peptides that show this pattern are GQYPnFVKI*STTHW and HHFTQRAGILKL*nP wherein cleavage occurs at the site marked with an asterisk. In these peptides, the lowercase n corresponds to norleucine, an isostere of methionine. The N-terminal cleavage products (highlighted in bold in Figure 2a,b) increased with time in the uninhibited reaction (0.1% DMSO) but did not increase in the presence of CFZ. BTZ also reduced cleavage of these sites, albeit with lower potency than CFZ. We generated a substrate specificity profile of the amino acids in the P4 to P4′ positions of these 64 cleavage sites and found a preference for hydrophobic amino acids at P4 and P1, N, H, and W at P3, and K and T at P2 (Figure 2c). On the prime side, A, R, K, and N were most frequently found at P1′ while W, F, and P were found at P2′, P3′, and P4′, respectively. Using this information, we synthesized the substrate, Ac-FNKL-amc, which contains several amino acids that are favored by Sm20S β5 in the P4 to P1 positions and compared its hydrolysis to that of the c20S substrate, Suc-LLVY-amc. At increasing concentrations, we show that cleavage of Ac-FNKL-amc is maximal at 25 μM and occurs at a ~4-fold higher rate than with Suc-LLVY-amc at the same concentration (Figure 2d).
3.3 Development of a Sm20S β1 substrate
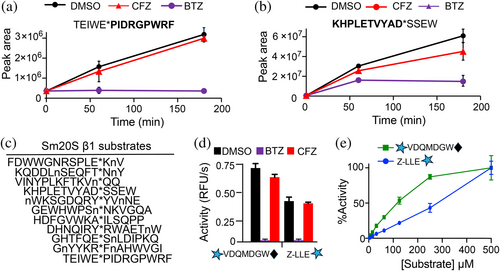
We next evaluated the MSP-MS cleavage products that were strongly inhibited by 500 nM BTZ relative to 500 nM CFZ, that is, isolating for β1 activity. We found 11 cleavage products that matched this profile. Example peptides that are cleaved under these conditions include TEIWE*PIDRGPWRF (Figure 3a) and KHPLETVYAD*SSEW (Figure 3b), whereby the cleavage products increase over time in the presence of vehicle (0.1% DMSO), but this product formation is reduced in the presence of BTZ and less so with CFZ. Many of these peptides had either Glu or Asp in the P1 position, a finding that is common for the β1 subunit of other proteasomes (Fajtova et al. 2024; Kisselev et al. 2003). We attempted to synthesize several tetrapeptide-amc substrates but had low yield and purity due to inefficient coupling of Glu and Asp to the amc group. Therefore, we searched for commercially available protease substrates containing E or D that could be cleaved by Sm20S β1. We found that the caspase-3 substrate, mca-VDQMD*GW-K(dnp) was hydrolyzed by Sm20S. Importantly, this activity was inhibited by 100 nM BTZ but not by 100 nM CFZ, which is similar to the inhibition profile generated when using the human c20S β1 subunit, LLE-amc (Figure 3d). When both substrates were evaluated over a concentration range of 2–500 μM, VDQMD*GW was cleaved with a ~2-fold greater efficiency between 16 and 250 μM (Figure 3e).
3.4 Development of a Sm20S β2 substrate
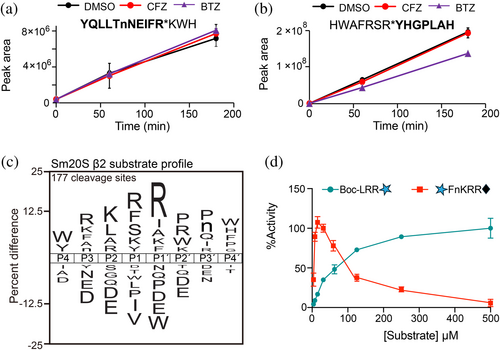
We next evaluated the MSP-MS peptide cleavage profiles that were neither inhibited by BTZ nor CFZ at 500 nM, that is, primarily due to the activity of the β2 subunit. We found 137 cleaved peptides that fit this criterion, exemplified by YQLLTnNEIFR*KWH and HWAFRSR*YHGPLAH (Figure 4a,b). The overall profile indicated a high frequency of cleavage when R is present at P3, P1, and P1′, and K is present at P2 (Figure 4b). Importantly, we show that L and D were not favored in the P1 position by the β2 subunit, which supported our choice of using these amino acids in the P1 position for the β5 and β1 subunits, respectively. As R in P1′ is the most frequently found amino acid across all eight positions (P4 to P4′), we decided to include this amino acid in the substrate design. Therefore, we made an internally quenched substrate consisting of the sequence mca-FnKRR-K(dnp) in which cleavage occurs between the two R residues. Cleavage of mca-FnKRR-K(dnp) and Boc-LRR-amc was then evaluated over a concentration range of 6.25–500 μM. For reasons yet unclear, Sm20S cleaved mca-FnKRR-K(dnp) with high efficiency up to 50 μM, after which a reduction in activity was measured up to 500 μM. By contrast, cleavage of Boc-LRR-amc steadily increased across the entire concentration range tested (Figure 4c).
3.5 Testing new substrates with single-step enriched Sm20S from cell extracts
The isolation of Sm20S to homogeneity is labor-intensive and costly due to the need for vertebrate animals to produce sufficient S. mansoni worms. Therefore, using our three new β subunit-specific substrates, we evaluated Sm20S activity in a S. mansoni protein extract subjected to only ammonium sulfate precipitation (30% and 60% saturation), the first step of the three-step purification process. Pepstatin, E-64, and AEBSF were included in the assay buffer to prevent substrate cleavage by S. mansoni aspartyl, cysteine, and serine proteases (Caffrey et al. 2004; Caffrey and Ruppel 1997; Horn et al. 2014), respectively, in the single-step enriched Sm20S. Our analysis of the enriched Sm20S extract revealed detectable β1, β2, and β5 peptidase activities. Notably, the reaction velocities (RFU/s) that were quantified using the new substrates were at least 9-fold greater than the old substrates (Figure 5a–c). Furthermore, we compared the inhibition profiles of the enriched extract to that of the purified enzyme, demonstrating a consistent inhibition pattern with 10 μM bortezomib (BTZ) and 10 μM carfilzomib (CFZ) (Figure 5d). These studies confirm that using the new substrates, the single-step-enriched Sm20S is sufficient to specifically measure the β1, β2, and β5 activities.
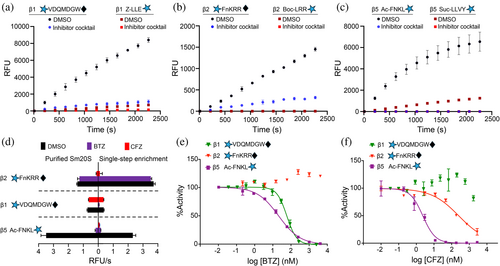
We next determined whether the potency of BTZ and CFZ could be directly measured in the single-step-enriched Sm20S. BTZ was found to have an IC50 value of 49.84 ± 0.24 nM for the Sm20S β1 subunit and 17.97 ± 2.05 nM for the β5 subunit (Figure 5e). No reduction of β2 activity was detected up to 5 μM. CFZ displayed an IC50 of 2.50 ± 0.51 nM for β5 and 288.0 ± 90 nM for Sm20S β2 (Figure 5f). The β1 subunit was inhibited by 15% at 5 μM. These data demonstrate that dose–response assays can be performed using proteasome inhibitors and the single-step enriched Sm20S. A comparison of the BTZ and CFZ dose–response curves, utilizing both the old and new substrates, reveals significant differences in subunit specificity. Specifically, 10 μM BTZ completely inhibited β2 activity with the old substrate, Boc-LRR-amc (Figure 1c). However, at the same concentration, BTZ did not inhibit β2 activity when using the new substrate, mca-FnKRR-K(dnp) (Figure 5e). This discrepancy suggests that Boc-LRR-amc is not exclusively cleaved by β2 and is processed by the β5 and/or β1 subunits. Similarly, 10 μM CFZ fully inhibited β1 activity with the old substrate, Z-LLE-amc (Figure 1b), but is only partially inhibitory with the new substrate, mca-VDQMDGW-K(dnp) (Figure 5f). This suggests that Z-LLE-amc is also susceptible to cleavage by β5 and/or β2. In summary, these observations demonstrate that the new substrates not only exhibit higher cleavage efficiency but also provide improved subunit specificity compared to the older substrates.
3.6 The subunit specificity of the 20S proteasome from three Schistosoma species
The new β1, β2, and β5 substrates were designed based on the specificity profile of the 20S from S. mansoni, which is the laboratory model. New anti-schistosomal drugs should be active against S. mansoni and the other two medically important schistosomes, S. haematobium and S. japonicum (Caffrey 2007; Caldwell et al. 2023). Thus, it is relevant to understand whether the substrates developed for Sm20S can also be used for the other two species. We first normalized proteasome amounts in extracts of all three schistosome species using the proteasome activity-based probe, Me4BodipyFL-Ahx3Leu3VS (Verdoes et al. 2006). This probe labeled three subunits of human c20S, with the bottom two bands co-migrating to yield one strongly fluorescent product (Figure 6a). The probe labeled two of the three β subunits in each of the schistosome extracts.
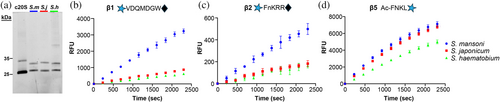
Once the proteasome amounts had been normalized, we evaluated the catalytic β subunit activities with the subunit-specific substrates. The specific activity using the β1 and β2 substrates was two- to three times higher for S. mansoni than for S. haematobium and S. japonicum (Figure 6b–d), whereas the activities measured using the β5 substrate were similar for the three species. It is unclear why substrate cleavage activity is lower for the β1 and β2 subunits of S. japonicum 20S proteasome (Sj20S) and S. haematobium 20S proteasome (Sh20S) considering that Sm20S has more than 94% sequence identity with Sj20S and Sh20S across all three catalytic subunits. For the β1 subunit specifically, Sm20S is 96% and 99% identical to Sj20S and Sh20S, respectively, whereas for the β2 subunit, Sm20S is 94% and 98% identical to Sj20S and Sh20S, respectively (Figure S2). In a structural overlay of the residues adjacent to the catalytic Thr, the β1 and β5 subunits are 100% identical, while the β2 site has a different residue in position 24 for each of the three species (Sm20S–Thr24, Sj20S–Asn24, and Sh20S–Ser24). Differences in the rate of substrate cleavage might, therefore, be due to other factors such as substrate access to the inner core of the proteasome which is controlled by several regulatory proteins in the 19S cap. Overall, the data reveal that the new substrates work well with the proteasomes of all three medically important schistosomes and can be used in the future for inhibition assays of the respective β catalytic subunits.
Schistosome extracts were then pre-incubated with 500 nM BTZ or CFZ to test their cross-species inhibitory effects. Similar inhibition profiles were recorded for the three species (Figure 7a–c). BTZ preferentially inhibited β1 and β5, whereas CFZ inhibited β5 and β2 more than β1. In addition, we tested MZB, a pan-catalytic β subunit inhibitor that is in Phase 3 clinical trials for the treatment of glioblastoma (Roth et al. 2024). MZB (250 nM) decreased the activity measured with each of the three substrates; specifically, a complete inactivation of β5, a 66 to 48% decrease in β2 activity, and a 61 to 34% reduction in β1 activity. Finally, our previous studies revealed that the proteasome inhibitor CP-17, an analog of the marine natural product, carmaphycin B, demonstrated potent anti-schistosomal activity (Bibo-Verdugo et al. 2019). Using the subunit-specific substrates, we evaluated the potency of CP-17 at 250 nM and determined that it preferentially targets β5 (>90% reduction) over β2 (44–48% reduction) with little inhibition of β1 being observed. These data are consistent with our previous studies using the purified enzyme (Bibo-Verdugo et al. 2019).
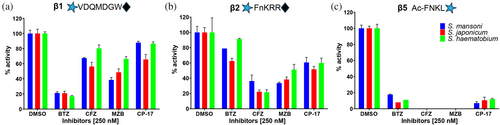
The similar inhibition responses observed across the three Schistosoma species for each catalytic β subunit suggest that proteasome inhibitors developed against one species will likely be effective against the other two. This discovery will significantly streamline the drug development process by reducing the need for extensive testing across all three species.
4 DISCUSSION
The reliance on praziquantel to treat schistosomiasis and the associated concerns regarding potential resistance, coupled with the drug's pharmaceutical and pharmacological limitations, encourage the exploration of alternative chemotherapeutics. Therefore, we focused on the 20S proteasome of the schistosome, given the preclinical and clinical success of targeting the orthologous protein in both Plasmodium and Leishmania (Liu et al. 2024b).
The 20S proteasome is a large multi-subunit, ATP-dependent proteolytic complex that regulates several cellular processes, including normal protein turnover and degradation of misfolded proteins. Inhibitors of c20S, for example, CFZ and BTZ, are key drugs for the treatment of blood cancers, and more recently, zetomipzomib (KZR-616) is one of several new inhibitors of i20S that are in clinical trials for the treatment of autoimmune diseases and immune-mediated disorders (Kirk et al. 2021). Importantly, anti-cancer proteasome inhibitors targeting Sm20S kill S. mansoni in vitro (Bibo-Verdugo et al. 2017). Also, inhibition of 19S proteasome deubiquitinating activity in the parasite induces a modest reduction in egg production in vitro, decreases viability, and is eventually lethal (do Patrocinio et al. 2020). Taken together, these data suggest that the schistosome proteasome is a drug target of interest for the potential treatment of schistosomiasis and encourage the characterization of the cleavage specificities of the individual 20S catalytic β subunits, information that would underpin a campaign to develop Schistosoma-selective proteasome inhibitors.
In eukaryotes, each of the 20S catalytic subunits has a distinct substrate specificity that varies between organisms (Maurits et al. 2020). Fluorogenic substrates such as Z-LLE-amc, Boc-LRR-amc, and Suc-LLVY-amc, which were developed for the β1, β2, and β5 subunits of c20S, respectively, are also cleaved by the respective subunits of parasite proteasomes (Li et al. 2014; Wyllie et al. 2019). However, using MSP-MS-directed substrate design, we show that these c20S substrates are not optimal for Sm20S β1, β2, and β5, and that we could optimize a new substrate for each β subunit that performed 2- to 8-fold better. These rationally optimized β subunit-selective substrates for Sm20S, therefore, should prove valuable in the design of inhibitors targeting each β subunit or combinations of β subunits. As a case in point, our MSP-MS studies suggest that the preferential cleavage of the sequence RKR*R (where * is the cleavage site) by Sm20S β2 (Figure 3b) is different from the cleavage preference of the c20S β2 subunit for which positively charged residues at P2 are not favored. For the β5 subunit, Sm20S prefers Thr at P2 and Asn at P3, both of which are not well tolerated by c20S at the same positions (Rut et al. 2018). Accordingly, our future inhibitor design efforts will focus on exploiting these differences to develop potent inhibitors of Sm20S that bind weakly to the host proteasome and, therefore, decrease cytotoxicity to mammalian cells.
We also developed a single-step enrichment of Sm20S activity from crude extracts using ammonium sulfate, which, when combined with the new specific substrates, allowed for a more streamlined evaluation of inhibitor engagement without the need for isolating pure Sm20S, a time-consuming process that would require the use of more vertebrate host animals. It was evident that the catalytic activity of β1 and β2 is higher for Sm20S than for Sj20S and Sh20S even though the protein sequences are >94% identical. It is possible that the 19S regulatory subunits that interact with each 20S proteasome may play a role in regulating substrate entry to the inner chamber of the proteasome and thus modulate catalytic activity with the new substrates.
Encouragingly, similar inhibition profiles were observed for the 20S proteasomes of S. mansoni, S. haematobium and S. japonicum, using both anti-cancer inhibitors and a carmaphycin B analog. This suggests that inhibitors that engage the 20S in S. mansoni, the most experimentally tractable species, could be broadly effective against the other two species. This would save time and money and align with the preferred target product profile for schistosomiasis drugs (Caffrey 2007; Caldwell et al. 2023), which calls for drugs that are active against all three major schistosome species.
In conclusion, our study employed mass spectrometry to characterize the substrate specificities of Sm20S β1, β2, and β5, leading to the design of new fluorogenic reporter substrates. These substrates enabled the development of a rapid enrichment method for the schistosome proteasome and revealed conserved inhibitor susceptibilities between the three important Schistosoma species. This paves the way for a program to develop broad-spectrum anti-schistosomal inhibitors that focus on the Sm20S proteasome.
AUTHOR CONTRIBUTIONS
Elany B. Silva: Methodology; investigation; writing – review and editing; validation; data curation; formal analysis. Zhenze Jiang: Writing – original draft; methodology; conceptualization; investigation; software; visualization; formal analysis. Chenxi Liu: Writing – original draft; methodology; investigation; formal analysis. Pavla Fajtová: Investigation. Thaiz R. Teixeira: Investigation; validation. Giovana de Castro Fiorini Maia: Visualization. Lawrence J. Liu: Investigation. Nelly El-Sakkary: Investigation. Danielle E. Skinner: Investigation. Ali Syed: Investigation. Steven C Wang: Investigation; methodology. Conor R. Caffrey: Conceptualization; funding acquisition; project administration; supervision; writing – review and editing; formal analysis; resources. Anthony J. O'Donoghue: Conceptualization; funding acquisition; project administration; supervision; visualization; writing – review and editing; data curation; formal analysis; resources.
ACKNOWLEDGMENTS
The research was supported by NIH awards R21AI133393 and R21AI171824 to AJO and CRC, and R01AI158612 and R21AI146387 to AJO. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PF received funding from the Program for Research and Mobility Support of Starting Researchers from the Czech Academy of Sciences (MSM200551901) and the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 846688, ProTeCT.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in MassIVE at https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp?redirect=auth, reference number MSV000096255.




