An enzyme-centric approach for constructing an amperometric l-malate biosensor with a long and programmable linear range
Abstract
l-Malate is a key flavor enhancer and acidulant in the food and beverage industry, particularly winemaking. Enzyme-based amperometric biosensors offer convenience for monitoring its concentration. However, only a small number of off-the-shelf malate-oxidizing enzymes have been used in previous devices. These typically have linear ranges poorly suited for the l-malate concentrations found in fruit processing and winemaking, making it necessary to use precisely diluted samples. Here, we describe a pipeline of database-mining, gene synthesis, recombinant expression, and spectrophotometric assays to characterize previously untested enzymes for their suitability in biosensors. The pipeline yielded a bespoke biocatalyst—the Ascaris suum malic enzyme carrying mutation R181Q [AsME(R181Q)]. Our first prototype with AsME(R181Q) had an ultra-wide linear range of 50–200 mM l-malate, corresponding to concentrations found in undiluted fruit juices (including grape). Changing the dication from Mg2+ to Mn2+ increased sensitivity five-fold and adding citrate (100 mM) increased it another six-fold, albeit decreasing the linear range to 1–10 mM. To our knowledge, this is the first time an l-malate biosensor with a tuneable combination of sensitivity and linear range has been described. The sensor response was also tested in the presence of various molecules abundant in juices and wines, with ascorbate shown to be a potent interferent. Interference was mitigated by the addition of ascorbate oxidase, allowing for differential measurements on an undiluted, untreated wine sample that corresponded well with commercial l-malate testing kits. Overall, this work demonstrates the power of an enzyme-centric approach for designing electrochemical biosensors with improved operational parameters and novel functionality.
1 INTRODUCTION
The global biosensor market is dominated by enzyme-based, amperometric devices (Turner, 2013). In these devices, an enzyme catalyzes a redox reaction on the analyte of interest, in such a way that electrons are transferred to or from an electrode. In some cases, an electroactive product of the reaction (such as a reduced cofactor) can interact directly with the electrode. More commonly, the device is designed such that the electroactive product of the enzyme-catalyzed reaction reduces or oxidizes an electron mediator, and this mediator then shuttles electrons to or from the electrode surface. Overall, catalytic turnover leads to a detectable, dose-dependent electrical current.
Enzyme-based, amperometric biosensors have numerous advantages over alternative analytical devices. Using an enzyme to drive a change in electrical current offers fast reaction times and exquisite specificity for the analyte of interest. Amperometric sensors are easy to miniaturize, manufacture, and integrate into handheld devices that are well-suited for in situ testing (Grieshaber et al., 2008). Ideal biosensors (such as blood glucose monitors) also offer true “sample in, answer out” operability; that is, no pre-processing or pre-treatment of the sample is required. In fields from point-of-care diagnostics to food and environmental analyses, it is acknowledged that complicated and/or tedious sample preparation can decrease real-world uptake of new biosensor technologies and increase the errors introduced by minimally trained users (Ashley et al., 2017; Campuzano et al., 2020; Chu et al., 2021; Park et al., 2020; Ribeiro et al., 2014).
Perhaps surprisingly, in biosensor design the choice of the enzyme itself often receives less attention than electrode construction, selection of an electron mediator, the enzyme immobilization strategy, flow cell design, and integration with instrumentation (Prodromidis & Karayannis, 2002). Previously, we highlighted l-malate biosensing as an example of this phenomenon. Four decades of research have yielded approximately three dozen enzyme-based amperometric biosensors for l-malate (Matthews et al., 2021). In all these devices, malate-oxidizing enzymes derived from only four named species (pig, cattle, chicken, and the bacterium Thermus flavus) have been used. We hypothesized that this historically narrow sampling of enzymes has imparted constraints on key device parameters such as linear range, sensitivity to interference, pH optima, or storage stability. The aim of this work was to address this hypothesis by identifying one or more new enzymes for l-malate biosensing, and to show that our enzyme-centric approach could yield a novel device that addressed one or more real-world problems.
l-Malate is a ubiquitous metabolite produced in the tricarboxylic acid cycle and it is the principal organic acid in many fruits and vegetables (Lobit et al., 2006). It is commonly used as a flavor enhancer and acidulant in the food and beverage industry and it is also used in household products such as cleansers and lotions, as well as in polymer synthesis, pharmaceuticals, textile finishing, metal cleaning, and animal feed (Wei et al., 2021; West, 2017). Monitoring l-malate is therefore an important aspect of many horticultural, fermentation, and biomanufacturing processes.
Two inter-related challenges have limited the “sample in, answer out” operability of previous l-malate biosensors. The first is that most previously described l-malate biosensors have linear calibration curves that lie in the micromolar range. However, samples such as fruit juices typically contain 2–200 mM l-malate (Li et al., 2020; Ma et al., 2018; Shekhar & Iritani, 1979). The broadest linear range reported for any device to date is 5–30 mM (Maines et al., 2000) but the vast majority of l-malate biosensors require careful and accurate sample dilution. In turn, this diminishes their usefulness in real-world horticultural and industrial settings, where users may lack the time, equipment, or technical expertise to laboriously construct dilution series. Much of the interest in l-malate biosensors stems from the importance of this compound in winemaking, and here there is additional complexity. During grape maturation, l-malate decreases rapidly from approximately 200 mM to 30–50 mM and this change can inform harvesting decisions (Olego et al., 2015; Volschenk et al., 2006). However, when malolactic fermentation is used during winemaking, it becomes important to monitor a different concentration range, from 5–10 mM down to 1 mM or less. No existing device spans these concentration ranges. Moreover, wines (and other juices) are complex mixtures, which can contain many compounds that interfere with biosensor performance (Matthews et al., 2021). When less-dilute samples are used, pre-treatments are often required to remove these interferents (Monošík et al., 2012; Mundaca-Uribe et al., 2017).
We set out to identify candidate enzymes for a new l-malate biosensor that could be used with undiluted, untreated samples. It is known that various factors determine the linear range of an amperometric biosensor, including the rates of electron transfer, the diffusive properties of electroactive compounds, and enzyme loading (Silverstein & Goodney, 2010). Critically, the Michaelis constant (KM) of the enzyme also influences the linear range because it helps to determine the point at which signal saturates (Baronas et al., 2021; Vasylieva & Marinesco, 2013). In theory, the linear range of a device should extend to analyte concentrations that are approximately one-tenth of the apparent Michaelis constant (KMApp) for the enzyme immobilized within the biosensor (Vasylieva & Marinesco, 2013).
The most commonly used malate oxidoreductase enzyme for previous biosensors has been the malate dehydrogenase (MDH) from pig heart. In solution, the KM of this enzyme for l-malate is approximately 0.2 mM (Passonneau & Lowry, 1993). Because undiluted sample matrices such as fruit juices contain l-malate at concentrations that are orders of magnitude above this KM, there is significant potential to saturate the enzyme and lose linearity in biosensor response (Maines et al., 2000).
Malate oxidoreductases are found across the tree of life, in a variety of physiological roles. For example, MDH enzymes catalyze the reversible oxidation of l-malate to oxaloacetate, accompanied by reduction of either NAD+ or NADP+. They appear in various metabolic pathways, most canonically the tricarboxylic acid cycle (Minárik et al., 2002). The malic enzymes (MEs) can also be NAD+- or NADP+-dependent, catalyzing the oxidative decarboxylation of l-malate in lipid metabolism (Chang & Tong, 2003). Malate:quinone oxidoreductases have also been described in some bacteria, which oxidize l-malate to oxaloacetate and donate electrons to the quinones of the electron transport chain (Kather et al., 2000). Inexpensive gene synthesis, combined with routine technologies for recombinant protein expression, now makes it possible to screen bespoke enzymes from any organism for their suitability in devices with improved performance. Here we identified, recombinantly produced, and characterized novel malate oxidoreductases with high KM values, to test whether they would yield amperometric biosensors with elongated linear ranges. We describe our pipeline for identifying enzyme candidates and screening them for practical utility in a biosensor. Using an engineered ME from the roundworm, Ascaris suum, on an unmodified gold electrode, we constructed a biosensor with the largest linear range reported to date (50–200 mM). We discovered that the linear range, sensitivity, and limit of detection (LOD) of the biosensor could be modulated by adding Mn2+ and/or citrate to the reaction buffer, yielding a single enzyme-based device that could be programmed to report on l-malate concentrations from 1 to 200 mM, corresponding to the range of maximum utility for horticultural and winemaking processes.
2 RESULTS
2.1 Selection of malate oxidoreductase candidates
To begin, we searched the Braunschweig Enzyme Database (BRENDA) (Chang et al., 2021) for any enzyme that used l-malate. At the time of our search (in late 2018), this returned enzymes in 29 different Enzyme Commission (EC) classes. Of these, 14 were oxidoreductases, although half were immediately excluded because they lacked entries with KM values for l-malate, catalyzed reactions that were not useful for amperometric biosensors, or had poorly defined reaction schemes.
The seven remaining EC classes included the three classes of enzyme that have been used in previous biosensors (Matthews et al., 2021). NAD+-dependent MDH enzymes are in EC class 1.1.1.37. MEs in EC 1.1.1.40 oxidatively decarboxylate l-malate to pyruvate and CO2 while reducing NADP+ to NADPH. And the malate:quinone oxidoreductases in EC 1.1.5.4 oxidize l-malate to oxaloacetate while donating electrons to a quinone.
The other four EC classes we identified were 1.1.1.38 (dual-specificity enzymes that oxidatively decarboxylate both l-malate and oxaloacetate), 1.1.1.39 (NAD+-dependent malic enzymes), 1.1.1.82 (NADP+-dependent MDHs), and 1.1.1.299 (MDHs that use NAD+ or NADP+).
BRENDA summarizes many functional parameters and molecular properties for each enzyme. We manually curated our list of candidates from the seven EC classes. Enzymes in EC 1.1.1.38 were discarded because of their dual substrate specificity. Of the remainder, we identified four enzymes with (a) high KM values; and/or (b) additional features that we hypothesized might be useful in a device (Table 1). Of particular note, none of these candidates were from EC classes that had previously been tested in amperometric biosensors. Our search strategy did not specifically exclude the EC classes used previously. Nevertheless, using enzymological knowledge—including kinetic parameters—to guide our search led us to classes of malate oxidoreductase that were wholly unexplored as biosensors.
| Malate oxidoreductase | EC class | KM for malate (mM) | Additional characteristics | Ref. |
|---|---|---|---|---|
| Ascaris suum malic enzyme with point mutation R181Q, NAD+-dependent | 1.1.1.39 | 57 | Enzyme kinetics modifiable by adding ammonium | Karsten and Cook (2007) |
| Azorhizobium caulinodans malic enzyme, NAD+-dependent | 1.1.1.39 | 27.6a | KM modifiable by adding acetyl-CoA | Zhang et al. (2012) |
| Sorghum bicolor malate dehydrogenase, NADP+-dependent | 1.1.1.82 | 12 | Chemically inducible on/off switch | Lemaire et al. (1996) |
| Aeropyrum pernix malate dehydrogenase, NAD(P)+-dependent | 1.1.1.299 | 0.12 | Thermo- and pH-stable | Kawakami et al. (2009) |
- a In the presence of 50 μM acetyl-CoA. KM = 2.6 mM in the absence of acetyl-CoA (Zhang et al., 2012).
The malic enzyme from the roundworm A. suum with arginine 181 mutated to glutamine [AsME(R181Q)], the ME from the bacterium Azorhizobium caulinodans (AcME), and the MDH from the plant Sorghum bicolor (SbMDH) were chosen because of their high KM values for malate, compared with the commercially available pig heart MDH (KM ~ 0.2 mM) (Passonneau & Lowry, 1993). The rationally designed R181Q mutant of AsME had the highest reported KM of any malate oxidoreductase, at 57 mM (Karsten & Cook, 2007). Small molecule effectors have been described for AsME(R181Q) and AcME; we hypothesized these may introduce a programmable element into any biosensor using them. On the other hand, SbMDH is activated by light, via the ferredoxin-thioredoxin system, in vivo. This switching behavior can be recapitulated for the purified enzyme with thioredoxin and dithiothreitol in vitro (Lemaire et al., 1996), which suggested a novel mechanism for controlling the activity of a biosensor.
While the MDH from Aeropyrum pernix (ApMDH) has a KM for l-malate (0.12 mM) that is similar to the commercial pig enzyme, it is from a hyperthermophilic archaeon. The enzyme is highly stable, retaining full activity after heat treatment at 100°C for 10 min (Kawakami et al., 2009). It was also reported that the optimum pH for l-malate oxidation was pH = 11, but that “when heated at 50°C for 30 min the enzyme showed no loss of activity at pHs between 5.5 and 10.5.” Its stability and apparent tolerance to changes in pH made ApMDH a candidate for a robust biosensor.
2.2 Biochemistry narrows the pipeline
Our approach for designing a new l-malate biosensor differed from previous work because we specifically set out to identify a broad suite of candidate enzymes. These need not have been commercially available, nor used previously in biosensors. The next step was therefore to screen our highly diverse set of four candidates for the properties that would make them useful and desirable in real-world devices.
Our first criterion for a usable enzyme was the ability to express it recombinantly in Escherichia coli. We were unable to achieve expression of soluble SbMDH, despite extensively and systematically varying the growth medium, inducer concentration, growth temperature after induction, and the duration of induction. Therefore, SbMDH was not considered further. The other three candidates were successfully expressed in soluble and active forms. AcME and AsME(R181Q) were purified using metal affinity chromatography and then size-exclusion chromatography, while metal affinity chromatography alone was sufficient for purifying ApMDH. Each protein was >95% pure, as judged by SDS-PAGE (Figure S1).
AcME proved to be unstable upon storage. While aliquots of soluble, active enzyme could be stored at −80°C, these aggregated and precipitated rapidly when thawed (on ice or at room temperature). Within 6 h, NAD+-dependent oxidative decarboxylation activity toward malate dropped to approximately 10% of the freshly thawed enzyme, rendering it unusable in a biosensor.
The purported advantage of ApMDH was its retention of activity across a broad pH range (Kawakami et al., 2009). Key sample types for l-malate biosensing, including wine and fruit juices, are all mildly acidic and typically in the range of pH 3–5 (Bridges & Mattice, 1939). Therefore, we characterized the pH dependence of ApMDH activity over the range of pH 3–10. Consistent with previous data (Kawakami et al., 2009), we saw the highest activity at pH 10. However, this decreased sharply with decreasing pH (Figure S2). At pH 5, the activity was only 4.5% of that seen at pH 10, and at pH 4 only trace activity could be observed, which could not be measured reliably. This severe pH dependence of activity meant that strong and precise buffering would be required to generate reproducible signals when testing samples of unknown malate concentration. For this reason, ApMDH was also discarded from our pipeline.
2.3 Characterization of the lead candidate, AsME(R181Q)
The steady-state kinetic parameters of soluble AsME(R181Q) have been investigated in detail (Karsten & Cook, 2007). Not only did it have the highest KM for l-malate among our candidates, but it was also known that its low activity (compared to wild-type AsME) could be rescued by adding ammonium (Karsten & Cook, 2007). We confirmed this phenomenon by using spectrophotometric assays to measure initial reaction velocities (V0) over a range of malate concentrations, in the presence of 0 mM, 4 mM, or 300 mM NH4+. Reaction mixtures were initially based on those described previously (Karsten & Cook, 2007), containing 25 mM Mg2+ and 10 mM NAD+. Adding NH4+ led to dramatic enhancements in enzyme-catalyzed reaction rates (Figure 1a,b). Across the range of assayed malate concentrations, adding NH4+ at 4 mM increased initial velocities approximately 6-fold, while adding 300 mM NH4+ increased them approximately 100-fold. However, adding NH4+ also caused the response to begin saturating at malate concentrations above 100 mM (Figure 1a,b). We discovered that these saturation kinetics could be avoided by increasing the Mg2+ concentration to 200 mM, albeit with adjusting the NAD+ concentration to a new optimum of 20 mM for assays with 4 mM NH4+, or 25 mM for assays with 300 mM NH4+ (Figure 1c).

MEs require a divalent metal ion for catalysis and structural stability (Chang & Tong, 2003). In other MEs, substituting Mg2+ for Mn2+ can have profound effects on enzyme parameters including reaction rates and the KM for l-malate (Casati et al., 1997; Lin & Davis, 1974). Moreover, while investigating potential interferents (Section 2.6 below) we discovered that citrate also increases the rate of the AsME(R181Q)-catalyzed reaction. Finally, then, we conducted further sets of assays in which Mg2+ was replaced with Mn2+, and where citrate (100 mM) was also added. In both cases, V0 increased for each given malate concentration (Figure 1d).
These results extended the previous analysis of AsME(R181Q) (Karsten & Cook, 2007). As expected from inspecting the datasets (Figure 1), fits to the Michaelis–Menten equation yielded unreliable estimates of KM (Table S1). Of the seven datasets collected, only two gave estimated KM values within the range of l-malate concentrations that we assayed and the errors on these were large (>30%). This confirmed that our modifications to the assay had increased KM such that the enzyme was not saturated at l-malate concentrations up to 200 mM. It was not possible to collect reliable activity data at higher l-malate concentrations because malate-induced changes in pH had confounding effects. However, our results implied that an amperometric biosensor based on AsME(R181Q), supplemented with 300 mM NH4+, would have desirable properties including an extended linear range and programmability through the addition or omission of Mg2+, Mn2+, and citrate.
2.4 Selection of an electron mediator
Using an electron mediator can improve the sensitivity and LOD of an enzyme-based amperometric biosensor. This is due to the rapid shuttling of electrons from the reduced cofactor (NADH in our case) to the surface of the electrode, as well as rapid transfer of the electrons to the electrode upon arrival of the mediator (Nagels & Staes, 2001). However, mediator performance is heavily dependent on factors such as pH and the electrode material so it can be useful to screen several candidates (Lupu et al., 2004).
Six different mediators were tested for their suitability in our biosensor but three were promptly eliminated. Phenazine methosulfate was unstable in the buffer used for enzyme assays (100 mM HEPES, pH 7), indicated by the solution containing the mediator turning rapidly from bright yellow to dark brown. Methyl red was insoluble in this buffer. Potassium ferricyanide produced highly variable current readings and fouled the electrode, forming a brown deposit on the reference electrode. Control experiments showed that this deposition was dependent on the mediator being reduced in the presence of the magnesium required for enzyme activity. For this reason, potassium ferricyanide was also ruled out.
For the remaining three mediators—hexaammineruthenium(III) chloride (HAR), gallocyanine (GAL), and 2,6-dichlorophenolindophenol (DCPIP)—the working potentials were chosen after obtaining cyclic voltammograms for each (Figure S3). These working potentials were +50 mV for HAR, +50 mV for GAL, and +150 mV for DCPIP. Using these working potentials, the analytical signals produced using each mediator in the presence of NADH were: HAR, 16 ± 1 μA cm−2; GAL, 4.0 ± 0.2 μA cm−2; and DCPIP, 29 ± 1 μA cm−2. With the largest analytical signal and a relatively low operating potential, DCPIP was chosen for use in the biosensor.
2.5 Biosensor construction and performance
The AsME(R181Q) enzyme and DCPIP were used as the basis for a new electrochemical biosensor constructed with unmodified, screen-printed electrodes. Our spectrophotometric activity data (Figure 1) led us to fine-tune the biosensor under three different sets of conditions.
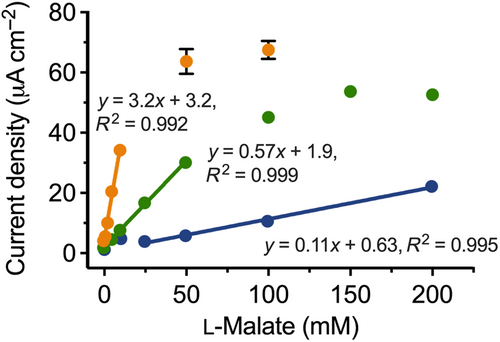
In the first iteration, the biosensor comprised the enzyme, NAD+, the mediator, 300 mM NH4+, and Mg2+ (200 mM) as the divalent cation. A calibration curve was constructed from 0 to 200 mM l-malate (Figure 2). The curve was highly linear in the range of 25–200 mM l-malate with a sensitivity of 0.11 μA mM−1 cm−2. As noted in Section 2.3, it became increasingly difficult to buffer the system at l-malate concentrations above 200 mM. Further, the linearity of the response reproducibly broke down at l-malate concentrations around 10 mM, where unexpectedly high current readings were obtained. Taking these factors into account, we therefore conservatively defined the usable linear range of the biosensor as 50–200 mM (Table 2). The breakdown in linearity also meant that we ascribed a conservative LOD of 50 mM to the biosensor under these conditions.
| Condition 1 | Condition 2 | Condition 3 | |
|---|---|---|---|
| Additive(s) | Mg2+ | Mn2+ | Mn2+ and citrate |
| Linear range (mM) | 50–200 | 5–50 | 1–10 |
| Sensitivity (μA mM−1 cm−2) | 0.11 | 0.57 | 3.2 |
| Limit of detection (mM) | 50 | 0.85 | 0.18 |
| Response time (s) | 125 | 125 | 125 |
Next, we tested the biosensor with Mn2+ (200 mM) in place of Mg2+. The resulting calibration curve showed that this gave a five-fold increase in sensitivity compared to using Mg2+ (Table 2). This was accompanied by a reduction in linear range, to 5–50 mM (Figure 2). While the use of Mn2+ increased the baseline noise slightly, a linear response was now observed down to an l-malate concentration of zero. Therefore, the blank measurement could be used to estimate the LOD at 0.85 mM (see Section 4.4 for details of the calculation).
Finally, we tested the sensor with Mn2+ (200 mM) and citrate (100 mM). Control experiments showed that the combination of Mn2+ and citrate was superior to Mg2+ and citrate for generating the greatest dose-dependent signals. The calibration curve was only linear from 1 to 10 mM l-malate (Figure 2). However, at 3.2 μA mM−1 cm−2, the sensitivity was 30-fold greater than the sensor with Mg2+ as the dication and 6-fold greater than when citrate was omitted. The LOD was also the best under these conditions, at 0.18 mM (Table 2).
2.6 Interference tests
As discussed in Section 1, key applications for any new l-malate biosensor would be in the food and beverage industry, particularly winemaking. Therefore, we tested a selection of the main compounds found in fruit juices, including grape juice (Gutiérrez-Gamboa et al., 2019; Li et al., 2020), for their potential to interfere with our biosensor (with Mg2+ as the divalent cation). The results are shown in Table 3.
| Compounda | Relative responseb (%) | Compounda | Relative responseb (%) |
|---|---|---|---|
| Ascorbate (5) | 1120 ± 50 | Glutamine (7.5) | 101 ± 3 |
| Ascorbate (0.1) | 147 ± 5 | Acetate (100) | 98 ± 5 |
| Citrate (100) | 151 ± 8 | Arginine (7.5) | 95 ± 6 |
| Fumarate (100) | 148 ± 8 | Pyruvate (100) | 93 ± 3 |
| Succinate (100) | 119 ± 6 | Glycerol (100) | 93 ± 2 |
| Potassium chloride (100) | 110 ± 1 | Alanine (7.5) | 93 ± 1 |
| Glucose (100) | 107 ± 7 | Lactate (100) | 91 ± 4 |
| Tartrate (100) | 106 ± 3 | Glutamate (7.5) | 85 ± 12 |
- a The tested concentration of each compound (mM) is given in parentheses.
- b All solutions contained 100 mM l-malate and were compared to the response of the biosensor with 100 mM l-malate alone, which was taken as 100%. Reported values are the mean ± SEM for triplicate tests.
Citrate, fumarate, and succinate increased the current response by more than 10%, although only at the high concentration of 100 mM. Measurements at concentrations typically found in non-citrus juices and wine (i.e., <5 mM) (Li et al., 2020; Robles et al., 2019) showed no interference in producing the malate-dependent signal. Interestingly, citrate is a known inhibitor of the ME from the yeast, Yarrowia lipolytica (Zhang et al., 2013). Therefore, it was surprising to discover it increased the activity of AsME(R181Q). As described in Section 2.5, this result led us to design the most sensitive version of our biosensor, using both Mn2+ and citrate as co-solutes.
Ascorbate was shown to be a potent interferent. Fruit juices including grape, apple, and citrus typically contain ascorbate at concentrations ranging from approximately 0.1 mM to over 6 mM (Apak et al., 2014; Dani et al., 2007; Ma et al., 2020). An ascorbate concentration of 5 mM was sufficient to completely saturate the sensor response and even reducing it to 0.1 mM still produced a response 1.5-fold higher than the malate-only control (Table 3). Ascorbate is known to directly reduce DCPIP (Maines et al., 2000), and control experiments showed that it was indeed interfering solely through the mediator.
The enzyme ascorbate oxidase has been shown to significantly reduce or eliminate ascorbate interference (Maines et al., 2000), including when it was used to pre-treat citrus juices in an MDH-based biosensor (Manzoli et al., 2004). However, the primary interest in l-malate biosensing stems from winemaking (Matthews et al., 2021). Therefore, we investigated whether ascorbate oxidase would be similarly useful for measuring the l-malate concentration in a wine sample. For this experiment, we obtained a winemaker's sample of Pinot Noir from near the onset of malolactic fermentation.
Spectrophotometric test kits from two commercial suppliers were used to estimate the l-malate concentration in the Pinot Noir sample at 3.6 ± 0.1 mM and 3.7 ± 0.1 mM, respectively. Using the biosensor with Mn2+ and citrate (Table 2) to analyze the untreated wine sample gave a signal corresponding to an apparent l-malate concentration of 17.0 ± 0.2 mM, consistent with substantial interference from ascorbate and/or other unidentified components of the sample matrix.
To investigate this interference, differential measurements were taken with ascorbate oxidase plus or minus AsME(R181Q). Adding 10 U of ascorbate oxidase to the assay mixture, together with AsME(R181Q), decreased the signal such that the apparent l-malate concentration was now 6.2 ± 0.9 mM. This implied that most or all of the interference from ascorbate had been eliminated. When ascorbate oxidase was present in the biosensor, but AsME(R181Q) was omitted, a background signal from the sample matrix was obtained equivalent to l-malate at 3.0 ± 0.8 mM. Subtracting this background gave an estimated l-malate concentration of 3.2 ± 1.2 mM in the Pinot Noir sample. Finally, we repeated this differential measurement scheme with a decreased amount of ascorbate oxidase (5 U) in the assay mixtures. This gave an estimated l-malate concentration of 3.1 ± 0.6 mM. In two-tailed, unpaired t-tests, neither of these estimates were significantly different from those obtained with the two spectrophotometric test kits (p > 0.4 for all pairwise comparisons).
These results suggest that differential readings, in the presence and absence of AsME(R181Q), will offer a generalizable approach for overcoming ascorbate interference in juice and wine samples. This strategy of taking differential readings to account for non-specific signals is standard practice in l-malate biosensing (Arif et al., 2002; Bucur et al., 2006; Gajovic et al., 1997, 1998; Lupu et al., 2004), although further testing will be required to validate the robustness of our approach with non-wine sample matrices.
3 DISCUSSION
3.1 A new enzyme for use in l-malate biosensors
We text-mined the BRENDA database (Chang et al., 2021) to identify malate oxidoreductases that had not previously been used in biosensors, but which might have useful properties. The ME from the roundworm, A. suum, bearing the R181Q mutation (Karsten & Cook, 2007), proved to have a number of advantageous characteristics. It was easy to express recombinantly and purify from E. coli. We developed assay conditions (Figure 1) in which reaction rates were linear with respect to l-malate, up to the maximum concentration we could assay (200 mM). Indeed, it is possible that the linear range is even higher but a different buffering system would be required to determine this. Critically for real-world implementation of a biosensor, 200 mM is the maximum concentration of l-malate found in grapes and fruit juices (Li et al., 2020; Volschenk et al., 2006). In turn, this means that our biosensor can be used to quantify l-malate in grape, wine, and juice samples without any need to dilute them first.
Our initial search of BRENDA was not limited to malic enzymes; however, this study has emphasized their significant untapped potential for use in l-malate biosensors. From the thermodynamic perspective, MEs should be useful in biosensors because they catalyze the oxidative decarboxylation of l-malate, ensuring the forward reaction is highly favored. In contrast, MDHs catalyze the reversible oxidation of l-malate to oxaloacetate. The equilibrium for this heavily favors the reverse reaction, meaning that a second enzyme such as a diaphorase is typically required to remove the reaction products (Matthews et al., 2021). Nevertheless, up until now MDH enzymes have dominated the field. In our recent review of l-malate biosensors, 25 of 34 made use of an MDH (Matthews et al., 2021). Only six previous studies have described ME-based amperometric biosensors (Arif et al., 2002; Esti et al., 2004; Gajovic et al., 1997, 1998; Lupu et al., 2004; Messia et al., 1996). Four of these studies made use of the commercially available enzyme from chicken liver (Arif et al., 2002; Gajovic et al., 1997, 1998; Messia et al., 1996), while the other two did not specify the source of their ME. The KM of the chicken liver ME for l-malate in solution is approximately 1 mM (Geer et al., 1980). Therefore, it is unsurprising that the broadest linear range for any previous ME-based device was 0.01–1.2 mM (Gajovic et al., 1998). By identifying AsME(R181Q), with its unusually high KM (Karsten & Cook, 2007), our pipeline delivered the first l-malate biosensor that combined the favorable thermodynamics of a single-enzyme, ME-based device with an ultra-long linear range. AsME(R181Q) is also the first NAD+-dependent ME (EC class 1.1.1.39) to be incorporated into a biosensor. All previous ME-based devices have used enzymes from EC class 1.1.1.40, which are NADP+-dependent (Matthews et al., 2021). Companies such as MilliporeSigma supply NAD+ for approximately one-tenth the price of NADP+, which offers a cost-of-goods advantage for commercial implementation of an AsME(R181Q)-based device.
3.2 Comparison with other amperometric l-malate biosensors
Our primary goal was to construct a biosensor with a long enough linear range that it might be used with undiluted samples from the food and beverage industry. At the same time, linear range trades off with sensitivity (Matthews et al., 2021). For example, the most sensitive MDH-based device described to date has an exquisite sensitivity of 1365 μA mM−1 cm−2 but a linear range of 0.1–1 μM; therefore, wine samples needed to be diluted 10,000- to 25,000-fold in order to use it (Giménez-Gómez et al., 2017). We have updated our recent literature survey (Matthews et al., 2021) to place the three versions of our biosensor in the context of previous design efforts (Figure 3).
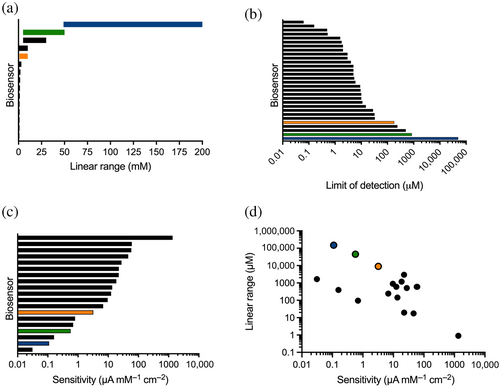
When Mg2+ was used as the dication, our biosensor had the longest linear range (50–200 mM; Figure 2) of any enzyme-based, amperometric l-malate biosensor described to date. The ability to monitor l-malate concentrations over a span of 150 mM is six-fold greater than the best-performing device reported previously (top black bar in Figure 3a). This previous biosensor used pig heart MDH and a diaphorase enzyme on a platinum electrode to report on l-malate concentrations up to 30 mM (Maines et al., 2000). Replacing Mg2+ with Mn2+ in our biosensor also yielded a device with a longer linear range than this (5–50 mM).
Conversely, our biosensors were among those with the lowest sensitivity and the highest LOD (Figure 3b,c), making them some of the poorest at resolving small differences in l-malate concentration. Nevertheless, these features of our devices make them highly suitable for monitoring the large l-malate changes that can be associated with food and beverage processing. For example, the operational parameters of our biosensor with Mg2+ make it appropriate for measuring the >5 mM per day change in l-malate that can occur as wine grapes grow and mature (Volschenk et al., 2006). On the other hand, using Mn2+ and citrate in our device showed improved sensitivity and an LOD of 180 μM while retaining a millimolar linear range (Figure 3d), making it better suited for monitoring the removal of l-malate during the winemaking process of malolactic fermentation.
It was important to investigate interference due to non-specific oxidation events, in order to validate our biosensor for the analysis of untreated samples. At the relevant concentrations for juice and wine samples, only ascorbate showed significant interference (Table 3), through its direct reduction of the mediator, DCPIP. This was effectively removed from a Pinot Noir wine sample by treatment with ascorbate oxidase. Although we did not extensively re-optimize our sensor with this extra enzyme, we expect that little further optimization will be necessary. Using ascorbate oxidase—especially in conjunction with DCPIP—is now a well-established method in a wide range of biosensing applications, including MDH-based l-malate biosensors (Maines et al., 2000; Manzoli et al., 2004), and also for analytes such as lactate, glucose, and cholesterol (Nah et al., 2016; Tzouwara-Karayanni et al., 1993; Uzunoglu et al., 2016). Using ascorbate oxidase is more efficacious than membrane-based methods that aim to separate l-malate and ascorbate via charge or diffusive properties (Maines et al., 2000). Further, we did not need to pre-incubate the wine samples with the ascorbate oxidase. Instead, the enzyme was simply added to the other components of the testing solution, all of which were pre-dispensed onto the electrode. Then a wine sample was added directly to this testing solution in order to initiate measurement. A simple differential measurement scheme, consisting of independent measurements plus and minus AsME(R181Q), made it straightforward to calculate the l-malate concentration in the wine sample.
3.3 Concluding remarks
We have demonstrated that an enzyme-centric approach to biosensor design can yield novel amperometric detection systems with beneficial properties. Careful characterization of AsME(R181Q) suggested it would be useful for constructing biosensors with long linear ranges and this was borne out with our prototype device. Uniquely, we showed that the operational parameters of our l-malate biosensor could be programmed through changing the dication and/or adding citrate. The ability to accurately analyze a minimally processed wine sample also represents a step toward genuine “sample in, answer out” operability for l-malate biosensing. Our measurements were conducted by adding 20 μL of wine directly to 30 μL of the testing solution that had been pre-dispensed onto the electrode surface. The wine sample was not decolorized or pre-treated in any way. This contrasts with previous studies in which pre-treatments with reagents such as iodine (Mundaca-Uribe et al., 2017), or with commercial kits for removing polyphenols (Monošík et al., 2012), were required prior to sample measurement. Moreover, all previous ME-based biosensors required accurate pre-dilution of juice or wine samples, before these diluted samples were in turn mixed with the testing solution on the electrode (Arif et al., 2002; Esti et al., 2004; Gajovic et al., 1997; Gajovic et al., 1998; Lupu et al., 2004; Messia et al., 1996). The pre-dilution factors in these studies ranged from 10-fold for potato and tomato samples (Arif et al., 2002) to 1000-fold for wine samples (Messia et al., 1996). The removal of pre-treatment and pre-dilution steps is useful for reducing liquid handling, reducing the time taken per test, and for increasing accuracy (particularly for non-expert users). Further work will focus on minimizing interference and addressing stability through electrode design and the use of enzyme immobilization strategies, including those specifically designed to remove ascorbate interference (Maines et al., 2000).
Over 20 years ago, the development of new techniques for protein engineering and directed evolution led to a call for biosensor developers to “adapt the molecule to the instrument, rather than the instrument to the molecule” (Hellinga & Marvin, 1998). Here, we have shown that the time has come to embrace this approach. The availability of comprehensive enzyme databases and the ease of gene synthesis makes it possible to identify and screen proteins that deliver new biosensor functionalities using generic detector technologies—rather than optimizing the non-biological sensor components for use with commercially available enzymes such as the chicken liver ME. In this work, we showed that a bespoke enzyme could be used to modulate linear range, yielding a device with a feature that is desirable for real-world application. The same enzyme-centric approach is equally valid for improving other aspects of enzyme-based biosensors. For example, we began with the assumption that a sensor's calibration curve is linked to the steady-state parameters of the enzyme (Baronas et al., 2021; Vasylieva & Marinesco, 2013)—and therefore an enzyme with a high KM should impart an elongated linear range. The same line of reasoning suggests that an enzyme with a high catalytic efficiency should produce a highly sensitive device. Shelf life could be improved by identifying and/or engineering highly thermostable enzymes. Similarly, screening bespoke enzymes for their specificities with alternate substrates could inform the design of devices with decreased interference effects. Protein engineering approaches have already shown promise for optimizing variables such as enzyme immobilization (Pei et al., 2022). With powerful new approaches such as AI-driven protein design on the cusp of becoming mainstream (Madani et al., 2023; Watson et al., 2023), we believe the use of bespoke enzymes in biosensors is a strategy ripe for much further exploration.
4 MATERIALS AND METHODS
4.1 Reagents and solutions
All chemicals were at reagent or laboratory grade purity and sourced from MilliporeSigma (St Louis, MO, USA), unless stated otherwise. Reagent solutions were made with deionized water. Where needed, solutions were autoclaved at 121°C, 100 kPa for 20 min.
4.2 Expression and purification of candidate malate oxidoreductases
The gene sequences for the four candidate enzymes were obtained from various repositories: AsME(R181Q), GenBank sequence M81055.1; AcME, KEGG genes, accession code AZC_3656; SbMDH, GenBank sequence X53453.1; and ApMDH, GenBank sequence AB263815.1. Genes with codons optimized for heterologous expression in E. coli were synthesized by Twist Bioscience (San Francisco, CA, USA), which also cloned them into the pET-28a(+) expression vector. This vector encoded an N-terminal hexahistidine (His6) tag for AsME(R181Q), AcME, and SbMDH. However, the crystallographically determined structure of the ApMDH tetramer (PDB ID: 2D4A; Kawakami et al., 2009) suggested that the C terminus of each subunit was more solvent accessible, and distant from the interfaces of the tetramer, than the N terminus. Therefore, we made use of a C-terminal His6 tag for ApMDH.
Recombinant protein expression was in E. coli strain BL21 (DE3) Gold. Enzymes were purified using immobilized metal chromatography. AsME(R181Q) and AcME were purified further using size-exclusion chromatography. Details are provided in the Supplementary Methods.
After purification, protein concentrations were determined by measuring the absorbance at 280 nm and using extinction coefficients predicted by ProtParam (Gasteiger et al., 2005). Aliquots of each enzyme were stored at −80°C until use.
4.3 Spectrophotometric activity assays
Enzyme activities were measured using a standard assay (Karsten & Cook, 2007). For each enzyme, oxidation of l-malate was accompanied by reduction of NAD+ to NADH. This was detected via the increase in absorbance at 340 nm. All assay conditions are described in the Supplementary Methods.
4.4 Electrochemical experiments
Electrochemical experiments used C220BT screen-printed electrodes from Metrohm Dropsens (Asturias, Spain). Each sensor had a planar gold working electrode with an area of 0.13 cm2 (Ø = 4 mm), a gold counter electrode, and a silver reference electrode. Measurements were performed with a DY2116B potentiostat from Digi-Ivy (Austin, TX, USA), with subsequent analysis using its integrated software. Experiments were performed at room temperature using 50 μL of the testing solution, which was sufficient to fully cover all three electrodes. All reported currents were normalized for the electrode area and reported using the IUPAC convention, which defines anodic current as positive.
Electron mediators were initially characterized using cyclic voltammetry. Each mediator (or NADH as a control), at a final concentration of 5 mM, was dissolved in 100 mM HEPES (pH 7.0), 200 mM MgCl2, and 300 mM NH4Cl, to replicate the conditions used with AsME(R181Q). Voltammograms were produced by sweeping the potential between +500 mV and −500 mV at a scan rate of 100 mV/s. Peak oxidation potentials were identified by the potentiostat's integrated software. Next, chronoamperometry was used to characterize the analytical signal produced by each mediator. Each mediator (5 mM) was tested in solution with 100 mM HEPES (pH 7.0), 5 mM NADH, 200 mM MgCl2, and 300 mM NH4Cl. The analytical signal was measured after a 2-min pre-incubation period and a 5-s pulse at the chosen operating potential. Experiments were performed in triplicate and the results are reported as mean ± SEM.
Calibration curves for each biosensor were constructed over the range 0–200 mM l-malate using testing solutions of total volume 50 μL, which contained 100 mM HEPES (pH 7.0), 10.2 μM AsME(R181Q), 10 mM NAD+, 0.5 mM 2,6-dichloroindophenol (DCPIP), 200 mM divalent metal ion (either Mg2+ or Mn2+, supplied as the chloride salt) and 300 mM NH4Cl. Where noted, assays also contained 100 mM citrate. DCPIP was unstable in solution containing HEPES; therefore, it was added last and measurements were made within 2 min of its addition. Each enzyme-catalyzed reaction was started by the addition of l-malate and was allowed to proceed for 2 min before current measurements were taken after a 5-s pulse at +150 mV. In Mn2+ and citrate-containing conditions, the LOD was determined using the 3sb/m criterion (Mundaca-Uribe et al., 2017), where sb was the standard deviation of the blank measurements and m was the slope of the calibration curve.
Tests for interference from common juice components were carried out using 100 mM HEPES (pH 7.0), 10.2 μM AsME(R181Q), 10 mM NAD+, 0.5 mM DCPIP, 200 mM MgCl2, 300 mM NH4Cl, and 100 mM l-malate. Measurements were made in the same manner as described for the calibration curves.
4.5 Determination of l-malate in a wine sample
We tested a sample of Pinot Noir that was obtained approximately two-thirds of the way through its fermentation. First, the l-malate in the sample was measured using the l-Malic Acid UniFlex Kit from Unitech Scientific (Hawaiian Gardens, CA, USA) and the l-Malic Acid Assay Kit from Megazyme (Bray, Ireland). These spectrophotometric kits were used according to their manufacturers' instructions.
Next, l-malate in same wine sample was measured amperometrically. Each testing solution contained 20 μL of wine in a final volume of 50 μL. The wine sample was undiluted and was not decolorized or otherwise pre-treated. The other components in the testing solution were 500 mM MES buffer (pH 6.7), 10.2 μM AsME(R181Q), 10 mM NAD+, 0.5 mM DCPIP, 200 mM MnCl2, 300 mM NH4Cl, 100 mM citrate and either 0 U, 5 U, or 10 U of ascorbate oxidase from Cucurbita sp. (MilliporeSigma). AsME(R181Q) was omitted from some tests, as described in Section 2.6. Enzyme-catalyzed reactions were initiated by the addition of the wine sample and measurements were made as described for the calibration curves. All tests on the Pinot Noir sample (spectrophotometric and amperometric) were done in triplicate, with results reported as the mean ± SEM.
AUTHOR CONTRIBUTIONS
Christopher J. Matthews: Conceptualization, Methodology, Formal Analysis, Investigation, Writing—Original Draft, Visualization. Wayne M. Patrick: Conceptualization, Formal Analysis, Writing—Review & Editing, Visualization, Supervision, Project Administration, Funding Acquisition.
ACKNOWLEDGMENTS
We gratefully acknowledge James Dicey and Matt Dicey (Dicey Wines, Cromwell, New Zealand) for their feedback on this research and for providing the wine sample used in the ascorbate oxidase experiments. This work was supported by the New Zealand Ministry of Business, Innovation and Employment via a Smart Ideas grant from the Endeavour Fund (grant number RTVU1806). Open access publishing facilitated by Victoria University of Wellington, as part of the Wiley - Victoria University of Wellington agreement via the Council of Australian University Librarians.