Promoting the activity of a receptor tyrosine phosphatase with a novel pH-responsive transmembrane agonist inhibits cancer-associated phenotypes
Review Editor: Aitziber L. Cortajarena
Abstract
Cell signaling by receptor protein tyrosine kinases (RTKs) is tightly controlled by the counterbalancing actions of receptor protein tyrosine phosphatases (RPTPs). Due to their role in attenuating the signal-initiating potency of RTKs, RPTPs have long been viewed as therapeutic targets. However, the development of activators of RPTPs has remained limited. We previously reported that the homodimerization of a representative member of the RPTP family (protein tyrosine phosphatase receptor J or PTPRJ) is regulated by specific transmembrane (TM) residues. Disrupting this interaction by single point mutations promotes PTPRJ access to its RTK substrates (e.g., EGFR and FLT3), reduces RTK's phosphorylation and downstream signaling, and ultimately antagonizes RTK-driven cell phenotypes. Here, we designed and tested a series of first-in-class pH-responsive TM peptide agonists of PTPRJ that are soluble in aqueous solution but insert as a helical TM domain in lipid membranes when the pH is lowered to match that of the acidic microenvironment of tumors. The most promising peptide reduced EGFR's phosphorylation and inhibited cancer cell EGFR-driven migration and proliferation, similar to the PTPRJ's TM point mutations. Developing tumor-selective and TM-targeting peptide binders of critical RPTPs could afford a potentially transformative approach to studying RPTP's selectivity mechanism without requiring less specific inhibitors and represent a novel class of therapeutics against RTK-driven cancers.
1 INTRODUCTION
Protein tyrosine phosphorylation is essential for initiating and propagating cell signal transduction in response to external stimuli (Manning et al., 2002; Ubersax & Ferrell Jr, 2007). The magnitude and duration of tyrosine phosphorylation are tightly regulated by the counterbalancing actions of receptor tyrosine kinases (RTKs) and receptor protein tyrosine phosphatases (RPTPs). Indeed, RPTPs dephosphorylate regulatory tyrosine residues on RTKs to attenuate their signal-initiating potency (Hunter, 2009; Tonks, 2013). It is now recognized that disrupted RTKs and RPTPs activity through oncogenic mutations results in aberrant protein tyrosine phosphorylation and is implicated in many cancer types (Cohen & Alessi, 2013; He et al., 2014; Libermann et al., 1985; Mok, 2011; Spano et al., 2005). Accordingly, targeted small molecule inhibitors and antibodies have been developed to suppress the oncogenic activity of RTKs, such as the epidermal growth factor receptor (EGFR). While gefitinib and cetuximab can be highly effective, at least initially (Ercan et al., 2012; Furcht et al., 2013; Lazzara et al., 2010), acquired resistance can arise through gatekeeper mutations or bypass signaling via alternative RTKs (Hata et al., 2016; Niederst & Engelman, 2013).
On the other hand, several RPTPs have been identified as suppressors of many tumor types, including colon, lung, breast, and thyroid (Iuliano et al., 2004; Keane et al., 1996; Nunes-Xavier et al., 2013; Ruivenkamp et al., 2002; Ruivenkamp et al., 2003). Therefore, promoting the phosphatase activity of RPTPs represents a possible alternative to reverse malignant phenotypes driven by aberrant RTK signaling and overcome common acquired resistance mechanisms. While there are clear advantages in enhancing RPTPs tumor-suppressing activity toward dysregulated RTKs (Farrington et al., 2020), allosteric modulators of RPTPs remain underdeveloped due to a gap in knowledge of their structure–function relationship.
In contrast to their RTK counterparts, the activity of RPTPs appears to be suppressed by homodimerization (Barr et al., 2009; Hower et al., 2009; Jiang et al., 1999). We previously reported that specific transmembrane (TM) domain interactions stabilize the homodimerization of protein tyrosine phosphatase receptor J (PTPRJ) (Bloch et al., 2019), a relevant tumor suppressor and essential down-regulator of growth factor signaling, including PDGFβR (Jandt et al., 2003; Kovalenko et al., 2000; Petermann et al., 2011), VEGFR (Lampugnani et al., 2003; Patel et al., 2012), METR (Lampugnani et al., 2003; Palka et al., 2003), EGFR (Avraham & Yarden, 2011; Tarcic et al., 2009), and FLT3b (Arora et al., 2011; Böhmer et al., 2013). We identified the TM residues G979, G983, and G987 as major mediators of PTPRJ's self-association through a specific contact interface. We showed that introducing glycine-to-leucine mutations disrupted PTPRJ oligomerization in cells, promoted PTPRJ association with EGFR, decreased EGFR phosphorylation at tyrosines 1068 and 1173, and antagonized EGFR-driven cell phenotypes. Schwarz et al. observed similar results toward the oncogenic mutant FLT3 (with internal tandem duplications) in acute myeloid leukemia cell models (Schwarz et al., 2022). These results suggest that disrupting the oligomerization of PTPRJ could prove a valuable therapeutic strategy to restrict oncogenic RTK activity in cancer cells.
In response to the lack of specific agonists, we also reported the screening and identification of a transmembrane peptide (RJbinder) that interacts with the TM domain of PTPRJ and disrupts its TM domain-mediated dimerization (Bloch et al., 2019). Treatment of EGFR-driven cancer cells with this peptide resulted in decreased PTPRJ self-association, reduced EGFR phosphorylation, and ultimately inhibited cell migration. This peptide represents a first-in-class agonist and possibly a new allosteric approach to target the activity of RPTPs. However, like for other TM peptides, poor solubility in aqueous solution, need for a delivery vehicle (i.e., detergent micelles), lack of insertion directionality, and indiscriminate insertion in healthy and cancer cells limit its efficacy and applications.
Here, to circumvent these hurdles and improve targeting and efficacy against cancer cells (where RTKs are dysregulated), we combined the properties of the RJbinder with those of the pH (Low) Insertion Peptides (pHLIP), a family of peptides with validated selective tumor targeting and promising therapeutic potential. The pHLIP peptides insert into the plasma membrane of cancer cells in a pH-dependent manner (Burns et al., 2015; Burns et al., 2016; Burns et al., 2017; Burns & Thévenin, 2015; Gerhart et al., 2018) by taking advantage of the inherent acidic microenvironment of solid tumors (Bhujwalla et al., 2002; Gerweck & Seetharaman, 1996; Vaupel et al., 1989; Warburg, 1956; Wike-Hooley et al., 1984; Zhang et al., 2010). We threaded the residues shown to provide pHLIP with aqueous solubility and pH-sensitive insertion into the sequence of RJbinder to engineer hybrid peptides. By making minimal sequence modifications, we show that the resulting lead hybrid peptide (hybrid7) is readily soluble in aqueous solution and inserts into membranes as a TM α-helix in a pH-dependent manner without affecting its ability to interfere with PTPRJ oligomerization. Treating cancer cells with hybrid7 resulted in decreased EGFR phosphorylation, attenuation of downstream signaling, and inhibition of cancer cell migration and proliferation, significantly improving the original, non-pH-sensitive RJbinder peptide.
2 RESULTS
2.1 Hybrid peptide design and synthesis
We considered several factors to thread residues conferring pHLIP-like properties into the sequence of RJbinder without affecting its properties. First, we only kept the region of RJbinder predicted to be the TM domain (LLLLSSIGAIMWVSLVCLIA; bold in Table 1). Second, we held the residues S9, A13, and V17 of RJbinder (Table 1; underline residues) constant. We previously identified them as crucial for its activity and likely part of the contact interface with PTPRJ (Bloch et al., 2019). Third, several residues are essential to the pH-responsive properties of pHLIP. For instance, the two aspartic acid residues at positions 14 and 25 (Table 1; red residues) confer pH sensitivity by being protonated at lower pH. Additionally, the proline residue at position 20 (Table 1; green residues) maintains a high energy barrier to membrane insertion until protonation by limiting α-helix formation. Notably, pHLIP insertion properties can be finely tuned by changing these residues to other amino acids. For example, replacing either D14 or D25 with glutamic acid or 2-aminoadipic acid and P20 with glycine increases the pK50 (i.e., the pH at which 50% of peptides are in the α-helical membrane inserted state) (Table 1) (Barrera et al., 2012; Musial-Siwek et al., 2010; Onyango et al., 2015; Vasquez-Montes et al., 2018). Fourth, the position and spacing of these residues are also crucial (Fendos et al., 2013) and were conserved in our design while not affecting the residues involved in the interaction of RJbinder with PTPRJ. Finally, given the hydrophobicity of RJbinder and to aid with solubility, insertion unidirectionality, and retention of the inserted state, we included an N-terminal Aspartic tag and the C-terminal region of pHLIP (DADEG) (Barrera et al., 2011; Cunningham & Deber, 2007; Melnyk et al., 2003). With this in mind, we designed and synthesized two hybrid peptides (Table 1; hybrids 1 and 2).
| Name | Sequence | pK50 |
| RJbinder | KKTTLLLL S SIG A IMW V SLVCLIAVKGSNKK | N/A |
| pHLIP(WT) | GGEQNPIYWARYDWLFTTPLLLLDLALLVDADEGTG | 6.16a |
| pHLIP(D14E) | GGEQNPIYWARYEWLFTTPLLLLDLALLVDADEGTG | 6.14a |
| pHLIP(D25E) | GGEQNPIYWARYDWLFTTPLLLLELALLVDADEGTG | 6.27a |
| pHLIP(D14Aad) | GGEQNPIYWARYAadWLFTTGLLLLDLALLVDADEGTG | 6.37a |
| pHLIP(D25Aad) | GGEQNPIYWARYDWLFTTGLLLLAadLALLVDADEGTG | 6.74a |
| pHLIP(P20G) | AAEQNPIYWARYDWLFTTGLLLLDLALLVDADEGG | 7.2b |
| pHLIP(WT-Ala) | AAEQNPIYWARYDWLFTTPLLLLDLALLVDADEGG | 6.1b |
| Hybrid 1 | DDDDDDTTLLLLS DIGA IMWVSLVDLIADADEG | This study |
| Hybrid 2 | DDDDDDTTLLLLS DIGA PMWVSLVDLIADADEG | This study |
| Hybrid 3 | DDDDDDTTLLLLS DIGA PMWVSLVELIADADEG | This study |
| Hybrid 4 | DDDDDDTTLLLLS DIGA GMWVSLVELIADADEG | This study |
| Hybrid 5 | DDDDTTLLLLS DIGA GMWVSLVELIADADEG | This study |
| Hybrid 6 | DDDDTTLLLLS DIGA GMWVSLVAadLIADADEG | This study |
| Hybrid 7 | DDTTLLLLS EIGA GMWVSLVELIADADEG | This study |
| Hybrid 7-control | DDTTLLLLS EIG L GMWVSLVELIADADEG | This study |
| Hybrid 8 | DDTTLLLLS EIGA GMWVSLVELIAEAEEG | This study |
| Hybrid 9 | DDTTLLLLS AadIGA GMWVSLVELIADADEG | This study |
2.2 Identification of pH-responsive hybrid peptides
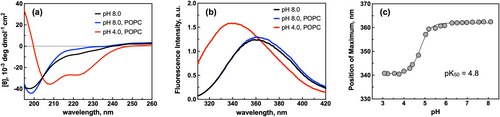
To assess whether the hybrid peptides can insert into lipid membranes as TM helices in a pH-dependent manner, we conducted far-UV circular dichroism (CD) and Trp fluorescence spectroscopy measurements with or without large unilamellar lipid vesicles (LUVs) consisting of palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) as a function of pH. We selected POPC because it allows comparison with the many reported biophysical measurements with pHLIP and other TM peptides (Alves et al., 2018; Nguyen et al., 2015; Vasquez-Montes et al., 2019; Vasquez-Montes et al., 2022). As observed by CD spectroscopy (Figure S1a), hybrid1 adopts a mostly disordered conformation at pH 8.0 with and without vesicles and exhibits a slight increase in helical content at pH 4.0 in the presence of vesicles. However, no change in Trp fluorescence emission is observed in either condition (Figure S1b). With emission maxima centered around 360 nm, these results indicate that the Trp resides in an aqueous environment and that hybrid1 does not partition into the lipid bilayer. On the other hand, hybrid2 exhibits spectroscopic signatures consistent with a pH-responsive insertion (Figure 1a,b). As observed by CD spectroscopy (Figure 1a), it adopts a mostly disordered conformation at pH 8.0 in the absence and presence of LUVs and shows an increase in helical content at pH 4.0 with vesicles (74% helical). In the presence of LUVs, hybrid2 exhibits a blue shift in the Trp emission spectrum from 362 nm at pH 8.0 to 341 nm at pH 4.0 (Figure 1b), indicating a transition of the Trp from a polar, aqueous environment to a more hydrophobic environment such as the lipid bilayer. From analyzing the complete pH titration from 3.0 to 8.0 and the corresponding positions of maxima, we determined a protonation-dependent membrane insertion pK50 of 4.8 (Figure 1c and Table 1). While these results are consistent with the desired behavior (forming a TM ɑ-helix upon acidification), we considered a pK50 of 4.8 too low for possible future applications.
Therefore, to increase the pK50 of insertion, we designed hybrids 3–9 with sequence modifications guided by those established in pHLIP variants (Table 1). All seven peptides exhibited, albeit to a different extent, CD (Figure S2) and Trp fluorescence (Figure S3) spectroscopic signatures similar to hybrid2 and consistent with a transition of disordered peptides at pH 8 to inserted ɑ-helices upon acidification. They all show a sigmoidal pH response in the presence of vesicles (Figure S4) with pK50 from 4.7 to 5.7 (Table S1). Notably, hybrid7 displayed the highest helical content (Table S1) at low pH in the presence of vesicles (97%) (Figure 2a), the largest blue shift (from 363 to 333 nm) (Figure 2b), and the highest pK50 value (5.68) (Figure 2c).
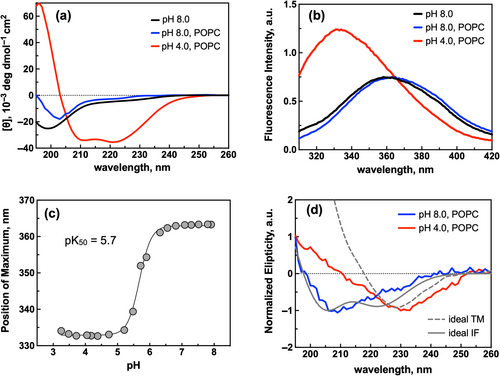
To determine the orientation hybrid7 adopts in the presence of vesicles at lower pH, we performed oriented circular dichroism (OCD), which allows for the differentiation between transmembrane and interfacial α-helices (Bürck et al., 2016; Wu et al., 1990). OCD measurements at low pH in the presence of POPC vesicles yielded a spectrum with a single minimum of around 230 nm (Figure 2d; red line), consistent with an inserted TM helix. On the other hand, the OCD spectrum at pH 8.0 has a single minimum at ~210 nm (Figure 2d; blue line), which is not consistent with either a TM or interfacial α-helix but is consistent with an unstructured peptide and the CD results (Figure 2a). Given its favorable properties, we selected hybrid7 as the lead peptide for further evaluation.
2.3 Hybrid7 associates with the transmembrane domain of PTPRJ
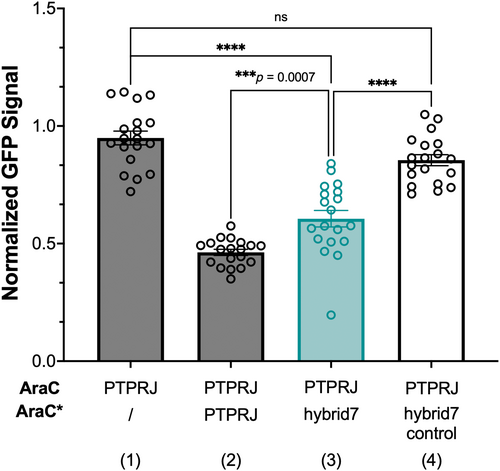
To assess whether the sequence modifications made to obtain hybrid7 affect the ability of the peptide to interfere with PTPRJ dimerization, we used the dominant-negative AraC-based transcriptional reporter assay (DN-AraTM) (Barton et al., 2015; Deng et al., 2014; Goh et al., 2018; Su & Berger, 2012; Su & Berger, 2013; Wonganu & Berger, 2016). This assay reports on the propensity of TM domains to self-associate and heterodimerize in cell membranes and allowed us, in a previous study, to identify the disruptive glycine-to-leucine TM mutations and the RJbinder peptide (Bloch et al., 2019). Briefly, the assay relies on a protein chimera containing the receptor domain of interest fused to either the transcription factor AraC (which is active at the araBAD promoter as a homodimer) or an AraC mutant unable to activate transcription (AraC*). Homodimerization of AraC (a result of receptor domain self-association) induces the transcription of the gene coding for the green fluorescent protein (GFP). On the other hand, preferential TM-mediated AraC-AraC* heterodimerization reduces the level of GFP transcription. Thus, the level of GFP fluorescence intensity is a measure of receptor domain homo- or hetero-dimerization.
Figure 3 shows that hybrid7 retains the ability to interact with the TM of PTPRJ. Indeed, the co-expression of hybrid7 as a competitor to PTPRJ-AraC led to a significant decrease in GFP expression (Figure 3; 1 vs. 3), indicative of a preferential heterodimer. This decrease is comparable to the one observed when the TM sequence of PTPRJ is co-expressed as a competitor (Figure 3; 1 vs. 2). As a control, we also tested the effect of mutating A13 to leucine in hybrid7. We previously showed that A13L causes RJbinder to lose its ability to out-compete PTPRJ oligomerization by interfering with helix-packing (Bloch et al., 2019). Similarly, the point mutation significantly hampers hybrid7 efficacy to interact with PTPRJ (Figure 3; 1 vs. 4), even though it exhibits an increased expression (Figure S6a). Noteworthy, neither hybrid7 nor hybrid7-control sequences show a propensity to dimerize (Figure S6b): Both show a low signal compared to the strongly interacting transmembrane domain of the leukocyte receptor tyrosine kinase (LTK) (Finger et al., 2009) when the AraC fusions are expressed alone (Figure S6b; 1 vs. 4 and 1 vs. 7). Co-expression of the AraC* fusion does not decrease that signal significantly either (Figure S6b; 4 vs. 5 and 7 vs. 8), indicating that there is no competition between the two fusions. Hybrid7 not having the propensity to self-interact is an advantageous property because its oligomerization would likely hinder its interaction with PTPRJ. A peptide corresponding to the A13L variant was synthesized and subsequently used as a negative control peptide (hybrid7-control). Moreover, the A13L substitution did not significantly affect the ability of hybrid7-control to form a pH-inducible TM α-helix or the pK50 of insertion (Table S1 and Figure S5).
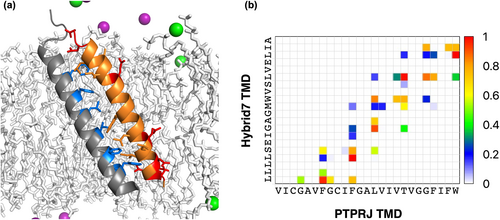
To better understand the molecular interactions between hybrid7 and the TM domain of PTPRJ, we also performed 70 ns replica exchange molecular dynamics (REMD), followed by 1 μs all-atom MD simulations in the POPC bilayer (Figure 4b and Figure S7). The resulting model shows a left-handed heterodimer with a crossing angle of +19 degrees that positions the acidic residues away from the contact interface (Figure 4a). The inter-TM contact probability map (Figure 4b and Figure S7c) shows contact mainly through residues Leu5, Leu 8, Gly12, Leu19, and Leu22 on hybrid7, and Phe12, Phe16, Leu19, Thr23, and Trp30 on PTPRJ. It suggests that PTPRJ interacts with hybrid7 through different residues than those we identified to mediate its homodimerization (Gly9, Gly13, and Gly17). It also indicates that, while Ala13 on hybrid7 does not have the highest probability of inter-TM contact, it is still a ‘hot spot’ for interaction with PTPRJ (Figure 4b and Figure S7c). It is also the case for Ser9, whose substitution for leucine disrupts PTPRJ TM-TM interaction (Bloch et al., 2019). This is consistent with the results obtained with the DN-AraTM reporter assay and the influence of A13 on hybrid7 interaction with PTPRJ. However, we recognize the differences between the results from MD simulations and experimental observations. A more in-depth understanding of the contact interfaces is the focus of ongoing work.
2.4 Hybrid7 inserts into cancer cell membranes without disruption
To assess the interaction of hybrid7 with cells, we treated HSC3 cells (an EGFR-driven human squamous cell carcinoma line we previously engineered to express full-length PTPRJ (Bloch et al., 2019)) with hybrid7 labeled at its N-terminus with fluorescein (FITC-hybrid7). Labeling hybrid7 with FITC does not significantly alter its pH-responsive structure, insertion, and pK50 (6.0 vs. 5.7 for hybrid7) (Table S1 and Figure S5).
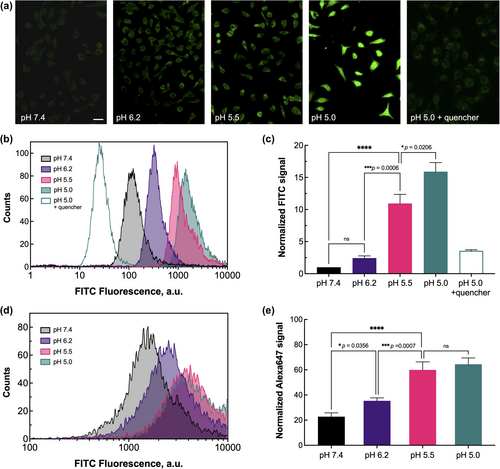
While FITC-hybrid7 showed minor interactions with cells at physiological pH 7.4, progressive acidification resulted in an increase of FITC fluorescence intensity (Figure 5a), attributed to the pH-dependent membrane insertion of hybrid7. We also quantified this interaction by flow cytometry. Consistent with what was observed qualitatively by microscopy, the relative amount of FITC-hybrid7 detected on the cells increased in a pH-dependent manner, with a 12-fold (pH 5.5) and 16-fold (pH 5.0) increase in fluorescence over that at pH 7.4 (Figure 5b,c). Moreover, in both microscopy and flow cytometry experiments, FITC fluorescence is quenched by the extracellular addition of trypan blue (a known quencher of fluorescein emission (Cowen et al., 1985; Hed et al., 1987; Van Amersfoort & Van Strijp, 1994)) (Figure 5b,c), indicating that FITC (and therefore the N-terminal end of hybrid7) is extracellularly exposed. Finally, the exposure of FITC to the extracellular environment was confirmed by flow cytometry, showing an increase in the recruitment of anti-FITC antibodies upon a decrease in pH (Figure 5d,e). This result is consistent with what we observed in another study using pHLIP labeled at its N-terminus with FITC (Wehr et al., 2020).
With confirmation that hybrid7 selectively associates with cell membranes in a pH-responsive manner, we sought to determine whether the insertion damages the integrity of the lipid bilayer. Following a 1-h treatment with 10 μM of hybrid7 at pH 7.4, 5.5, or 5.0, the media from the HSC3 cells was analyzed for the release of the intracellular enzyme lactate dehydrogenase (LDH), a proxy for membrane integrity. Neither hybrid7 nor low pH treatment causes a significant release of LDH (Figure S7), indicating that the peptide does not cause membrane disruption, consistent with our previous results obtained with pHLIP (Burns et al., 2015; Burns et al., 2016; Burns et al., 2017; Burns & Thévenin, 2015).
Altogether, these results indicate that hybrid7 inserts in the plasma cell membrane in a pH-dependent manner with its N-terminal end remaining extracellularly (like pHLIP) without disrupting the cell plasma membrane.
2.5 Hybrid7 inhibits EGFR phosphorylation, EGFR-Driven cell migration, and proliferation in a pH-dependent manner
Next, we determined the functional effect of hybrid7 on the activity of PTPRJ in cells. We hypothesized that treating cells with hybrid7 at lower pH would decrease EGFR signaling and, ultimately, inhibit EGFR-driven cell migration and proliferation while having no effect at higher pH.
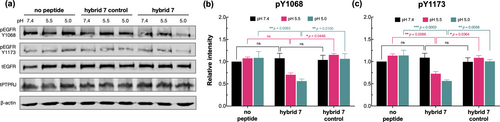
Figure 6 shows that 10 min treatment of HSC3 cells expressing wild-type PTPRJ with 10 μM hybrid7 resulted in a significant pH-dependent decrease in EGFR phosphorylation at both Tyr-1068 and Tyr-1173 (30% and 45% reduction at pH 5.5 and pH 5.0, respectively). On the other hand, no changes in phosphorylation level were observed after treatment with hybrid7-control.
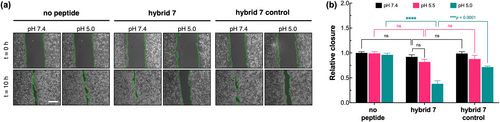
Additionally, results in Figure 7 show that similar treatment with hybrid7 inhibits EGFR-driven cell migration in a pH-dependent manner. At pH 7.4 and 5.5, hybrid7 had no significant effect on cell migration but decreased cell migration by 60% at pH 5.0. While treatment with hybrid7-control does not result in any effect at pH 5.5, a 30% inhibition in cell migration was observed when cells were treated at pH 5.0.
Lastly, hybrid7 inhibits cell proliferation in a concentration and pH-dependent manner (Figure 8a). At pH 7.4, hybrid7 had minimal effect on cell viability, with a maximum decrease of 30% at 10 μM. On the other hand, a significant concentration-dependent decrease in cell viability was observed at pH 5.5, with only 20% viability at 10 μM and an IC50 of 0.54 μM. Noteworthy, hybrid7-control had no effect at either high or acidic pH (Figure 8b), and hybrid7 did not affect the parental HSC3 cells expressing a minimal level (undetectable by immunoblot) of PTPRJ (Figure S8) (Bloch et al., 2019). Crucially, cell treatment at lower pH did not affect either EGFR phosphorylation (Figure 6), cell migration (Figure 7) or proliferation (Figure 8).
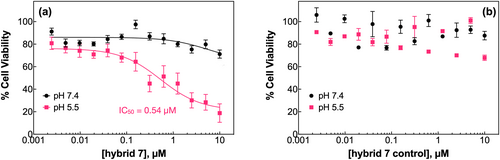
Taken together, our results strongly indicate that hybrid7 disrupts, in a pH-dependent manner, the TM domain-mediated dimerization of PTPRJ, thereby enhancing PTPRJ's ability to dephosphorylate EGFR and attenuating downstream signaling cascades and EGFR-driven phenotypes.
3 DISCUSSION
Because of the lack of clear agonists for PTPRJ and its tumor-suppressor role, promoting its activity toward EGFR and other substrate RTKs represents a much-needed and unique way to study PTPRJ signaling mechanisms and a promising therapeutic modality in oncology. In this work, we engineered an allosteric agonist of PTPRJ, hybrid7, that is readily soluble in aqueous buffer and inserts as a helical TM domain into cell membranes without disrupting them under acidic conditions that resemble the microenvironment of tumors.
Our results also support that hybrid7 interacts with PTPRJ and promote its activity toward EGFR, leading to reduced EGFR phosphorylation and inhibition of EGFR-driven cell migration and proliferation. Hybrid7 disrupts these processes more efficiently than the previously reported G-to-L TM point mutations and the RJbinder peptide. For example, treatment with RJbinder at the same concentration (10 μM) decreased phosphorylation at Tyr-1068 and Tyr-117 by only ~20%–30% (45% reduction for hybrid7 at pH 5.0) and cell migration by 20% (vs. 60% reduction for hybrid7 at pH 5.0) (Bloch et al., 2019). RJbinder also required 1-h treatment to achieve these results, while cells were only treated with hybrid7 for 10 min. Hybrid7 also inhibits cell proliferation with a promising IC50 of 0.5 μM.
Although our results indicate that the TM Glu residues are not part of the contact interface between hybrid7 and PTPRJ, acidic residues may provide additional specificity to TM interactions (Bañó-Polo et al., 2013; Gratkowski et al., 2001; Zhou et al., 2001). The addition of these residues may afford hybrid7 additional efficacy and could explain some of the small effects hybrid7-control had on pEGFR and cell migration. Moreover, it is likely that the single point mutation in hybrid7-control hinders the ability to interact with PTPRJ but does not entirely abrogate it. Based on our MD simulation results, the residues neighboring Ala13 (mutated to a Leu in hybrid7-control) may also contribute to the association with PTPRJ. It is also possible that the presence of PTPRJ in the cell membrane may favor the inserted state, raising the apparent pK50 of insertion. It could also explain the observed efficacy of hybrid7 in cells even at a pH relatively far from its determined pK50 in vesicles. This was observed with the TYPE7 peptide designed to bind to the TM of EphA2 in a pH-dependent manner, for which the presence of EphA2 in vesicles increased the pK50 from 6.18 to 6.85 (Alves et al., 2018). However, based on this result, the efficacy of TYPE7 in cells was only tested at normal physiological pH, making it impossible to assess its pH-mediated selectivity. Nevertheless, improving the efficacy of hybrid7 by tuning its pH50 of insertion and its contact interface with PTPRJ is the focus of ongoing efforts in our laboratory.
In conclusion, hybrid7 represents a first-in-class tumor-targeting allosteric agonist of RPTPs, and we expect that the basic framework developed here can be extended to other RPTPs (and, therefore, other RTK substrates). Indeed, modulation of RPTP activity through dimerization of TM domains is a general theme throughout the family, and targeting the TM domains could, therefore, offer a level of specificity that cannot be achieved by targeting either the extracellular or PTP domains alone. While this strategy is therapeutically relevant to tumors driven by oncogenic RTK signaling, it also presents an alternative approach for RPTP activation to study their function and identify new substrates in cells.
4 EXPERIMENTAL SECTION
4.1 Solid-phase peptide synthesis
Hybrid peptides were prepared by solid-phase peptide synthesis, purified and analyzed by RP-HPLC, and confirmed via MALDI-TOF mass spectrometry, as previously reported (Bloch et al., 2019; Gerhart et al., 2018; Vasquez-Montes et al., 2019; Vasquez-Montes et al., 2022; Wehr et al., 2020).
4.2 Sample preparation of hybrid peptides for circular dichroism (CD) and tryptophan fluorescence measurements
All peptides were first solubilized to 20 μM in 5 mM sodium phosphate (pH 8.0). Each peptide was diluted to a final concentration of 7 μM before analysis. 100 nm 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) large unilamellar vesicles were prepared by freeze–thaw and extrusion, as previously reported (Bloch et al., 2019; Gerhart et al., 2018; Vasquez-Montes et al., 2019; Vasquez-Montes et al., 2022; Wehr et al., 2020). Peptides were incubated with the resulting vesicles at a 1:300 ratio. The pH was adjusted to the desired experimental values with HCl, and the samples were incubated for 30 min at room temperature prior to spectroscopic analysis.
4.3 CD spectroscopy
4.4 Oriented circular dichroism (OCD)
OCD measurements were performed using a JASCO-810 spectropolarimeter (JASCO, Easton, MD). Spectra were obtained by creating a stack of oriented multilayers on a quartz disc as previously described (Vasquez-Montes et al., 2019; Vasquez-Montes et al., 2022; Wimley & White, 2000). Briefly, 0.1 mM hybrid7 and a 10 mM POPC were co-dissolved in methanol (1:100 peptide to lipid ratio). The multilayer stack was created by placing 2.5 μ l of the peptide/lipid mixture in the center of a 2.5 cm disc. The solvent was then air-dried to a 1 cm diameter and hydrated using warm air at ~100% relative humidity. A drop of 5 mM HEPES buffer pH 4 or 5 mM HEPES buffer pH 8 was added between each stack layer and completely dried before continuing with multilayer stacking. The disc containing the multilayer stack was mounted on a sealed tube with the sample side pointing inwards. An average of 50 scans was collected at eight different orientations at 45 degree intervals along the central axis. The collected spectra were averaged, and the background signal (determined by collecting the spectra of a multilayer stack in the absence of peptide) was subtracted. The presented spectra have been normalized to the ellipticity of their respective minimum due to difficulties calculating the peptide concentration along the beam path of each stack.
4.5 Tryptophan fluorescence spectroscopy
4.6 Determining pK50 of hybrid peptides
4.7 Computational methods
4.7.1 PTPRJ-hybrid7 heterodimer assembly in implicit bilayer
To model the PTPRJ-hybrid7 heterodimer, we employed replica exchange molecular dynamics (REMD) simulations (Sugita & Okamoto, 1999). The sequences of PTPRJ and hybrid7 used are given below, and the TM domains are underlined. Considering the low pH environment, aspartic and glutamic acids (marked in bold) were all protonated.
PTPRJ Acetyl-1PQDPGVICGAVFGCIFGALVIVTVGGFIFWRKKRK35-CONH2.
Hybrid7 NH3-1DDTTLLLLSEIGAGMWVSLVELIADADEG29-CONH2.
The initial configuration of the PTPRJ-hybrid7 heterodimer for each replica was prepared as follows. The principal axes of PTPRJ and hybrid7 TM helices were aligned to the membrane normal (z-direction), and the C-termini of TM helices were inserted into the cytosolic side. The helices were separated by 30 Å with random orientations (rotations along each principal axis). For 52 replicas in a temperature range of 300–1000 K, a 70-ns REMD simulation was performed in the GBSW implicit membrane model (Im et al., 2003) using CHARMM (Brooks et al., 2009). The Langevin dynamics simulation was performed with a collision frequency of 5 ps−1, and the integration time step was set to 2 fs with the SHAKE algorithm (Ryckaert et al., 1977) for constraining covalent bonds involving hydrogen atoms. For the implicit membrane, the GBSW default options provided in Implicit Solvent Modeler in CHARMM-GUI (Jo et al., 2008) were used except for an empirical surface tension coefficient (0.03 kcal/mol/Å (Manning et al., 2002)) for the nonpolar solvation contribution. Replica exchanges were attempted every 1 ps, controlled by the CHARMM REPDSTR module (Woodcock et al., 2007). The centroid structure obtained from the cluster analysis (see below) was used to model the initial structures of PTPRJ-Hybrid7 assembly in an explicit bilayer.
4.7.2 Molecular dynamics simulations in explicit lipid bilayer
The stability of the obtained structure model (centroid) of PTPRJ-Hybrid7 heterodimers from REMD simulations was examined by molecular dynamics (MD) simulations of the TM assembly in an explicit POPC bilayer, consisting of the heterodimer, 90 and 89 POPC lipids in the upper and lower leaflets, respectively, and bulk water with 150 mM KCl. The membrane system was prepared using the CHARMM-GUI Membrane Builder (Wu et al., 2014), and equilibrated by a series of short constant volume and temperature (NVT) and constant temperature and pressure (NPT) simulations, during which various restraints were gradually relaxed. A 1-μs restraint-free NPT simulation was followed for production. For better statistics, we carried out five independent simulations. All simulations were performed using OpenMM (Eastman et al., 2017; Lee et al., 2016) with the C36 protein (Huang & MacKerell, 2013) and lipid (Klauda et al., 2010) force fields and TIP3P water model (Jorgensen et al., 1983; MacKerell et al., 1998). Integration time steps for equilibration were set to 1 fs and 2 fs for NVT and NPT simulations with SHAKE algorithm (Ryckaert et al., 1977), respectively. For the production run, the integration time step was set to 4 fs with the hydrogen mass repartitioning technique (Gao et al., 2021; Hopkins et al., 2015). The van der Waals interactions were smoothly switched off over 10–12 Å by a force-based switching function (Steinbach & Brooks, 1994), and the electrostatic interactions were calculated by the particle-mesh Ewald method (Essmann et al., 1995). The temperature (T = 303.15 K) and the pressure (p = 1 bar) were controlled by Langevin dynamics with a friction coefficient of 1 ps−1 and a semi-isotropic Monte Carlo barostat (Åqvist et al., 2004; Chow & Ferguson, 1995) with a pressure coupling frequency of 100 steps, respectively.
4.7.3 Analysis
The sampled conformations from REMD were evaluated by hierarchical clustering, where initially, all sample conformations were assigned to different clusters. Starting from these initial clusters, two clusters were merged when the average root mean square deviation between Cα atoms (Cα-RMSD) for all inter-cluster conformation pairs was less than a cut-off value of 3 Å. The clustering was iterated until there was no cluster closer than the RMSD cut-off. The clustering was done for seven 10-ns blocks of trajectories from the replica at T = 300 K. The centroid of the largest cluster during the last 40 ns was chosen as the structural model of PTPRJ-hybrid7 TM assembly. For TM models in explicit POPC bilayer, we calculated Cα-RMSD from their initial structure and inter-TM contact probability. For a conformation at a given time frame, the contact state between a pair of residues is assigned to 1 when the minimum distance between heavy atoms in the residue pair is smaller than 4.5 Å and 0 otherwise. The last 800-ns trajectories from each replica were analyzed. Also, representative conformations of PTPRJ-hydrid7 TM dimer in a POPC bilayer were obtained from the cluster analysis based on the pairwise Cα-RMSD between conformations with the same cut-off.
4.8 Cell culture
Engineered human tongue squamous cell carcinoma HSC3 cells previously engineered to express wild-type PTPRJ ectopically (Bloch et al., 2019) were cultured in DMEM high glucose supplemented with 10% FBS, 100 units/mL penicillin, and 0.1 mg/mL streptomycin, in a humidified atmosphere of 5% CO2 at 37°C.
4.9 Cell treatment and assessment of EGFR Tyr phosphorylation level
HSC3 cells were seeded in 12-well plates at 80,000 cells/well and incubated overnight. The cell media was replaced with serum-free media for 2 h before peptide treatment. Hybrid7 and hybrid7-control were solubilized in an appropriate amount of serum-starved media, pH 7.4, so that upon pH adjustment, the desired treatment concentration (10 μM) was obtained. The peptides were added to serum-starved cells and allowed to incubate for 5 min at 37°C. The media was then adjusted to the desired pH using a pre-established volume of serum-free media buffered with citric acid, pH 2.0. After a 10 min incubation at 37°C, the treatment media was removed and replaced with fresh serum-free media containing EGF (10 ng/mL), and the cells were incubated for another 10 min at 37°C. Cells were solubilized by the addition of cell extraction buffer supplemented with broad-spectrum phosphatase and protease inhibitors (Pierce #88667), spun down, and the supernatant was mixed with loading buffer. Samples were boiled for 10 min at 95°C and resolved by SDS-PAGE on an 8% tris-glycine gel. Subsequently, the samples were transferred onto a 0.45 μm nitrocellulose membrane (GE Healthcare #1060002) at 100 V for 1 h at 4°C. Membranes were blocked with 5% bovine serum albumin (BSA) in tris-buffered saline Tween 20 (TBS-T) for 1 h at RT and then blotted for phosphorylated EGFR (pY1068: RRID:AB_2096270; pY1173: RRID:AB_331795). When blotting for total proteins (EGFR:RRID:AB_2246311; β-Actin: RRID:AB_2242334; PTPRJ: RRID:AB_2935775), the membranes were blocked with 5% dry milk in TBS-T for 1 h at RT. Following blocking, the membranes were incubated with primary antibodies in 5% BSA TBS overnight at 4°C (pEGFR at 1:1000 dilution, total EGFR 1:2000, PTPRJ, 1:200, β-Actin 1:4000). Membranes were then incubated with the appropriate secondary antibodies in TBS-T for 30 min at RT at 1:4000 dilution (Anti-rabbit: RRID:AB_2099233; Anti-mouse: RRID:AB_330924). Following washes with TBS-T, the immunoblot was visualized by chemiluminescence after incubation with Clarity Western ECL Substrate (Bio-Rad). Images were quantified using ImageJ (Abramoff, 2007) and plotted as normalized (ratio of phosphorylated to total intensity) mean values (Li et al., 2004; Russ & Engelman, 1999).
4.10 Wound healing scratch assay
HSC3 cells were seeded in 6-well plates at a cell density necessary to reach confluency after 24 h and then serum-starved in serum-free media for 2 h before peptide treatment. Peptide preparation and cell treatment were performed as described above. After scratching the confluent cell monolayer with a 1 mL pipette tip, the media was replaced with complete media containing EGF (50 ng/mL) at 37°C for 10 h. Scratch areas were quantified with ImageJ using the MRI Wound Healing Tool (https://github.com/MontpellierRessourcesImagerie/imagej_macros_and_scripts/wiki/Wound-Healing-Tool), and the closure percent corresponds to the percent change in the area between the initial and final time points. Percent scratch closure was normalized to wild-type cells treated at pH 7.4.
4.11 DN-AraTM assay
The DNA sequences coding for the TM domains of interest (see below) were subcloned into either pAraTMwt (coding for AraC) or pAraTMDN (coding for the inactive form of AraC, AraC*) plasmids, using standard molecular biology techniques. All constructs were verified by DNA sequencing.
PTPRJ QDPGVICGAVFGCIFGALVIVTVGGFIFWRKKRKDAKNNEVSFSQIKPK.
LTK KPPGPLVLMVAVVATSTLSLLMVCGVLILVKQKKWQGLQEMRLPSPELE.
Hybrid7 DDTTLLLLSEIGAGMWVSLVELIADADEG.
Hybrid7-control DDTTLLLLSEIGLGMWVSLVELIADADEG.
The AraC and AraC*-based plasmids were co-transformed with the reporter plasmid (pAraGFPCDF) into the AraC-deficient E. coli strain SB1676 (the E. coli Genetic Stock Center at Yale University) and streaked onto selective lysogeny broth (LB) plates (100 μg/mL ampicillin, 50 μg/mL kanamycin, and 100 μg/mL spectinomycin). Colonies were picked from each construct and tested, as previously described (Bloch et al., 2019). The results are reported as the ratio of GFP fluorescence emission at 530 nm to absorbance at 580 nm and normalized to the negative control (empty plasmids and reporter plasmid). Immunoblotting was performed using HRP-conjugated anti-Myc (1:5000; RRID:AB_2148465), and anti-HA (1:5000; RRID:AB_10978021) followed by secondary anti-mouse HRP-conjugated (1:5000; RRID:AB_330924).
4.12 Peptide interaction with cells by fluorescence microscopy
HSC3 cells were seeded on 22 mm × 22 mm coverslips pretreated with poly-L-lysine in 6 well plates at 300,000 cells/well to be ~60% confluent after 16 h. Cells were treated for 10 min with 5 μM of FITC-hybrid7 as described above. Following two washes with PBS (same pH as treatment), the cells were fixed with non-permeabilizing 4% PFA in water for 10 min at 4°C. Fluorescence quenching was performed by treatment with 0.4% Trypan blue for 5 min. All samples were washed with PBS prior to mounting. The coverslips were mounted on slides with Fluoromount (Sigma-Aldrich #F4680) before being imaged by a Nikon Eclipse Ti microscope with a 20× objective.
4.13 Peptide interaction with cells by flow cytometry
FITC-hybrid7 was solubilized in treatment media (supplemented with 10 mM sodium bicarbonate pH 7.4) to a final concentration of 1 μM. 300,000 HSC3 cells per sample were treated with peptides, washed, and fixed, as described above. After washing with PBS (pH 7.4), the samples were blocked with 1% BSA in PBS for 10 min at 4°C. The cells were labeled with 100 μL of anti-FITC-Alexa647 antibody (RRID:AB_2339044) in 1% BSA PBS (pH 7.4; final concentration of ~0.5 μg/mL) for 1 h at 4°C. After washing in PBS, samples without Trypan blue quenching were resuspended in PBS for analysis. Trypan blue samples were successively treated with 0.4% Trypan blue for 5 min before analysis. The cells were analyzed using a BDFacs Canto II flow cytometer equipped with a 488 nm argon laser with a 530/30 bandpass filter, and a 633 nm HeNe laser with a 660/20 bandpass filter. A minimum of 3000 events were counted for each experimental condition. The data were analyzed using FACSDiva. The fluorescence data are expressed as mean arbitrary fluorescence units and were gated to include all intact cells.
4.14 Lactate dehydrogenase release assay
HSC3 cells were seeded in 24-well plates at 200,000 cells/well and incubated overnight at 37°C. The cells were then treated with 10 μM hybrid7 previously described for immunoblot analyses. The amount of lactate dehydrogenase (LDH) release was analyzed using the cyQUANT LDH Cytotoxicity assay (ThermoFisher, cat#C20301), as previously described (Gerhart et al., 2018). LDH release was normalized to cells treated with media only at pH 7.4 (spontaneous release) and cells lysed with the provided lysis buffer after treatment with media only at pH 7.4 (maximum release).
4.15 Cell viability assay
HSC3 cells were seeded in 96-well plates at 5000 cells/well and incubated overnight at 37°C. The cells were treated with hybrid7 and hybrid7-control, prepared as previously described for immunoblot analyses. Following the treatment, the cells were washed once and then recovered in 100 μL of complete DMEM media for 72 h at 37°C. Cell viability was assessed via MTT assay, as previously reported (Bloch et al., 2019). Cell viability was normalized to cells treated with media only at pH 7.4 (100% viability). Data were fitted to a sigmoidal dose–response curve and EC50 values were determined using Prism 9 (GraphPad).
AUTHOR CONTRIBUTIONS
Sophie Rizzo: Investigation; methodology; validation; formal analysis; writing—review & editing; writing—original draft; data curation; visualization. Eden Sikorski: Investigation; writing—original draft; formal analysis; data curation; visualization; validation; methodology. Soohyung Park: Writing—review & editing; formal analysis; methodology; data curation; visualization; validation; investigation; software; funding acquisition. Wonpil Im: Writing—review & editing; formal analysis; software; methodology; validation; resources; supervision. Victor Vasquez-Montes: Data curation; methodology; formal analysis; validation; visualization; investigation. Alexey S. Ladokhin: Resources; supervision; methodology; writing—review & editing; formal analysis; validation; funding acquisition. Damien Thévenin: Conceptualization; funding acquisition; writing—review & editing; visualization; validation; methodology; formal analysis; project administration; resources; supervision.
ACKNOWLEDGMENTS
This work was supported by the National Institute of General Medical Sciences: R01GM139998 to Damien Thévenin and R01GM126778 to Alexey S. Ladokhin, and National Science Foundation MCB-2111728 to Wonpil Im.