Knitting and untying the protein network: Modulation of protein ensembles as a therapeutic strategy
Abstract
Proteins constitute the working machinery and structural support of all organisms. In performing a given function, they must adopt highly specific structures that can change with their level of activity, often through the direct or indirect action of other proteins. Indeed, proteins typically function within an ensemble, rather than individually. Hence, they must be sufficiently flexible to interact with each other and execute diverse tasks. The discovery that errors within these groups can ultimately cause disease has led to a paradigm shift in drug discovery, from an emphasis on single protein targets to a holistic approach whereby entire ensembles are targeted.
Protein Networks
Proteins are the main players within the cells. They execute myriad functions, such as structural support, cell-cycle control, enzymatic activity, cell signaling and immune response. The functional diversity characteristic to so many proteins depends on their ability to bind other molecules such as other proteins, other biomolecules, small ligands or simple ions.
Within the cell, protein–protein interactions are organized into an exquisite, highly complex network known as the interactome,1 in which proteins can be depicted as nodes, and their interactions, as edges (see Fig. 1). Essential proteins are more connected (hubs) than non-essential ones; hence, they constitute the structural basis of the network. This phenomenon has been recently named as the centrality–lethality rule, because deletion of a hub protein is likely to be lethal for the organism.2, 3 Nonetheless, deletion of non-hub proteins can also prove fatal.
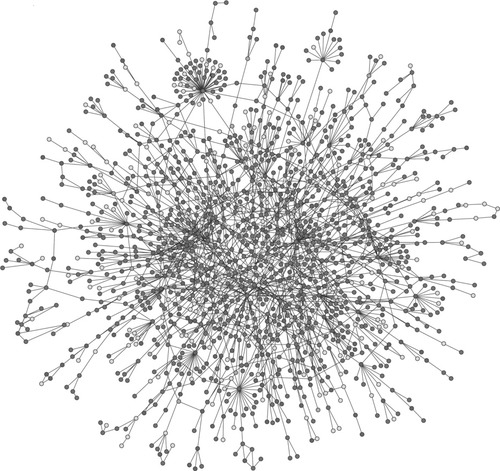
Yeast protein interaction network map. (Reprinted by permission from Macmillan Publishers Ltd. Science, 411: 41–42, © 2001).
There are only a few essential proteins with tens, hundreds or thousands of links. These levels of hub connectivity are somehow reflected in the protein structure, since protein promiscuity and diversity can be only achieved by a certain intrinsic structural flexibility.4-8
The scale-free connectivity network can be dissected into functional sub-networks which represent unique signaling pathways: ensembles of distinct proteins that act in concert to transduce information. Many interactions within the network undergo temporal and spatial dynamic regulation, which depends on signal-induced or context-dependent post-translational modifications. The transient nature of the interactions that lead to post-translational modifications makes them difficult to detect; hence, they have been described as soft wires.9 In contrast, physical interactions that are more robust, and therefore, are more readily identified, have been dubbed hard wires. However, the hardness of a wire is not related to the importance of the interaction. Furthermore, each task has an optimal affinity, and evolution does not always lead to tighter interactions.
Homo-oligomeric proteins are particularly relevant in the protein network. In fact, it is estimated that over 35% of proteins must self-assemble to function.10 The majority are dimers and tetramers11; higher order oligomers are less common (except in the case of some structural proteins), and odd-number stoichiometries are also rare. These defined oligomeric structures are characterized by their high degree of symmetry.10 Many functional advantages have favored oligomeric states throughout evolution; self-assembly confers an additional level of control and regulatory flexibility, as well as structural stability and resistance to degradation, denaturation and mutations, because the shorter the sequence the lower the chance of transcription errors. Nevertheless, oligomerization may also be disadvantageous and is not always the evolutionary direction.
Protein–Protein Interactions
Protein–protein interactions have garnered ever increasing attention in the past few years. This is due to their central role in the emerging field of systems biology, which studies biological systems as the association of complex interaction networks, thereby introducing a new perspective in understanding life processes: integration instead of reduction.
How proteins interact
Regardless of the biological importance of an interaction between proteins, they interact with high specificity under a wide range of affinities, from the high millimolar to the low femtomolar.12
Protein–protein complexes can be characterized according to three parameters13: (i) the nature of the units: homo-oligomeric (same) or hetero-oligomeric (different); (ii) the life-time of the complex: permanent (very stable; only exists the complex form) or transient (associates and dissociates in vivo, whether in dynamic equilibrium or under the influence of a molecule which triggers an equilibrium shift); and (iii) the existence of the units: obligate (partners do not exist independently, and dissociation leads to denaturation; therefore, they are permanent) or non-obligate (partners exist independently). Many interactions do not fall into any distinct type, and a continuous exists between obligate-non-obligate and/or permanent-transient states.14
The stability of a complex very much depends on its environment. Indeed, all interactions are ultimately driven by the concentration of the components and by the free energy change resulting from formation of the complex. Thus, protein–protein interactions can be controlled by altering the local concentration (gene expression, protein degradation, temporary storage or co-localization in time and space) and by influencing the binding affinity (local change in physiological conditions or physicochemical modifications of the interface).
At the interface
A key challenge in the study of protein–protein interfaces is their diversity, which is a direct consequence of the variety of biological roles resulting from protein–protein interactions.15 Every protein complex has adopted different solutions to fulfill the binding requirements for specificity and affinity. Consequently, no universal statements can be made about the composition and architecture of protein–protein binding sites.
Specificity derives from the complementarity of physical shape and chemistry of the binding interfaces, although structural hindrances of the protein as a whole may also condition the interaction.
Protein–protein interfaces range in area from 600Å2 to >4000 Å2; the average is about 1500Å2,16 but the interface area clearly depends on the dimensions of the protein and on the nature of the complex.15, 17
The size and the shape of the interface do not directly correlate with its binding energy.14, 18 Interfaces are not homogeneous and their stability usually depends on only a few, crucial residues known as hotspots, which contribute the most to the binding energy, whether packed together or dispersed over the surface.17, 19 The surrounding residues protect the hotspots from the bulk solvent; this is required to generate a local environment of lower dielectric constant and thereby facilitate tight interactions. Paradoxically, water molecules also form part of many protein-protein interfaces, fitting into small cavities of the surface and establishing hydrogen bonds between the two sides.20-23
Generally, “interfaces are more hydrophobic than protein exteriors but more polar than protein interiors.”24 The residue composition of interface regions does not differ greatly from that of protein surfaces, although aromatic residues and arginine appear somewhat more frequently.25 Actually, the most frequent hotspots are tryptophan, arginine and tyrosine—all of which can establish multiple types of favorable interactions, whereas others such as valine, lysine or serine rarely serve as hotspots.15, 26 The hydrophobic effect frequently has a relevant role in the interaction between protein surfaces, although, in contrast to the case of protein cores, hydrogen bonds, salt bridges and water molecules are also highly implicated.27, 28 Carbonyl groups from the backbone—which comprise nearly 10% of the total interface area—also contribute to stabilization.29
It is worth noting that the nature of the complex conditions the nature of the interface.12, 15, 17, 18 Thus, for instance, many permanent and obligate protein complexes present large interfaces of high hydrophobicity whose interaction results in tighter binding, whereas the interfaces of proteins interacting only transiently display a greater abundance of polar contact.17, 18, 30 This is totally coherent, since in transient complexes proteins exist as free entities, and therefore, most of time their interfaces are solvent exposed. However, due to the floppiness of proteins, there may be exceptions to this trend.31
Structural rearrangements of the unbound proteins are part of the binding process. They range from minor movements of the side chains to major reorganizations in the backbone, such as loops switches, domain conformational changes or even folding of a disordered sequence.6-8 These rearrangements can be induced by the binding event itself or promoted by post-translational modifications or by environmental changes. The energetic costs associated with the structural change—for instance, a disorder-to-order transition of an unstructured region would cause a large decrease in conformational entropy—uncouples binding strength from specificity and causes highly specific interactions to become reversible. Whatever the trigger and the energetic cost, protein conformation also is a selection mechanism for protein–protein interactions and hence, for activity.
Dynamics
Regardless of the high specificity, each protein–protein interaction (hence, each biological response) has its optimal affinity (hence, an optimal duration: permanent or transient complexes, namely, hard wires or soft wires in the network). In fact, biological responses depend on the kinetics of the encounter between the interacting partners.32 For instance, a specific and rapid association of the protein complex is crucial in biological processes such as signal transductions, redox reactions or the regulation of enzymatic activities.
Protein partners must diffuse through the media and come across in the right orientation. The rate of association of a protein complex is usually limited by diffusion and geometric constraints of the binding sites (i.e. diffusion control). For these cases, typical association rates are in the range of 105–106 M−1s−1.33 The reaction may be further slowed by subsequent chemical processes (i.e. reaction control), such as large conformational rearrangement. In contrast, faster rates (>109 M−1s−1) have been measured for interactions for which the speed of the process is of functional relevance. In these cases, the rate of association is enhanced by strong, favorable, long-range electrostatic forces.34-37
Holes and Knots in the Network: When Something Goes Wrong
The protein network must be perfectly balanced for the organism to operate normally. This in turn demands that the conformation, concentration, localization and timing of interacting proteins must be orchestrated flawlessly. Should any of these conditions not be satisfied, the system would fail, and disease would eventually set in.38-41
There are many factors at the molecular level which can alter the harmony of the protein network. Disturbance may imply the loss-of-function of a protein or a pathway (a hole in the net), the gain of an undesired one (a knot in the net) or both phenomena simultaneously. Clearly, the more central the perturbed hub, the worse the consequences.3
Protein networks can suffer from exogenous or endogenous perturbations. In the former, a pathogenic external factor (e.g. bacteria or viruses) directly interferes in the protein-protein connections, cutting established wires and establishing new ones; whereas in the latter, the problem lies in malfunctions of the organism itself, usually at the genomic level.
A point change in the nucleotide sequence of a gene (i.e. a mutation) can have catastrophic consequences for the organism; indeed, many diseases are associated with dysfunctional mutated proteins. Substitutions, deletions or truncations in the sequence of a protein can abolish or diminish (loss-of-function), or even enhance (gain-of-function), pre-existing protein–protein interactions. The mutation can directly alter the binding interface or the structure of the binding domain, but can also affect other distant residues which, despite not interacting physically with the partner proteins, play a key role in controlling modifications required for the binding, expression or localization of the protein. Besides affecting pre-existing connections, mutations can also promote new undesired interactions with other, new partners (i.e. gain-of-function), which typically has negative implications for the organism.
There are other endogenous mechanisms which can impair network traffic. This is the case of the well-known, and regrettably frequent, misfolding disorders.42, 43 Whether or not due to a mutation, an error in the folding process of the nascent polypeptide chain also results in loss-of-function of the corresponding protein, and therefore, generation of a hole in the network. Mutations are a common cause of misfolding, but other environmental factors such as oxidative stress, pH changes, osmotic shock, as well as many others that remain poorly understood, can also compromise the structure of a protein. Moreover, accumulation and deposition of misfolded proteins in the organism can frequently result in the formation of highly ordered aggregates, namely amyloid fibrils and plaques, which can prove fatal to an organism.44
Protein misfolding disorders
Protein expression is carried out by the ribosomes. Many newly synthesized polypeptides are directly released to the cytosol, but many others—mainly transmembrane and secreted proteins—are selectively captured by the endoplasmic reticulum during or after their synthesis, where they find the optimal environment for folding and post-translational modifications. The folding processes are thoroughly supervised by a complex quality control system that is mainly ruled by endogenous molecular chaperones, which assist in protein folding and prevent accumulation of defective proteins.45 In fact, the biosynthesis of chaperones is controlled by a feedback system that responds to any increase in misfolded proteins.
A point mutation in the protein sequence or the perturbation of the folding process by any environmental trigger (e.g. protein overexpression, oxidative stress or defects in the chaperones pathways) can lead to a misfolded protein, which is then polyubiquitylated by the quality control system and destroyed by the proteasome.46, 47 Consequently, the protein and its function are lost. Diseases caused by impaired intracellular trafficking of prematurely degraded misfolded polypeptides48 include cystic fibrosis, familial hypercholesterolemia, retinitis pigmentosa (inherited blindness), nephrogenic diabetes insipidus, Fabry's disease, and others.49
Furthermore the clearance mechanism of unfolded species can fail, causing the species to accumulate and ultimately aggregate into cytotoxic macro-structures, whether highly-organized or amorphous. This chain of events causes diseases such as α-antitrypsin deficiency and early onset cataracts.
Neurodegenerative disorders like Alzheimer's, Parkinson's or prion diseases are also caused by accumulation and aggregation of misfolded proteins, although in these maladies, the native protein is converted into structured amyloid fibrillar aggregates, either intra- or extra-cellularly.50, 51 Rather than a problem in the protein folding process, the accumulation in these cases results from unbalanced kinetics of the protein misfolding and aggregation and the clearance of aberrant species.52
Protein misfolding pathologies can also lead to connective tissue diseases, which often result from incorporation of toxic or imperfect conformations into a functional protein structure. Besides environmental factors, genetic mutations may also affect collagen and keratin resulting in defective constructions.53, 54 Inherited connective tissue diseases include Marfan syndrome, osteogenesis imperfecta and pediatric rheuma.
Untying Knots and Knitting Tears: Strategies to Mend and Control the Protein Network
Understanding the molecular basis of a disease, namely, where and how the protein network fails, is crucial for developing a therapeutic strategy. Thinking in network terms expands the possibilities of drug discovery, because restoration or blockage of a pathway does not always require acting on the target node; better results may be achieved by focusing on the partners of the target protein.39, 55, 56
Targeting protein–protein interfaces is not trivial and designing drugs able to selectively recognize a protein surface implies many challenges. These surfaces are known for lacking any distinct recognition motif and for being diverse (i.e. each is unique), shielded by solvent molecules and ions, and generally large and/or non-continuous. Moreover, conformational flexibility of the protein in the unbound state and post-translational modifications further complicate targeting.57 In addition, biophysical techniques are usually employed to characterize the interaction, although these assays are typically performed in artificial environments that scarcely represent physiological conditions. Indeed, the hurdles of targeting protein surfaces are the reason that protein–protein interactions have remained under-explored and under-exploited as drug targets for so long. It was not until the beginning of the genomic–proteomic–interactomic era that protein–protein interactions became an attractive target for drug discovery.
Conceptually, it is much easier to develop inhibitors of a protein–protein interaction than agents for enhancing or restoring the activity or stability of a system. This is because stimulation requires not only binding but also accurate mimicry of the interaction that triggers a response, whereas inhibition can be accomplished in a less exact manner via any strategy that effectively prevents the binding of any of the partners. This premise is reflected in the wealth of literature on inhibitors of protein–protein interactions58-62 and the relative scarcity of articles on the stabilization of protein complexes.63-65
Inhibiting protein–protein interactions
Many strategies for designing inhibitors of protein–protein interfaces have been described to date. The challenge can be approached from a biological perspective, and there have been reports of success through the use of artificial antibodies (i.e. immunotherapy),66 miniature proteins,67, 68 functional oligonucleotides,69 and high throughput screening of phage display libraries.70, 71
For chemists, the rising interest in targeting protein interfaces has translated into a growing number of novel strategies for the design of drug-like compounds for surface recognition, in addition to the already well-known high throughput screening of synthetic62 or natural compound libraries, as well as computational assisted methods.72, 73 Noteworthy approaches include the use of peptidomimetics (peptides modified with non-natural amino acids, non-natural backbones or other synthetic properties)74, 75 and proteomimetics (designed small synthetic constructions)76-78 to mimic the structural and functional features of a target protein.
The design of low molecular weight drug-like molecules able to selectively and efficiently recognize a large, featureless protein surface is sometimes unattainable. These limitations have inspired novel rational strategies in which larger scaffolds are employed to attach multiple anchoring groups that are specially selected to interact with unique features of the protein surface. The area covered by these potential ligands is large, and even if the protein–protein interface does not present any relevant anchoring point, other residues out of the boundary can also be used. In addition to the anchoring groups, the scaffold itself can be also functionalized to achieve tighter binding. The concept of multivalency79 is applied to the design of these ligand molecules: a tighter interaction can be obtained from the simultaneous contribution of multiple, low to moderate affinity interactions. In addition to increasing the thermodynamic and kinetic stability of the complex, multiple contributions also lead to higher specificity and easier induction of conformational changes.
This multivalent approach has been successfully applied in the surface recognition challenge, usually, by taking advantage of the symmetry of the target molecule (see Fig. 2). Branching chelators are selected on the basis of the chemical characteristics of the target residues on the surface. The scaffold, whether natural or synthetic, can also actively participate in the binding or simply remain as a rigid support whose entropic penalty is low.
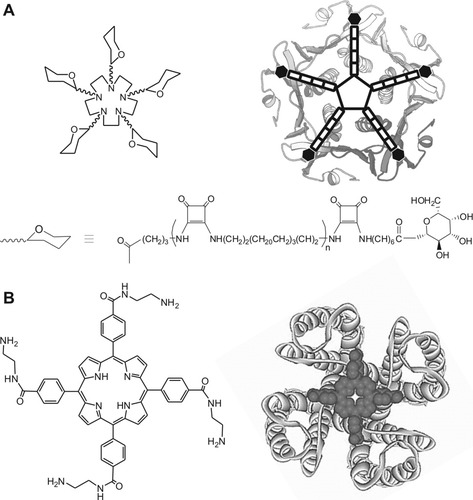
A: Pentavalent saccharide-inhibitor for the receptor binding site of cholera toxin B-pentamer; at the right, a schematic representation of the ligand binding mode (Ref.80). B: Porphyrin-based tetravalent ligand for blocking human Kv1.3 potassium channel; at the bottom, the complex protein-ligand (Reproduced from Ref.81, with permission from the American Chemical Society, USA, © 2003).
A small selection of reported multivalent ligands for surface recognition is shown in Figures 2 and 3; many other scaffolds, both smaller and larger, have also been described. Despite their huge size—far from that of the classic drug-like molecule—these ligands have proven successful in their inhibitory tasks in vitro and in vivo, thus opening new doors in rational drug design.
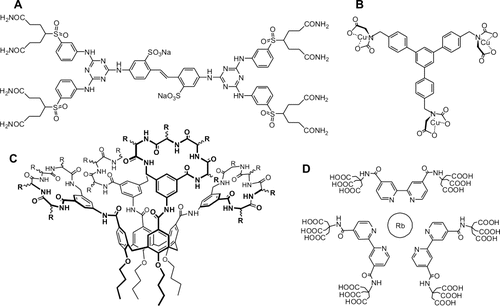
A: Bistrazine compound that inhibits the tetrameric assembly of RS virus fusion protein.82 B: Cu2+-IDA-containing trivalent ligand that targets the imidazole side-chain of three histidines at the surface of carbonic anhydrase.83 C: Calixarene-based ligand functionalized with multiple recognition elements designed for disrupting the interaction of the homodimer PDGF with its receptor.84 D: [Rb(bpy)3] complex for positively charged cytochrome c surface recognition.85
Stabilizing protein–protein interactions
Stabilization of protein–protein interactions holds potential as a therapeutic strategy, yet it has garnered little attention. Block et al. recently claimed that stabilization could prove even more effective than inhibition.64 They found that a number of therapeutically interesting targets form a “druggable” cavity at the boundaries of the protein complex, in which fitting of a small molecule should be thermodynamically favorable. In fact, some drugs currently on the market (e.g. rapamycin, tacrolimus, brefeldin A, taxol, forskolin) act by stabilizing pre-existing protein–protein assemblies.
From the design perspective, almost all the work has been focused on cell surface receptors which need to oligomerize for signal transduction.86 Several dimerizers, composed of two anchoring groups linked by an appropriate spacer,63 have been reported to date, but they are only used in laboratory practices.87
Stabilizing obligate oligomers can be also of great importance. In this context, it is worth mentioning the small stabilizers of the transthyretin native tetramer described by Kelly and co-workers88, which by stabilizing, act as inhibitors of the formation of amyloid fibril of the transthyretin (see next section). Our group has also recently reported calixarene molecules that rescue and stabilize the functional tetrameric state of certain mutants of protein p53.89
Dealing with misfolding disorders
Misfolding can result from various factors and can lead to diverse pathologies; hence, it can be treated at different levels. Protein surface recognition is still a requisite for most of the approaches indicated for misfolding or aggregation disorders.
One possibility is to work at the genetic level. If a misfolded mutated protein is prematurely degraded, then the wild type gene can be introduced into the cell by transfection (i.e. gene therapy). Conversely, if the problem is that a misfolded species is not conveniently removed, then improvements can be made by up-regulating endogenous molecular chaperones or aggregate clearance mechanisms.90
At the protein level, aggregation can also be inhibited by molecules which bind to the growing aberrant amyloid structures to ultimately inhibit self-assembly.91 Another option is the use of kinetic native-state stabilizers, introduced by Kelly and coworkers,52, 88 which are drug-like molecules designed to specifically and efficiently interact with the native state of the protein to stabilize it and prevent its misfolding, thereby precluding toxic aggregation.
Misfolded nascent mutated polypeptides can also be saved through the use of exogenous chaperones: specific or non-specific molecules that aid proteins to fold correctly and consequently, to evade quality control and be shuttled to their functional location.49, 92 Artificial chaperones can be classified according to their specificity. Chemical chaperones are low molecular weight chemicals (e.g. glycerol, deuterated water, DMSO, 4-phenylbutiric acid) that act as osmolytes; they drive the folding of the defective protein towards the native state by increasing the hydrostatic pressure (and through other not yet well understood mechanisms).93, 94 Despite their efficacy, the clinical application of such unspecific molecules is rather unlikely.
More promising are the so-called pharmacological chaperones or pharmacoperones (see Fig. 4),48, 95 small template molecules that specifically bind the protein in its native conformation, thereby shifting the folding equilibrium towards the correct structure. Most of the reported examples correspond to the rescue of misfolded mutated enzymes by—ironically—inhibitors. This approach has become very promising for genetic diseases, especially for those cases in which structural instability of a protein is caused by a mutation.96 Of course, not all genetic mutations are treatable, only some of those that affect protein folding or stability, but not those that affect protein activity or integrity.
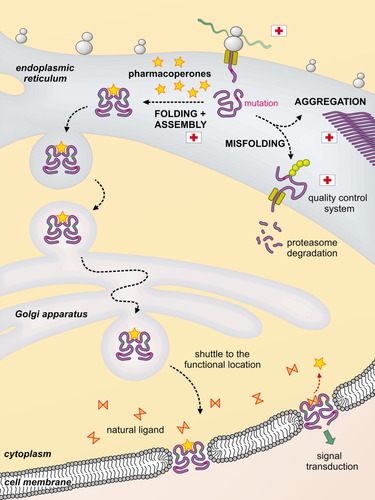
Pharmacoperones in action: a misfolded protein imported to the ER can be rapidly removed by the quality control system, in order to avoid it being crushed into toxic aggregates. An exogenous chaperone (the pharmacoperone) can act as a template and aid the protein to fold correctly. Once properly folded, the protein can bypass the quality control system and travel to its final location, where it can perform its function. In contrast to endogenous molecular chaperones, pharmacological chaperones remain associated once the protein has been folded. In the example illustrated here, a mutated receptor is rescued by an inhibitor (the pharmacoperone) which is then displaced by the endogenous natural ligand.
The Case of p53
The tumor suppressor protein p53 perfectly illustrates the relevance of the protein network, the drastic consequences which result from its perturbation, and the strategies which can be followed to restore it.
p53 is intimately linked to cancer; nearly 60% of human tumors present alterations in the p53 pathway. This proportion, which far exceeds that for other proteins, is testament to the central role of p53 and its pathways (a huge hub in the network) in the regulation and growth of the organism. Hence, p53 is a clear example of the centrality–lethality rule.3
p53 is a transcription factor of genes which control the cell cycle and preserve the genomic integrity of the organism.97, 98 It can be activated by numerous factors, primarily, DNA damage and other situations of cellular stress, and can respond by triggering a variety of events, including cell-cycle arrest, DNA repair and apoptosis [Fig. 5(A)].100, 101 Because it plays a key role in life, p53 is one of the most—if not the most—extensively studied proteins and is the target protein par excellence in cancer therapy.
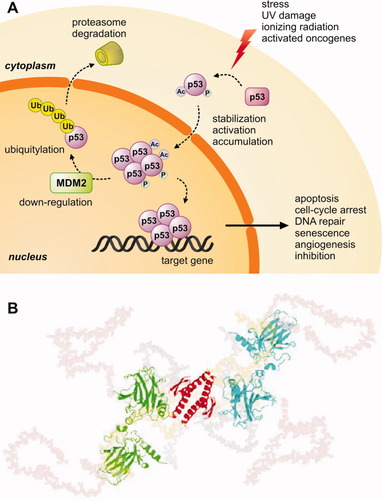
Protein p53. A: Simplified scheme of the p53 pathway. B: SAXS models of free p53; the structured core DNA binding domain (green and blue) and tetramerization domain (red) are displayed in cartoon representation, and unstructured connector linkers (grey), N-termini (salmon) and C-termini (yellow) in semitransparent space-fill mode (Reproduced from Ref.99, © 2007 National Academy of Science, USA).
From a structural point of view, the active form of p53 is a homotetramer constituted by the self-assembly of four monomers of 393 residues each. Five main domains can be distinguished in each monomer102: the N-terminal acidic transactivation domain (residues 1–42), the Pro-rich domain (residues 61–94), the DNA binding domain (residues 102–292), the tetramerization domain (residues 326–357) and the C-terminal regulatory domain (residues 360–393). By far, the most important is the large, central DNA binding domain, which is actually responsible for the transcriptional function of the protein. Nevertheless, the rest of domains should not be overlooked, since they are all essential for the correct function of the protein (e.g. functional tetramer assembly, regulatory post-translational modifications, binding to multitude of partners).
In line with its central position within the cellular network, p53 presents several regions of intrinsic disorder [Fig. 5(B)].103, 104 These are mainly the N- and C-terminal regulatory domains,99 although the folded DNA binding domain is also somewhat structurally unstable,105 which confers the promiscuity required for binding various partners such as DNA and proteins—most of which have yet to be identified.106 The activity of the protein is regulated by numerous intricate mechanisms which control expression levels, degradation rate, localization, structure, post-translational modifications or interactions with biomolecules.
The fine balance of the p53 pathway can be altered by many factors. The most common impairment is the direct mutation of the p53 gene, which in more than 95% of cases affects the prominent DNA binding region.107, 108 Single missense mutations can compromise the structure of the protein or modify key residues either directly involved in the interaction with other biomolecules or required for post-translational modifications. Besides loss-of-function, mutations can also result in a protein with undesired oncogenic activity by promoting the transcription of oncogenic genes.
In about 10% of p53-associated tumor cases, the pathway fails due to alterations in direct or indirect partners of p53, the most relevant of which is the protein MDM2.109 Interactions with viral proteins (e.g. human papilloma virus E6 protein, HIV1 Tat protein) have also been reported to neutralize p53 activity and establish conditions that favor cancer.110, 111
Reconstruction of the p53 tumor suppressor pathway has become one of the most exciting concepts in cancer therapy; hence, a growing number of p53-targetting strategies have been proposed in the past few years.107, 112, 113 For cases in which the wild-type p53 gene is present (ca. 10%), enhancement of protein activity is usually sought. The main target for this aim is the p53–MDM2 interaction, although it is not the only target, because p53 interacts with a wide spectrum of other proteins. MDM2 down-regulates p53 by direct binding; hence, inhibition of their interaction results in accumulation of active p53. Actually, the inhibition of p53-MDM2 interaction has been proposed as the model of study for the design of inhibitory strategies for protein–protein interactions, and many reviews have been written on the matter.114-117 Given that in the unbound state the p53 binding sequence (at the N-terminus) is not structured, most efforts have been focused on the MDM2 surface. The search for inhibitors of the p53-binding site on MDM2 has employed almost every current strategy: antibodies, oligonucleotides, α-peptides, β-peptides, retro-enantio-peptides, peptidomimetics, peptoids, mini proteins, small molecules from natural product or synthetic libraries, in silico screening, etc. The reader is referred to a complete review recently published by Murray and Gellman which describes almost every inhibitor reported for the p53-MDM2 interaction.116
For cases in which the p53 gene is mutated (ca. 90%), efforts have been made towards the pharmacological rescue of the non-functional protein through use of small binding molecules. Several molecular chaperones have recently been reported to recover the folded structure and the activity of some inactive unfolded mutants of the DNA-binding domain.118, 119
Given that mutations mainly affect the DNA-binding domain, little attention has been paid to other regions, despite the fact that mutations in other domains are also found in human cancers. For instance, regarding the tetramerization domain, our group recently reported a designed multifunctional calix[4]arene ligand which can stabilize the tetrameric assembly of mutated tetramerization domains.89
Of course, in the case of mutations affecting key residues for the direct function, or hotspots implied in protein–biomolecule interactions, regardless of whether they enable correct folding, functionality cannot be rescued through pharmacological chaperones. For these cases and many others, restoration of p53 activity has been attempted via genetic therapy, using suitable viral vectors to introduce intact or improved p53 genes into the tumor tissue.120, 121 Success has been reported for some clinical trials; however, this strategy is still too limited for immediate practical application.
Epilogue
The breakthrough concept that proteins function as a contact network rather than as independent individuals is not only one of the most important advances in our comprehension of living systems, but also translates to a new era in drug discovery. The few reported examples of diseases caused by “impolite” protein social behavior certainly represent only the tip of the iceberg. Therapeutic intervention through molecules designed to selectively modulate the strength and specificity of protein–protein interactions is becoming a reality. This will not only feature molecules with inhibitory capacity: equally or even more interesting are those compounds which can rescue pre-established interactions or structures whose loss results in disease.
Acknowledgements
The authors are indebted to Sílvia Pujals, Nessim Kichik, Dr. Teresa Tarragó, and the reviewers for their critical reading and helpful feedback.




