Lessons from a quarter century of being human in protein science
Funding information: Directorate for Education and Human Resources, Grant/Award Number: iUSE-1947102
Abstract
Over the past quarter century, my engagement with the protein society has allowed me to witness first-hand the evolution of our deepening understanding of the complexity of protein folding landscapes. During my own evolution as a protein scientist, my passion for protein folding has deepened into an obsession with mapping and decoding the thermodynamic and kinetic secrets of protein landscapes—especially those of rebel proteins, whose “nontraditional” behavior has challenged our paradigms and inspired the expansion of our models and methods. It is perhaps not surprising that I see parallels in the evolution of the landscape framework and in the development of our own trajectories as humans in Science, Technology, Engineering and Mathematics (STEM). Just as with proteins, however, we need to recognize that our individual human landscapes are not isolated from our local departmental and institutional communities, and are integrated into the larger networks of our STEM disciplines, academia, industry, and/or government, not to mention society. My experience with hundreds of participants in the Being Human in STEM (HSTEM) initiative that Amherst College undergraduates and I co-founded in 2016 has helped me find hope for STEM and humanity. If we commit to reconciling our identities as scientists with our responsibilities as human beings, together we can accelerate the evolution of individual, community, and societal landscapes to contribute to addressing the dire challenges facing our planet.
1 LANDSCAPE EXPLORATION AS A DEVELOPING PROTEIN SCIENTIST
Following Anfinsen's experiments with RNase A,1 the default for protein folding was accepted as the spontaneous and deterministic pathway from an unfolded amino acid chain to the specifically folded three-dimensional protein structure designed for its particular function. When I first began to learn about protein folding, theorists were conceptualizing the downhill nature of protein folding toward lower energy as concomitant with narrowing of the configurational space explored by the folding chain until the protein attains its final thermodynamically stable native state.2-4 Similarly, the narrative presented in the recitation of CVs that we typically provide as an introduction to our seminar speakers lends itself to this perception of a deterministic set of educational and career steps that naturally follow on each other, leading, inevitably, to the speaker's current position, and status (Figure 1). Using my own journey in STEM as a case study, it is very easy to curate snapshots from my development as a scientist that align with the narrowing of exploration and gaining of credentials, recognition, and expertise that conclude with my current image as a confident science researcher and faculty member, fulfilling my natural destiny.

I was operating with a smooth funnel assumption for the progress of my own life toward earning a Ph.D. and eventual faculty position when I began graduate school. Conversely, I was immediately fascinated by alpha-lytic protease (αLP), which possessed a very nontraditional landscape and most definitely was not sliding down a smooth funnel toward a functional native state. Anfinsen's classic approach of challenging a solution of purified protein with denaturant, then removing the denaturant, led αLP to refold to an inactive partially folded intermediate.5 It turned out that αLP had evolved to fold to the active state only as a precursor with an N-terminal piece called the pro-region. Once folded, αLP cleaved the bond connecting the pro-region to the mature protease, leaving the pro-region, unstable on its own, to be digested.6 Sorting out how the thermodynamics and kinetics of αLP's distinct landscapes enabled folding in one context, and function in another, was the puzzle my Ph.D. would help unravel. Now that I am a parent of a college student, the idea of a pro-region (parents) facilitating the initial exploration of a protein's (child's) landscape to ensure attainment of the properly correctly folded structure capable of (in theory) functioning on its own has taken on a whole new meaning!
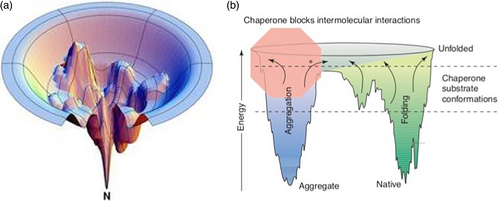
I was fortunate that I began my career in protein folding in the 1990's at the University of California at San Francisco (UCSF), where I got to mix with students and postdocs from the computational theory research groups of Ken Dill, Tack Kuntz, and Peter Kollman. I remember discussing αLP's folding oddities with Hue Sun Chan and others from Ken's group, and whether there was any way that their HP model7 could predict this. Like many experimentalists, my imagination was captured by the Dill group's iconic bumpy funnel image8 of the protein folding (Figure 2a). Folding landscapes for proteins may include multiple pathways, deep wells, and many barriers that can delay—temporarily or permanently, the attainment of the lowest energy state during folding (or refolding). This bumpy protein landscape mirrors the complexities, challenges, ambiguous choice points, and backtracking that almost every human encounters as they move through life, whether or not in development as a scientist.
Indeed, in graduate school, I failed my oral qualifying exam despite my love of protein folding, my extensive preparation, and the accumulation of aced exams since childhood. It was Ken who opened the line of questioning about thermodynamic concepts integral to the approach that I was proposing, that I could not answer. After I snuck down a side staircase and fled home, I slid into a deep kinetic trap of humiliation, imposter syndrome, and depression, where I remained for weeks.
When we do not routinely and openly acknowledge such failures and setbacks as part and parcel of our own journeys in STEM,9 it is no wonder that students and postdocs, especially those who hold identities not well-represented in the ranks of “successful” scientists, pathologize their experiences of hitting barriers and becoming stuck in nonfunctional kinetic traps as their own individual failings. It is tempting to focus on the snapshot of a final functional and stable human, just as it is tempting to reduce the complexity of a protein to its easily digestible cartoon representation. But in both cases, this shorthand obscures how the dynamics of exploration, discovery and failure are essential, inescapable, and persist throughout a lifetime. In the case of humans, those dynamics provide the most important lessons that enable us to stop chasing external validation by hopping onto whatever ladders we see others climbing, to taking agency for shaping our own landscapes and setting our own destinations because they have meaning to us.
As with life, in science reassessment is a continuing process, and around this time, other developments in the field led researchers to question whether traditional folding experiments were sufficient to truly decipher the protein folding mystery. How reliable were the secrets extracted from an isolated protein solution forced to independently reenact its folding in a test tube in response to inducing and then withdrawing harsh conditions? Cell biologists and geneticists like Carol Gross, with whom I rotated at UCSF, were piecing together why in vivo, many proteins required assistance in reaching their native states from other proteins, such as chaperones and chaperonins, while other proteins, that is, proteases and proteasomes, cleared out the debris of proteins who were hopeless at folding. In various labs, including those of Jonathan Weissman and Stanley Prusiner on other floors at UCSF, researchers were demonstrating the importance of studying protein aggregation and not just successful folding. Proteins with no structural homology were shown to end up in highly stable and nonfunctional structures ordered amyloid fibrils, including even the most well-behaved spontaneously folding model proteins not associated with any disease. Dobson and colleagues hypothesized that the propensity to aggregate is universally encoded into the amino acid sequences of all proteins.10
The inherent tension between functional folding and aggregation was eventually summarized beautifully in a double funnel model of protein folding landscapes by Patricia Clark, Figure 2b.11 The functional funnel leading to the natively folded conformation, which our experimental focus on spontaneously folding proteins had biased us toward imagining was all that mattered, actually intersects with an aggregation funnel. Alternative fates for the amino acid chain in the dysfunctional side of the double funnel could be even more stable than the functionally folded protein. Depending on the specific protein, and the cellular conditions, the tendency for an amino acid chain to explore its aggregation funnel varied. The fact that it is inherent to the nature of a polypeptide chain to encode aggregation as well as function, had been dealt with in cells through the coevolution of proteins alongside the cellular chaperone and proteolysis machinery.
Developing as a protein scientist while the field evolved to better understand and integrate these new ideas into our models and approaches has helped me learn from analogies between the human and protein landscapes. As an early graduate student teetering between my own functional and aggregation funnels following the destabilizing orals experience, I was simply living out what it means to be human. Like proteins, function and dysfunction, strength and vulnerability are equally encoded into our beings. Like proteins, we have evolved to be interdependent with our local community and the larger ecosystem of society.
Had I undertaken graduate school with the isolated protein in a test-tube mindset, believing that success relied solely on my inner brilliance, grit, and confidence, it is very likely that after failing my orals, I would have continued my slide toward dead-end aggregation, giving up on science, and probably my belief in myself. Instead, I asked for help and activated my robust homeostasis network—some components of which had simply coevolved through proximity (lab mates, advisor, family), others which I had intentionally cultivated (close classmates, women in life sciences peers, friends outside of graduate school). With their help, I was reminded that this setback was not a matter of life and death, did not indicate I had reached the limits of my potential, and I reached equilibrium again.
Importantly, I also decided to take more ownership of my project and my learning. I vowed to push myself outside of temptingly comfortable wells of doing what was asked of me and what was working for others, to instead asking myself what I needed to do. It was up to me to ensure I was exploring with as much curiosity and rigor as I could—asking question after question until I truly understood. Only by taking responsibility for creating my own landscape for learning could I have confidence—not that I knew everything or was perfect—but that I had done the best I could.
Given its nontraditional folding behavior, investigating αLP meant taking less for granted about what should work and what we should observe. We had to take a more open stance to learn from αLP and let αLP share its secrets with us, since through the traditional denaturation regime we could only pry some of its secrets loose. Being more open meant getting more creative with our experiments and letting go of limiting assumptions about how a natively folded protein should behave, and what characteristics of the folding landscape were most relevant for function and longevity.
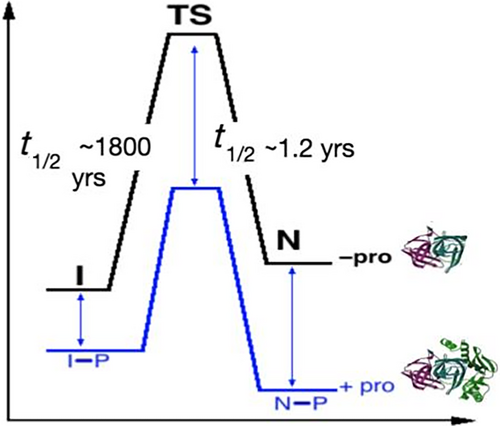
What we learned about αLP was that the folding landscape of the mature protease did not resemble a funnel whose contours guided folding polypeptides toward the folded functional structure. The mature protease conformation is separated by a huge activation free energy barrier from its unfolded states, which would take millennia to surmount in the absence of the pro region. Furthermore, that folded active conformation is thermodynamically less stable than its unfolded states.12
How was this possible? This extracellular protease secreted by the soil bacterium Lysobacter enzymogenes, only has to fold once—during its initial synthesis as a precursor. With the pro-region there, αLP's original folding landscape is actually that of a traditional spontaneous folder (lower blue representation in Figure 3). Once the mature but thermodynamically unstable protease is secreted into the soil, it persists and continues degrading polypeptides of other microorganisms to provide nutrients to its host through reliance on a giant kinetic barrier to unfolding to prevent equilibration with its lower energy states (upper black representation in Figure 3). Although not as massive as the millennium scale barrier to folding, the unfolding half-time of a year is good enough to ensure αLP's longevity as a functional protease.
Because my orientation to αLP had shifted to one of reverent close attention and openness to learning its lessons, I was analyzing everything I could. At one point, I ran a protein gel on a set of fluorescence samples after I had monitored their unfolding kinetics. When I noticed some proteolytic fragments in the unfolded samples, I was at first terrified that what I had been monitoring and interpreting as unfolding, was in fact just αLP getting chewed up through proteolysis. In the most exhilarating 2 months of my Ph.D., I raced to sort out what was really happening—was my whole Ph.D. based on a proteolytic artifact, or was this a clue to something important about how αLP's landscape had evolved to provide optimal function?
In the end, at my colleague Julie Sohl's suggestion, I set up a survival assay to directly compare αLP's ability to remain active with that of its mammalian homologs. In what was to me the battle of the ages (and pre-dated the TV reality show ‘Survivor’ by several years), αLP outlasted trypsin and chymotrypsin.13 Its longer lifetime coincided exactly with its own global unfolding timescale. αLP's functional landscape is so perfectly evolved, that it only becomes vulnerable to proteolytic attack once it is in the globally unfolded conformation. This was confirmed through native-state hydrogen exchange NMR studies that identified a large core of protected sites in the protease that (as shown by a lack of exchange) did not undergo the type of conformational dynamics that render most proteins sensitive to local proteolysis even in the native state.14 This made functional sense because unlike its mammalian homologs, which are subject to proteolytic degradation to curtail their activity outside of post-meal time in the intestines, there is no need for the secreted αLP to be subject to regulation.
I was still fascinated by what thermodynamic and kinetic factors differ in the landscapes of the model well-behaved proteins and those of the rebels, which danced on the edge between functional and aggregation funnels—chaperone substrates and disease-related misfolding proteins alike—when it came time to decide on a postdoc. I was lucky that In addition to my supportive local Agard lab and UCSF communities, since my first summer as a graduate student I had been welcomed into a supportive larger global network of Protein Scientists. Many members of the Protein Society have served as informal mentors to me, giving feedback on my science and advice and support at every step. I have been very fortunate that the global protein science network is populated with many inspiring and generous women scientists, whose talks and science helped me envision myself 1 day giving a Plenary Award talk, which did come to pass this past July (2021). Having met and interacted with Sue Lindquist, I was very intrigued by what the Hsp104 chaperone could possibly be doing to the landscape of aggregated proteins to actually pull them back out of the aggregation funnel.15 I was also captivated by the unfolding of Jeff Kelly's transthyretin story and his lab's beautiful in vitro characterization of how disease-related mutants were impacting the landscape.16 It was a heady time of pulling out of my personal and scientific well of senior graduate student stability to explore the possibility of a postdoc at the University of Chicago or at Scripps.
However, when I mentioned to David that my soon-to-be illegal* wife Meg and I were hoping for at least one postdoc option located on the East Coast near her family, he encouraged me to reach out to Andrew Miranker at Yale University. (*Note: I referred to Meg at that time as my “illegal” wife because our March 5, 2000 wedding was not recognized by California or the US government. One benefit of that was that when we were actually legally married on June 17, 2008 our children were present. That benefit was erased by them having to protest for our family's right to exist as the same-sex marriage ban was debated and then passed in California on November 4, 2008, while I was on the academic job market for a faculty position.) In Chris Dobson's group, Carol Robinson, Sheena Radford, Andrew, and others had just started demonstrating the potential of applying the hydrogen exchange mass spectrometry (HXMS) method for directly observing intermediates during refolding17 and while interacting with chaperonins.18 However, most exciting to me was the potential of native-state HXMS (NHXMS) to complement and extend the insights from native-state HXNMR as pioneered by Clare Woodward,19 Steve Mayo,20 Walter Englander,21 and my outside Ph.D. thesis committee member Susan Marqusee across the Bay at UC Berkeley.22
Joining Andrew's lab, I became hooked by the siren song of native-state HXMS. Monitoring how a protein sampled its landscape from the native state, thanks to the sensitivity of hydrogen exchange, circumvented the limitations of only being able to learn from proteins that refolded spontaneously from denaturant. Sticking to experimental conditions of mild denaturant or none at all would decrease the potential stampede toward the aggregation funnel when more than 10% of the molecules were simultaneously induced to unfold by the traditional high denaturant concentrations. The low concentrations of protein required for MS compared to NMR could make it possible to study proteins that are not available in significant amounts. For αLP, obligate chaperone substrates, or other rebel proteins prone to misfolding and aggregation, a native-state HXMS approach could unlock otherwise inaccessible details on the native side of the barrier.
2 EXPANDING THE UNDERSTANDING OF PROTEIN LANDSCAPES
Beginning my scientific career through intimately observing a rebel protein like αLP, whose behavior defied the prevailing paradigm of thermodynamic stability, has driven me to want to develop a detailed understanding of the landscapes of as many proteins as possible in order to understand not just which trends and patterns but how and why some protein defy the trends. In graduate school, this meant measuring the temperature dependence of αLP's unfolding kinetics and conducting archaeological excavation of other such studies in the literature to enable a comprehensive comparison of the activation parameters of αLP vs. spontaneous folders.23 While we discovered dramatic differences in the features of the refolding barrier, the details of the unfolding barrier for αLP, which is actually the key to αLP's functional longevity, were just slightly exaggerated compared to spontaneous folders. The painstaking nature of collecting the temperature and denaturant dependence of the unfolding and folding kinetics mean that there were just over a dozen proteins available for this analysis, making it difficult to further probe the underlying thermodynamic distinctions responsible for tuning barriers. The desire to stay on top of and apply methods to better understand the information contained in existing and future measurements of protein landscapes, motivated my Amherst colleague Amy Wagaman in the Mathematics and Statistics department and me to create “ACPro” a curated database of protein folding kinetics that our undergraduate researchers can add to from literature searches and use to learn and understand more about protein folding.24
In addition to always seeking to have a comprehensive understanding of what we know so far, I have been focused on being cognizant of what lessons we are unable to learn and which landscapes we are yet unable to access due to the experimental limitations. On a more meta level, I am constantly on the lookout for what is missed when we focus on what we expect based on our incomplete understanding, and on how to help ourselves remain open to the unexpected. Had David Agard been less stubborn when time after time expression of the mature protease did not lead to a folded protein that could be purified, he might have moved on to a more “traditional” and “well-behaved” protein system with which to investigate serine protease mechanism through mutagenesis. This would certainly have been the more prudent choice as an assistant professor. Thankfully he stuck with it.
When we run into behavior that does not match our expectations and experience, how often do we question our own assumptions and limitations? When do we choose curiosity over irritation and the desire for a quick answer, and doggedly persist with the conviction that it is worth our time and energy to learn something new that we do not yet understand? In fact, as scientists, we probably are more open to taking this approach in our research than we are in the other arenas that we navigate as humans with limited experience and knowledge. I have definitely caused irritation in advisors, collaborators, and students as I have followed my curiosity in pursuit of a comprehensive understanding of all protein landscapes and the enhancement of native-state HXMS as an approach to access a much greater diversity of protein landscapes with dogged persistence but a slow pace.
As a postdoc with Andrew and then as a Beckman Senior Research Fellow with Judith Frydman at Stanford, I sought to understand the scope of what would be possible to learn about protein landscapes from native-state HXMS by simulating the predicted HX behavior of proteins undergoing global unfolding. Drawing on the most comprehensive set of landscape data under standard conditions I could (thanks to Kevin Plaxco for spearheading an effort by members of the Protein Science community to collect and report unfolding and folding kinetics at 25°C, pH 7),25 I calculated the extent to which tuning pH could shift the HX behavior from the EX2 regime that reports on stability, to the EX1 regime that reports on the kinetic barrier of unfolding—the star of the landscape for αLP and other kinetically stable proteins.26 For some proteins, the pH shift predicted to enable mapping the full landscape via HXMS, might be minor enough to not also distort the landscape. But for most, native-state HXMS pH tuning can only lead to a partial determination of the landscape.
What was exciting as I was transitioning to starting my own lab at Amherst, is that I discovered that for most characterized proteins, global unfolding at pH 7 would lead to HX behavior intermediate between these two extremes. This regime is typically avoided in HX studies because the measured rate constant of HX does not directly report on just the kinetic barrier of unfolding (as in the EX1 regime) or the stability (as in the EX2 regime). Instead, both aspects of the landscape contribute to HX. What if we could take advantage of that to simultaneously extract both stability and barrier values from HXMS under native conditions? Imagine replacing the painstaking time- and sample- consuming separate sets of equilibrium and kinetic unfolding and refolding (Chevron) experiments performed as a function of denaturant concentration with one or a few HXMS time courses.
However, the tantalizing potential of EXX is tempered by the challenges of devising ways to untangle the combined contributions of stability and kinetics to the observed HXMS time course for the globally exchanging residues. In fact, we demonstrated that a simple analytical model for the exchange of a 2-state protein undergoing HXMS due to global unfolding came very close to replicating the apparent 3-state experimental HXMS behavior for the amyloidogenic protein β2M under near-native equilibrium conditions.27 My computational undergraduates (mostly those who are majoring in Mathematics or Computer Science in addition to Chemistry or Biochemistry and Biophysics) have built a numerical simulations approach to fit complex HXMS time course data and clearly distinguish whether the spectra arise from a 2-state (no intermediate) or 3-state (possesses a folding intermediate) folder. My wet-lab students have been using model proteins to develop the experimental NHXMS workflow. We are now poised with our combined computational and experimental HXMS approach to move from focusing on development and validation to putting our Native-HXMS method to the test: will we be able to truly explore a fuller diversity of the protein landscapes evolved in nature?
3 LANDSCAPE EXPLORATION AS A DEVELOPING FACULTY MEMBER
When I arrived as an assistant professor in the Chemistry Department at Amherst College, it was a dream come true. I was going to be mapping diverse protein landscapes while teaching and mentoring diverse undergraduates. My children would be growing up in a quaint New England college town and getting to know their cousins, grandparents, aunts, and uncles. I had made it and was looking forward to sliding down the smooth funnel toward tenure.
As I ought to have expected, it turns out the new faculty landscape at a liberal arts research college is beyond bumpy! My first two attempts to get a major research instrumentation grant to buy a new mass spectrometer were unsuccessful. I was staying up until 2–3 a.m. overpreparing the first five slides of a lecture and then throwing something together for the final 15 in the half-hour before the lecture. I could not figure out how to show slides, use the chalkboard to work problems, and keep track of my notes. My students confused me and the other biracial young female professor I was co-teaching with and wrote nasty personal attacks in addition to legitimate critiques of my sleep-deprived uneven attempts at performing as a “sage on the stage.”28 In addition to her part-time job, my wife was working more than full-time navigating the learning challenges and health conditions of our children. In the full college faculty meeting, which took place 7:30–9:00 p.m. (and still do!) biweekly on Tuesdays in the “Red Room,” when I still had 8 h of prep for my 9 a.m. lecture, a bunch of senior colleagues wielded Robert's rules like weapons, while I tried to keep track of committee acronyms and to perfect a noncommittal mumble during the oral votes on questions I did not have the time, energy or background to comprehend.
On one rare occasion when I actually read the faculty meeting agenda ahead of time, I was pleased to see that the “Committee of 6” was bringing forth a motion developed by the “CPR” to finally provide paid parental leave. But when I read further, I discovered that full coverage of parental leave would only be available to women who had given birth to an infant. This was justified as specifically necessary for biological mothers, due to the physical consequence of undergoing such an intensive medical procedure as childbirth. With ears ringing and heart pounding, I raised my hand and made a statement about how this policy would have impacted me, as the primary caregiver when each of our children had come into our family (3 years apart) as neither biologically birthed nor as infants. Instead, at 2 and 3 years old they had been navigating the trauma of being separated from their country, their culture, and the only family they had known—their caregivers and the other children in their children's’ homes. I did not know enough to realize I would need to be prepared to “propose an amendment” and get a “second,” and likely have strategized with potential allies to truly change anything about the motion that night. However, I was proud to use my trembling voice to call out the invisible assumptions in the policy, and for a brief moment to expose the entire faculty body to the reality of how individual blindspots lead to institutional structures that actively exclude humans like me.
In my second year, a full-time position was advertised at the community college in the Bay Area at which I had served as an adjunct between the end of my research fellow position at Stanford and the beginning of my job at Amherst. I wrote a fantastic cover letter describing how I was ready to focus on teaching and let go of research, because I was committed to teaching and mentoring students. I knew I would be better able to navigate the barriers and challenges in the new faculty landscape if I were back in the diverse community that had been my home for nearly two decades. However, my wife and I decided that sticking it out at least for the remaining 5 years until a tenure decision was the best option for our family, so I never followed through with that application. By my third-year review, I had no grants from ~5 proposal submissions, no papers and a mediocre teaching record. I was well on my way into the aggregation funnel.
There were two saving graces for me—my lab and the protein science community. The Jaswal lab community that I had dreamed of the building had become a vibrant reality. I had followed my plan of scaffolding the development of hands-on skills alongside deepening understanding of the scientific concepts, intentionally setting goals for lab and leadership progress with individual students, and offering them opportunities to experience the incredible research and community of protein scientists. The undergraduates in my lab were smart, eager, and willing to try anything. They believed me when I told them that it was as important in science to contribute to a collaborative and inclusive lab community as it was to be individually organized, committed and productive. Learning about all of the research being done by others at the Protein Society Symposia made the dry science from lectures and textbooks come to life, and opened a whole new world of what it means to be a scientist. They were terrified going into their first poster sessions, but without fail, after presenting non-stop to good-hearted Protein Society members who offered their full attention and constructive feedback, they were proud and excited. The realization that the work they had done in our lab positioned them as scientists considered peers by researchers from around the world, was as potent as the Best Poster Awards that several of them garnered over the years in catalyzing their science identity. They transformed from students seeking to be told what to do, to scientists with agency and confidence to take ownership of their projects.
I treasured each of the stages with them as they transitioned from accepting my micromanagement of their lab notebook habits, to catching on to the fact that the ideas I tossed out in our hurried conversations squeezed between my meeting with a search candidate and frantically revising my kinetic molecular theory of gases class design (in vain) one more time, were meant to be questioned and challenged. Indeed to this day, my standard of success in guiding fledgling scientists remains that a senior gives me the side-eye and delivers some version of “Dr. J, that is ridiculous,” before proceeding to demolish—with evidence from their own experiments and the literature—my suggestion.
I take joy and pride in how I have co-created with my students a lab environment that fosters curiosity together with humility, and collaboration together with independence. Students gain an appreciation of the importance of challenging and growing oneself as a researcher and as a whole person, while also recognizing one's responsibility for the collective health and productivity of the lab and our larger community. As they leave the lab and Amherst heading to research technician positions, medical school, graduate school, or in directions I never imagined with confidence in themselves and a commitment to collaboration and community, they constitute truly the most significant output from my scholarship.
Nonetheless, for their sake and mine, I needed to ask for more help to get the results of our work together into the form of scholarship beyond poster and seminar presentations. At the end of my third year, as I was heading into my pre-tenure sabbatical, I stumbled on the National Center for Faculty Diversity & Development (NCFDD), which had recently been founded by Kerry Ann Rockquemore, the author of “The Black Academic's Guide to Winning Tenure without Losing Your Soul.” After accepting an invitation to a “Writing Challenge,” I found myself pledging with a group of online strangers that I would write for 30 min and check off a little box for 14 days. I was shocked by how much I got done, how good it felt to have prioritized my writing first thing in the morning, and how energized I was by seeing those boxes fill in. In just days, my panic and dread about facing a year dedicated to only writing papers and grants had transformed into hope, relief, and even excitement.
I enrolled in the NCFDD's 12-week “faculty success program” that June. I continued with 30 min of writing to begin my workday, and dutifully—if skeptically—tried each week's homework curated by NCFDD from the buffet of evidence-based practices for sustained writing productivity alongside my small group of three other science faculty and a faculty coach. Figuratively kicking and screaming, I forced myself to make a 3-month plan. I consulted it to create weekly plans that progressed from blatantly unrealistic to reasonable. Especially after the painful time tracking exercise that revealed how much time things were actually taking and how much time I was invisibly wasting, making my weekly plan became a motivating habit.
Key to sustaining my several new habits was the accountability and support of the online community. Pre-Covid, the idea that energy invested in digital exchanges with strangers (not even in the same discipline) could contribute in any way to one's own scholarship was totally novel and unfathomable to most of my colleagues. Of course, I only admitted to a few that I had resorted to this external chaperone system to help get me into a functional scholarly production landscape. I was initially convinced that most rigorous innately brilliant and productive scholars would rightly condemn this as evidence that I did not deserve tenure because I could not hack it on my own.
However, over those 12 weeks, I began more honestly engaging with the prompts about each day's writing session—in writing my own reflections and reading those of others. I realized that my resistance to writing, my procrastination, my imposter syndrome, and my harsh critique of any first draft, were all not unique to me nor evidence that I do not belong in science and academia. The smooth funnel I envisioned for everyone else in their writing process, was actually bumpy, sharing many of the same barriers, traps, and excursions to nonproductivity that mine did.
Not only did I experience validation, but I found that I was talented at responding to the challenges of others with the ability to reframe, offer potential next steps and creative hacks that helped them overcome limiting assumptions that were making them stuck. Discovering that I possessed this “moonlighting function” that could coax others off their aggregation edge into a functional funnel also helped me get better at coaching myself. That summer was the turning point that reminded me that barriers are inherent to pursuing any worthwhile goal, and that I wasn't in this alone. Part of being a brilliant scholar and educator is to let go of the ego that thinks that success requires the ability to “John Henry” (use your brute force on your own) your own inherently bumpy funnel into smoothness at the cost of your health, relationships, and the original love and passion you had for your science.
In addition to the community I gained through NCFDD, beginning that summer and continuing to this day, I connected with other protein scientists in the region. Using funds from Amherst, I was able to work with Celia Schiffer at UMass Chan Medical School in Worcester as an external research mentor. During my pre-tenure sabbatical year, I escaped once or twice a week to a desk tucked away in her lab space. I could focus on advancing my writing projects during my 25 min timed blocks while surrounded by the hum of R1 research activities and drawing on the editing prowess of Nese Kurt Yilmaz. I have benefited from interacting with Bob Matthews and his lab, learning from their exquisite mapping of multiphasic kinetics of crazy bumpy funnels. I have been lucky enough to be welcomed to the Gierasch lab just down the street at UMass Amherst. In addition to presenting at and attending lab meetings, members of my lab drop in to use the lyophilizer and get help from Steve Eyles in the mass spec facility. Lila also humored me when I would record meetings going over my grant drafts using my fancy “Livescribe” recording pen, which was a time machine that let me revisit my illegible notes and by pressing the pen to the page, play the audio of the conversation at that point! Anne Gershenson let me camp out in her office and has remained a reliable writing accountability buddy for years. By June 2015, when I made a date with my lifelong mentor Susan Marqusee to go over my tenure letter at the Proteins Gordon conference, I was proud of what the lab had accomplished, proud of the papers I had completed and submitted, and excited about moving the lab into our next phase.
4 EXPANDING THE UNDERSTANDING OF HUMAN LANDSCAPES
In the fall of 2016, my tenure package was submitted and I was teaching Biochemical Principles of Life at the Molecular level to 50 students. On the morning of Thursday November 12, 2015, I somehow missed seeing the email from the Dean of Faculty announcing that students had organized a 1-h sit-in to show solidarity with other Black Lives Matter protests around the country, and had requested that faculty and staff join students at 1 p.m. in our campus library to show our support. I needed to be super-efficient about getting my work done so I could meet the kids getting off the bus after school since my wife was traveling for work. However, at 3 p.m., I noticed an email from the Dean and in my quick scan saw something like ”the students are still here and really really want faculty to show up.” I decided to swing past the library and was dumbfounded to see the mass of bodies spilling out. I do not really remember much of that afternoon but I did remember to meet the bus (in order to pick up my kids), and I must have helped with the usual homework. I took the kids to the campus cafeteria for a quick dinner and then finally made it to the library. While my kids sat in a corner with books, I caught the end of what had been hours of testimonials by students of color about how experiences in our classrooms, labs, departments, and campus community had led them to feel marginalized, isolated, excluded, and not welcomed with their whole identities.
Their stories got me thinking about how our campus structures and academia in general were evolved through systematic exclusion of women, Black, Indigenous, People of Color, non-Protestants, queer, trans, nonbinary, low-income, first-generation, disabled, and other “nontraditional” folks. The contours of the resulting educational landscapes enable certain students but not others to thrive. In particular, the assumptions about access to quality pre-collegiate education, parental ability to be involved in their children's education and provide enrichment opportunities, the students’ independence from responsibilities at home, and their ability to ignore societal factors and focus on academics while at college do not match the realities of many students.
Furthermore, in the absence of a framework and vision for accelerating the pace of campus evolution, students holding nonmajority identities not only experience a campus landscape not shaped for them, but one that is rigid, with no apparent mechanism for being reshaped by their voices, needs, and contributions. Students are welcomed and celebrated for the diversity of identities, experiences, and voices they bring to our community, but not included unless they manifest the traditional behaviors and patterns that lead to success in the existing rigid landscape. Especially in our STEM disciplines, where we pride ourselves on the objectivity of our experimental pursuits of knowledge and natural truth, many cling to a belief that our existing landscape as evolved prior to welcoming our current population of students provides the only worthy routes to excellence and academic rigor.
We do not understand why what worked to educate a homogeneous group of primarily straight, white, privileged, able-bodied men should need to be different. While we acknowledge that the leaky pipeline leading to demographics in STEM that resemble the general population less at each stage exists, we do not view that leaky pipeline as evidence of a flawed system. Rather, just as in the early days of focusing on the individual protein in a test tube, we tend to view failure to advance to the next level (fold into a state that is functional for STEM success) as simply evidence that an individual's landscape does not encode the correct priorities, interest, passion, grit, and resilience needed to succeed in STEM.
Was not my own success story as a biracial queer daughter of an immigrant in STEM who was on the brink of becoming a tenured professor proof that the system is navigable to those who demonstrate the work ethic, persistence, and discipline to do what it takes? However, when I considered my own pre-collegiate and collegiate landscapes in comparison to most of my students, I was forced to acknowledge just how many advantages that had nothing to do with my intellect, effort, or resilience had lowered barriers for me at each step of the way. Born to two college professors—one in STEM at a research university, the other in the arts at a liberal arts college, I grew up with high academic expectations and support and have always felt at home in academic settings and interacting with college and graduate students, postdocs, and professors. I was born into a zip code that afforded me good health care and public schools. My parents had the flexibility and financial stability to enroll my siblings and me in music lessons, youth symphony, sports teams, language camps, and at 16 I even accompanied my dad for his year-long sabbatical in Vienna, Austria.
By the time I applied to college I was the equivalent of αLP with my parents as the pro-region—who had tuned my barrier to that of a microsecond folder and stabilized me as a highly competitive college applicant. Thanks to that exogenous tuning, I was also primed to easily navigate academic structures with the confidence to engage peers and faculty, and the cultural capital to use resources to the fullest. Truthfully, although my interactions with the parental pro region have become more transient and infrequent since I was the age of the students protesting in the library, I continue to more easily scale the barriers at each stage of my career thanks to them. With our close-knit family, it is impossible for them not to assume defacto roles as academic career coaches, since they understand and have themselves experienced and navigated similar academic landscapes from student to new faculty to department chair to administrator. Reflecting on my own journey helped me better realize how systems that have been evolved with biases not only persist in “disadvantaging” individuals and groups, but they also persist in “advantaging” individuals and groups who have access to opportunities and family and cultural knowledge that invisibly lower barriers.
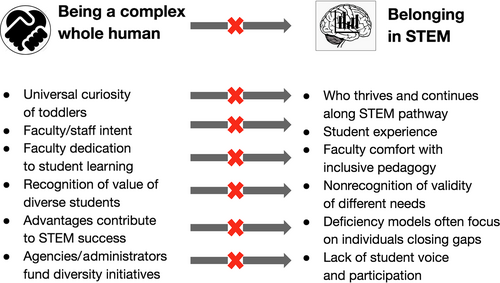
As I was reflecting with STEM students during and after the protest, we identified a number of additional disconnects in STEM, summarized in Figure 4, that do not stem from any one individual, department, or institution. While all toddlers are innate scientists whose curiosity and experimentation are well documented, the humans learning and doing STEM get less and less diverse at each step. The disconnect between the painful testimonials of students and the heartfelt letters of response from STEM departments at Amherst underscored the fundamental disconnect between faculty intent and student experience. Related to this is the fact that while the majority of faculty are dedicated to student learning, far fewer are comfortable with learning and implementing inclusive pedagogy. Institutions welcome and acknowledge the value of diverse students without simultaneously recognizing the validity of different needs. Although it's very clear that advantages accumulate and contribute to students finding success and thriving in STEM and academia, our deficit mindset and initiatives focus on helping individuals close gaps. Finally, proposals for remedying what we perceive as student deficiencies are generally devised by faculty and staff and funded by agencies and administrators. Rarely do we include students in defining the disconnects that they experience nor in discussing and designing efforts to bridge disconnects.
In some ways, what started that day and grew into a 4-day occupation of the library, termed “the Amherst Uprising,”29 was such an intense rupture on campus that virtually all participants who showed up at the library were profoundly destabilized back to a vulnerable and uncertain (unfolded) state. At the same time, we felt open and capable of imagining a different landscape together. It felt like it might be possible to capture the essential values and spirit of our rigorous intellectual residential learning enclave, while stretching and growing and making room for all of the many different starting points, enabling many paths, and allowing for wells that might not have been there before.
A few weeks after the Uprising, I was approached by a group of students, including Sanyu, one of the Black women who organized the original sit-in and other women, most of whom identified as Black Indigenous People of Color (BIPOC), queer, first-generation or low-income, about co-designing a special topics course to investigate these disconnects at Amherst and beyond. Although this was far outside my comfort zone, I felt the exhilaration and the possibility of starting a new as a nascent human being ready to collaborate in exploring what possibilities might exist to reshape our STEM landscape together. Thus, the course “Being Human in STEM” was conceived as a pilot experiment in our own local campus community to begin to bridge the overarching disconnect between what it takes to belong and thrive in STEM (and academia), and being welcomed and embraced for your full humanity with all of your identities, complexities, flaws and deviations from the mean on whichever axes.
What began as that first special topics course with 9 students in the spring of 2016 has exploded since then into a national movement with the mission to empower students, staff and faculty to reshape their classrooms, laboratories, and departments to create an inclusive and equitable STEM community that enables humans of all identities to thrive and flourish (Figure 5).30 We are in our 10th iteration at Amherst this January with an interdisciplinary facilitation team of four students who are HSTEM alumni, faculty from Biology, Chemistry, Mathematics and Statistics, Music, and Philosophy, and an enrollment of nearly 50 students. Faculty, staff, and students from other institutions have sought us out to learn more and adapt the HSTEM model to their own campuses. To date, 30 total iterations of HSTEM have been conducted at Amherst, Yale, and other public and private institutions and inquiries come in regularly from others. We held our first national HSTEM conference, funded with an award from the NSF improving undergraduate STEM education program, June 10–12, 2021 with close to 70 student, staff, and faculty participants from each of the 12 institutions that have offered HSTEM alongside HSTEM-curious folks.
While the details have evolved in each iteration at each institution, the blueprint devised by the HSTEM founding students has remained the same: (a) investigate the student experience in STEM by seeking and sharing narratives about the lived experience of BIPOC and other marginalized students, (b) review academic literature on identity, marginalization, and systems of oppression in society and STEM, (c) use the shared foundation in lived experience and academic research to propose campus interventions that are locally relevant and evidence- based, and (d) students share their stories of being human in STEM, their lessons from the course, and their proposals for concrete actions to advance antiracism, equity, and inclusion with their local STEM community through presentations and magazines with student-authored articles and resources. That core is supplemented with intentional community building throughout to make the class a space where students can feel brave enough to take risks, and make mistakes and learn from them as they practice having difficult discussions across differences.
Being Human in STEM has created an in vitro evolution approach that can accelerate the slow pace of campus and individual change by fixing certain parameters like building community and trust, requiring individual reflection, and engaging in authentic partnerships with others on campus. That very first HSTEM cohort created the website www.beinghumaninstem.com (which, because we are humans doing STEM, has a rather sporadic timeline of being updated!). Some student projects remain simply proposals based in academic and Amherst-specific research that are shared with campus stakeholders and reviewed by future classes. Some are implemented by the students themselves, like the first-ever GeoLatinas panel that brought four LatinX Amherst geology alums together for a Geology department event. Some are piloted and revisited by successive iterations, like a facilitation guide and materials for a workshop designed to be integrated into a lab or discussion section of an introductory STEM course that introduces students to Being Human in STEM topics and practices. Some have snowballed beyond the class to attract faculty and staff engagement, like the student-drafted tips on inclusive practices that grew into a 20-plus page HSTEM inclusive curricular practices handbook (http://www.beinghumaninstem.com/inclusive-curricular-resources.html). Now in its third edition, this resource that gives step-by-step instructions alongside the rationale and literature to back up a range of easy-to-integrate practices has been downloaded hundreds of times by educators teaching STEM from high school to graduate school, from first-time graduate student teaching assistants to educators with decades of experience. Importantly, the handbook and the growing set of resources generated through HSTEM research and collaboration are shared with the Amherst STEM community through new faculty orientation and other activities.
The way that HSTEM has contributed to an ongoing sustained conversation is as important as the specific projects proposed by students. Welcoming new participants into this ongoing process each year—as students or facilitators in the academic course, campus partners that students reach out to and interview or share ideas for feedback, other members of the campus community who want to understand what their friend, advisee, or classmate is learning in a course called Being Human in STEM, or who happen to attend the end of the semester salon—keeps the conversation about diversity, equity, and inclusion (DEI) alive, structured and connected to the academic literature as well as the broader context of the historical DEI efforts on campus and beyond.
With the international reckoning around racism in every dimension of society that began after George Floyd's murder at the hands of police in May 2020, we have been thinking about what lessons the HSTEM model might offer, as more people than ever before have committed themselves to reshaping the landscapes of STEM and academia to be antiracist, equitable, and inclusive. What enabled this watershed student protest that woke virtually the entire Amherst community to the urgency for all of us to reconcile our identities as scholars with our responsibilities as human beings, to birth and sustain HSTEM as a hub of constructive DEI engagement and progress? What can we learn from this homegrown, student-centered model created by BIPOC, and women undergraduate students?
We have realized that the transformation from protest to progress at Amherst occurred through a process that was organic in the moment, but has since proved to be the central process of the Amherst HSTEM initiative—guiding our interactions with each other and our approach to working with others for change on campus. The first step was listening, by the administrators, faculty, staff, and students who took in the hours of painful testimony. The second was to validate. Following individual outreach by a Black neuroscience student (Louise Atadja, who became one of the HSTEM founding students) and a gender nonbinary Biochemistry and Biophysics student detailing challenges in their Amherst STEM experience, each STEM department wrote a letter of support for the student protesters, pledging to do better (http://amherstuprising.org/documents). Taking that one step further, in our discussions during and after the protest, the students and I challenged ourselves to reflect on our own intersecting identities, biases, and privileges. This led me to probe more deeply into my own STEM narrative, and together with the students to identify the disconnects we then set out to consider bridging. The partnership we were forged, thanks to having first listened, validated, and reflected, was stronger and more effective than would have been otherwise possible.
It is instructive to consider this cycle in the context of what typically happens in STEM (and other areas) when student activism around social justice takes place (Figure 6). Typically, we do not even make it to the space to listen in the first place. Instead of validating the experiences of others, we are unable to accept the validity of an experience that differs from our own. Rather than reflect, we succumb to the reflexive human reaction of defensiveness. Beyond avoiding engaging in partnership toward action ourselves, we try to dissuade others from undertaking what we consider to be irrelevant advocacy that does not belong in STEM.
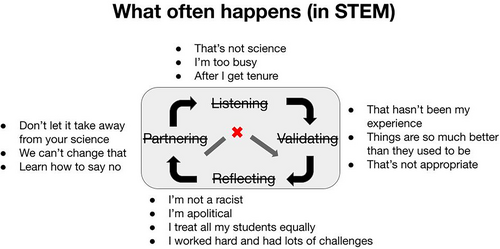
To be clear, in the past, I have thought many of these phrases to myself as a means to rationalize not leaving the landscape I had chosen as a protein scientist to explore the unfamiliar and scary landscape of engaging with the full spectrum of human identity and experience. During the brief excursions I had made out of my well of confidence and expertise, to consider the larger systems of inequity and racism and my role in them, I had grappled with feelings of guilt, discomfort, powerlessness, and fear of doing or saying the wrong thing. Who was I, and who were students—or anyone—to expect me, who has never taken a class in women's studies, Black studies, queer studies, disability justice, sociology, organizational change, and so on, to get involved in talking about, let alone taking responsibility for, shaping an antiracist and equitable STEM community or society? However, after witnessing the courage and vulnerability of our students who loved Amherst enough to stand up and shine the light on our failure to live up to our promise of an accessible education, when my students invited me to join them in the Being Human in STEM experiment, I agreed, as part of the experiment, to try letting go of those assumptions.
That experiment as paid off for me individually beyond anything I could have predicted at that moment. Instead of guilt and powerlessness, HSTEM has helped me feel agency and empowerment to shoulder responsibility. The tools, practices, and community that we have built with HSTEM have helped me to persist through the inevitable discomfort of engaging honestly across differences, and to value my mistakes as opportunities to learn. Other HSTEM alumni have shared my sense that by participating in HSTEM, it feels more possible to merge our scientist and human identities, feel belonging in the STEM spaces we inhabit, and feel committed to actively coevolving with the flawed institutions that we love enough to critique.30
At the core of everything we do in the course at Amherst, and the most effective tool that participants can take away to guide their continuing practice of being human in STEM after the course is our HSTEM process. Many of us desire a script we can memorize to make sure we say the right thing, act the right way, and minimize harm while maximizing impact as we try to shoulder our responsibility for combatting racism. We want to be ready to perfectly respond at all levels—from witnessing micro aggressions to taking principled stands and actions to identify and dismantle systemic oppression. What we have learned over 6 years of HSTEM is that there is no script, but we can follow a process, which HSTEM alum Aidan Park affectionately coined “The HSTEM central dogma” of Listening, Validating, Reflecting, and Partnering.
This simple cycle pushes against our reflexive “fight or flight” response to uncomfortable situations which ordinarily leads us to skip immediately to taking action (or disappearing). Any action that short circuits the full cycle is typically informed by neither a clear understanding of where the other party is coming from, nor a clear understanding of our own motivations and desired outcome. When the first step is to truly listen, you are freed from the pressure of the perfect reaction, connection, or synthesis. Following up with validating what you heard as opposed to agreeing, disagreeing, judging, or condemning, allows for clarification and builds trust because you have demonstrated that you actually see the other person's lived experience, even if it differs from yours. Taking time to reflect on your own experience and reaction and how they fit with the larger context sets you up to take next steps that align with your values and makes more room for curiosity and humility to edge out defensiveness. Intentionally moving through these steps when confronted with the complexity of real-life encounters with difficult conversations or problematic interactions strengthens the potential that the final step, partnering across differences to continue the dialogue or take collective action that is informed by multiple perspectives, will be effective.
In class, we practice drawing on the HSTEM central dogma as each of us improvises to write our own script time and time again. It guides us as we practice engaging authentically in honest conversation about the academic readings and our own varied identities, backgrounds, and experiences in STEM. The trust engendered by committing to this structure, which we buttress by developing shared community agreements for creating a brave learning space that challenges us to be honest, has enabled us to disagree with respect, without devaluing individual experiences. When students move from building an academic foundation to proposing campus change, adhering to the HSTEM central dogma helps them to integrate more than their own opinions and experiences.
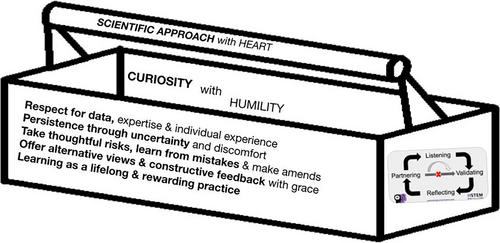
I would argue that as scientists, we already have the basic toolbox necessary for engaging in a continuing practice of being human in STEM that fosters the education and collaboration necessary to partner for change in landscapes at the individual, community, and global level. If we align our interactions with the HSTEM central dogma, we can simply apply our familiar scientific approach, expanded to include a bit more heart (Figure 7). In learning about individual and systemic racism, adding heart to our scientific approach requires pairing our curiosity with humility. We need to respect not only quantitative data, but the expertise of social scientists, indigenous ways of knowing, and fields that seek to critique and hold our STEM communities accountable for our impact on individuals and humanity, as well as the truth of an individual's lived experience. Just as in our research, where we revel in tackling what we do not know and will follow the problem we are passionate about, regardless of the new territory we find ourselves in, we also need to persist through the discomfort of gaining the expertise we lack in navigating the complexity of reshaping our landscapes for greater equity and inclusion. As researchers, we constantly take risks without being guaranteed of the outcome and accept that making mistakes is a natural part of the process of doing significant science. What we are not so good at is making amends when we make mistakes as we are learning how to shift our habits and becoming aware of how often our impact does not live up to our intent. We are great at discussing controversial topics in our fields, offering alternative interpretations, and feedback as we strive to advance our disciplines. In moving from words to action in our stated commitments to dismantle racism, we need to be better at focusing on being constructive. We have to show grace to others and ourselves, acknowledging that despite all good intentions, missteps and harmful impact will occur when we are truly stretching to dismantle racism in ourselves and in the systems of which we are a part. Finally, we prize learning and understand that it is a lifelong and rewarding process. The same is true for learning about the lived experiences of BIPOC and other marginalized individuals and groups and the ways in which our systems continue to exclude them.
In closing, I believe that at this moment in history, keeping our individual and collective human and STEM landscapes separated in an illusion of objectivity is no longer an option. That approach has not only led to the continued exclusion of marginalized groups, but to a state of affairs where more and more people refuse to accept scientific evidence. Continued “business as usual” in science as we are experiencing global warming, a global pandemic, global inequity and racism, and a global crisis in mental health is ludicrous. Science has had answers but has not provided solutions that can heal our earth and humanity. Embracing being human in STEM and beyond may be one of the last plays we have to make. Let us lead the way as humans in protein science.
ACKNOWLEDGMENTS
I am deeply indebted to the many humans who have indelibly shaped my development and continuing evolution in science, academia and life: Alice (the original Dr. J) and Sitaram Jaswal (the original human in STEM), Julie Sohl, David Agard, Ken Dill, Carol Gross, Susan Marqusee, Andrew Miranker, Lynne Regan, Judith Frydman, Celia Schiffer, Anne Gershenson, Lila Gierasch, Bob Matthews, members of the protein science community who have inspired me with their science and generously supported mine - Sheena Radford, Manajit Hayer-Hartl, Betty Craig, Helen Saibil, Sue Lindquist, Carol Robinson, Jane Clarke, Liz Meiering, Patrica Clark, and Tricia Serio, the fifty-plus past and present members of the Jaswal lab, my Amherst colleagues, in particular the Chemistry Department, my student, staff and faculty HSTEM collaborators at Amherst and beyond, with particular thanks to my long-time Amherst partners Megan Lyster and Sarah Bunnell, and the Protein Society for recognizing my intertwined efforts in research, education and service with the Carl Branden Award. I offer sincere thanks to Leah Schmalzbauer, Airlie Rose, and Anne Gershenson for reading and providing thoughtful comments that improved this manuscript.




