Structural investigation on SPI-6–associated Salmonella typhimurium VirG-like stress protein that promotes pathogen survival in macrophages
Shilpa Ray and Nishant Kumar Pandey contributed equally to this study.
Funding information: Council of Scientific and Industrial Research, India, Grant/Award Number: Senior Research Fellowship; Department of Biotechnology, Ministry of Science and Technology, Grant/Award Numbers: BT/IC-06/003/91-flagship proposal, Builder program BT/INF/22/SP42155/2021, ESRF BM14 access program; International Centre for Genetic Engineering and Biotechnology, Grant/Award Number: ICGEB New Delhi core fund; Kalinga Institute of Industrial Technology, Grant/Award Number: Core budget
Abstract
Enteric microbial pathogenesis, remarkably a complex process, is achieved by virulence factors encoded by genes located within regions of the bacterial genome termed pathogenicity islands. Salmonella pathogenicity islands (SPI) encodes proteins, that are essential virulence determinants for pathogen colonization and virulence. In addition to the well-characterized SPI-1 and SPI-2 proteins, which are required for bacterial invasion and intracellular replication, respectively, SPI-6 (formerly known as Salmonella enterica centisome 7 island [SCI]) encoding proteins are also known to play pivotal role in Salmonella pathogenesis. However, the underlying molecular mechanism of these proteins remained elusive. To gain molecular insights into SPI-6–associated proteins, in this study, a SPI-6 Salmonella typhimurium VirG-like protein (STV) is characterized using interdisciplinary experimental approaches including X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy and infection assays. The high-resolution crystal structure, determined by the single-wavelength anomalous dispersion (SAD) method, reveals that STV belongs to the LTxxQ motif family. Solution-state NMR spectroscopy studies reveal that STV form a dimer involving interconnected helices. Interestingly, functional studies show that STV influence pathogen persistence inside macrophages in vitro at later stages of infection. Altogether, our findings suggest that STV, a member of the LTxxQ stress protein family, modulates bacterial survival mechanism in macrophages through SPI-1 and SPI-2 genes, respectively.
1 INTRODUCTION
With the emergence of pathogens, the mechanism of host restriction and adaptation has become a serious endeavor. Enteric pathogens infect and colonize the intestinal cavity employing a myriad of strategies to compete with commensal bacteria and modulate eukaryotic hosts to initiate pathogenesis.1, 2 Many pathogens encode large clusters of gene cassettes that help them survive against the adverse host environment such as Salmonella enterica serovar Typhimurium, the chief cause of human gastroenteritis.3-5 The Salmonella genome harbors complex virulence determinant proteins called Salmonella pathogenicity island (SPI) acquired by horizontal gene transfer either from plasmids or phages that synergize to allow bacterial entry, survival, and persistence in unique niches.6 The most essential Salmonella virulence genes are distributed in 12 pathogenicity islands, of which only five (SPI-1, SPI-2, SPI-3, SPI-4, and SPI-5) have been well characterized.7 The two most characterized virulence determinants of Salmonella are SPI-18 and SPI-2,9 which are responsible for pathogen entry into host cell and further infection establishment, respectively.10, 11 Both SPI-1 and SPI-2 encode a highly structured and well-characterized Type III secretion System (T3SS)12-14 which contributes to Salmonella pathogenesis by translocation of secreted proteins across the target cell membrane for manipulating the host cytoskeleton during invasion15 and for survival of the bacterial pathogen.16 Thus, T3SS is also known as injectisomes.17 Additionally, the genes clustered in SPI-3,18 SPI-4,19 and SPI-520 encode virulence factors associated with different stages of enteric infection.
Salmonella typhimurium genome bears a well-conserved SPI-6 present in different serotypes which encodes certain members of the Type VI secretion system (T6SS).21-23 The T6SS system24 mimics an inverted contractile bacteriophage tail25 which ejects effectors mediating interbacterial interactions26 and host functioning.27-29 In Salmonella, the T6SS is encoded by genes located in five differentially distributed SPIs, namely SPI-6, SPI-19, SPI-20, SPI-21, and SPI-22. SPI-6, previously known as Salmonella enterica centisome 7 genomic island (SCI) is a 47-kb island adjacent to tRNA encoding gene aspV and includes adhesin and invasin genes such as pagN and safABCD fimbrial cluster30 along with the transcriptional regulator sinR. Recent studies in S. Typhimurium have shown that SPI-6 antibacterial activity and T6SS-dependent killing of commensal bacteria is essential for infection establishment in the host gut.31 The SPI-6–encoded T6SS30 also plays a pivotal role in Salmonella's virulence.32-34
STV (previously known as SciZ) is a periplasmic protein within the SPI-6 locus (SL1344_0303).30 The tactical location of STV encoding gene within the virulence and invasion-associated gene cluster intrigued us to investigate its structural and functional properties using interdisciplinary experimental approaches. This study aimed to inspect the role of STV as an essential virulence determinant in Salmonella pathogenesis and also questioned the possible contribution of STV for Type VI effector secretion. We determined a high-resolution crystal structure of STV along with biophysical characterization using nuclear magnetic resonance (NMR) spectroscopy. We were able to determine that STV plays an important role in the survival of S. Typhimurium in macrophage.
2 RESULTS
2.1 Crystal structure of STV
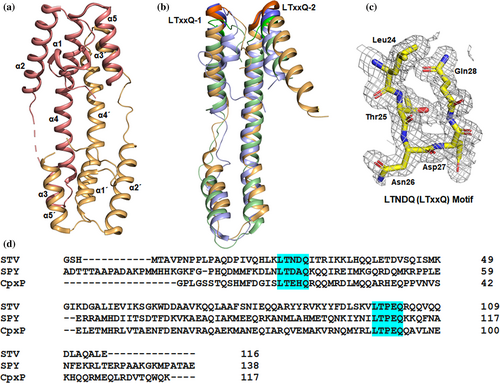
We have determined the three-dimensional structure of STV using X-ray crystallography. We cloned the matured STV encoding gene (SL1344_0303) lacking periplasmic signal and overexpressed in Escherichia coli. Homogenous STV was purified by nickel-nitriloacetic acid (Ni-NTA) affinity followed by size exclusion chromatography (SEC; Figure S1A). Diffraction quality crystals were obtained by crystallization of STV using sitting drop vapor diffusion method (Figure S1B). The crystal was diffracted to 2.19 Å resolution and the data were scaled in P21212 space group. The crystal structure of STV was solved using single-wavelength anomalous dispersion (SAD) on iodine-soaked crystal (protein data bank [PDB] id 5IO8). There were two molecules of STV present in an asymmetric unit. The high-resolution structure of STV was determined at 1.58 Å resolution by molecular replacement method using model coordinates obtained from SAD phasing (PDB id 5IHF). Crystallographic data processing and refinement statistics are summarized in Table 1. The final refined model contains two chains: chain A comprises 105 of 116 residues (1–5, 49–53, and 116 are missing) and 109 of 116 residues in chain B (1–5, 115, and 116 are missing). The overall structural architecture of each monomer protein looks like a hairpin loop or a “crowbar” (Figure 1a). Each STV monomer consists of five α-helices while N-terminal region is unstructured (Figure 1a). These helices are termed as α1 (Leu12–Gln20), α2 (Thr25–Gln45), α3 (Ala55–Gly63), a long and slightly curved helix α4 (Asp66–Leu99) and α5 (Thr100–Leu115) with a small loop region (Ile46–Gly54) between α3 and α4 of which no electron density was seen for Lys49–Asp53. For sake of clarity, chain B helices are termed as α1′ (a short helix Asp16–His21), α2′ (Thr25–Gln45), α3′ (Gly54–Gly63), α4′ (Asp66–Leu99), and α5′ (Thr100–Gln113) with a small loop region (Gln45–Asp53) between α3′ and α4′. The superposition of two monomers shows Cα root mean square deviation (RMSD) of 0.726 Å. α1–α2 and α4–α5 run antiparallel to each other and projected outward at an angle of 19.5° and 17.5°, respectively (Figure 2b). This V-shaped orientation of helices is stabilized by H-bond between backbone oxygen of Pro10 and side chain of His36 and Glu40 and Asn9 through two water molecules (HOH 303 and HOH 330) in α1–α2 and backbone oxygen of Ser96 and side chain of Arg104 in α4–α5.
| Parameter | ||
|---|---|---|
| Crystal | I-SAD | Native |
| Space group | P21212 | P21212 |
| Unit cell parameters | ||
| a, b, c (Å) | 60.11, 60.27, 2.60 | 58.96, 59.71, 63.13 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 |
| Matthews coefficient (Å3Da−1) | 2.15 | 2.11 |
| Solvent content (%) | 42.74 | 41.56 |
| Monomer in ASU | 2 | 2 |
| Data collection | ||
| Wavelength (Å) | 1.7712 | 1.7717 |
| Resolution range (Å) | 50.0–2.19 (2.23–2.19) | 63.13–1.58 (1.66–1.58) |
| Unique reflections | 12,430 (1,752) | 31,355 (4,502) |
| Total reflections | 172,098 (23,226) | 23,3,810 (31,923) |
| I/σ | 13.2 (6.2) | 9.7 (1.2) |
| Completeness (%) | 99.7 (98.7) | 99.7 (99.8) |
| Rmergea (%) | 5.8 (40.3) | 6.9 (68.0) |
| Redundancy | 7.5 (6.9) | 3.8 (3.6) |
| Refinement | ||
| Resolution range (Å) | 31.30–2.19 (2.29–2.18) | 43.38–1.58 (1.66–1.58) |
| Rworkb(%)/Rfree (%) | 20.44/24.54 | 20.30/21.30 |
| Root mean square deviation | ||
| Bonds (Å) | 0.012 | 0.004 |
| Angles (°) | 1.193 | 0.785 |
| Mean B-factor (Å2) | 49.71 | 44.72 |
| Protein atoms | 1,571 | 1,651 |
| Ramachandran plot (%) | ||
| Favored | 97.8 | 95.3 |
| Allowed | 2.2 | 4.7 |
| Outliers | 0 | 0 |
| PDB | 5IO8 | 5IHF |
- Note: The values in parentheses correspond to the values in the highest resolution shell.
- Abbreviation: ASU, asymmetric unit; PDB, protein data bank.
- a Rmerge = ∑hkl∑i│Ii(hkl) − <I(hkl)>│/∑hkl∑iIi(hkl), where Ii(hkl) is the intensity of the ith observation of reflection hkl and <I(hkl)> is the average intensity of the i observations.
- b Rwork = ∑hkl|Fo(hkl) − Fc(hkl)|/∑hkl|Fo(hkl)|, where Fo and Fc are observed and calculated structure factors, respectively.
2.2 STV is a member of LTxxQ motif family
STV protein has shown a very low amino acid sequence identity with available crystal structures in PDB, therefore, distance matrix alignment server search for structural similarity yielded similarity with LTxxQ motif-containing proteins.35 LTxxQ motif has been found in periplasmic proteins involved in stress response mechanism. Since STV was also predicted to be related with the survival of pathogens, therefore, we carried out a structural comparison with LTxxQ motif family proteins (Figure 1b). LTxxQ proteins are classified as LTxxQ motif family at InterPro (ID-IPR012899) and Pfam (ID-PF07813) database. In STV, LTxxQ motif has LTNDQ amino acid sequence (Figure 1c). The sequence similarity among members of this family is very low, however; structural similarity of STV with CpxP is closer than spheroplast protein Y (SPY) (Figure 1d). Cα RMSD of STV alignment to CpxP is 2.6 Å while to SPY is 2.7 Å.36 Taken together, structural analysis shows that LTxxQ motif family proteins, including STV, are stress response proteins that are secreted in the periplasm.
2.3 STV forms dimer of interconnected helices
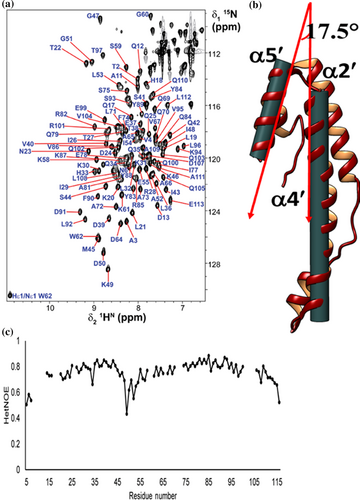
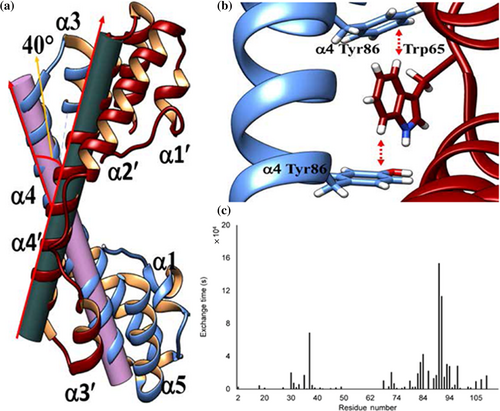
Solution-state NMR spectroscopy confirms the integrity of secondary structural elements in solution (Figure 2a). The majority of the protein molecule was rigid, barring a flexible loop connecting α1 helix to α2 helix, as evidenced by low values of 15N-{1H}-nOes (Figure 2c). Two STV monomers run antiparallel to each other forms a shape of “handshake” or “hand cup” with α4 and α4′ helices come close and many residues are involved in interaction. The oligomeric state of STV was characterized by SEC and solution-state NMR spectroscopy (Figure 2). The Stokes radius calculated by SEC was 2.21 nm which estimated the molecular weight of eluted fraction was to be 26 kDa that corresponds to the theoretical molecular weight of two monomers of STV (Figure S1C). The two monomers axis cross each other at an angle of 40° forming a concave surface at one side and a convex surface on another side (Figure 3a). The electrostatic surface view revealed negative patch at the edge and positive patch in the center of concave surface while the convex surface was largely hydrophobic. The STV dimer was stabilized by strong intermolecular interaction through one salt bridge and two π–π stacking. The salt bridge is formed between Glu81 and Arg85 in helix α4. Dimer is further stabilized by hydrogen bond interaction between Asp67 and Tyr86. The π–π stacking occurs between Trp65 of one chain is sandwiched between Tyr86 and Phe93 of another chain. Trp65 is making T-stacking, which is considered one of the strongest π–π interactions, with other two residues (Figure 3b). Many other van der Waals interactions between the two monomers provide additional stability. The major residues involved in van der Waals interactions are Arg85, Tyr86, Val89, Lys90, Phe93 of A chain to Val59, Lys61, Gly63, Trp65, and Asp67. Moreover, protein interfaces, surfaces and assemblies analysis (interface area) and H-D exchange studies by NMR showed that the C-terminal helix of STV was involved in dimer formation and hence, less solvent-exposed region of the protein (Figure 3c).
2.4 Role of STV in bacterial survival in macrophages through SPI-2/SPI-6 coregulation
Macrophages are used as a vehicle for systemic dissemination of Salmonella and cellular proliferation. To investigate the role of stv within phagocytic cells, murine macrophages, that is, RAW264.7 were infected with wild-type and mutant strain (STM ∆stv) at multiplicity of infection (MOI) 10. The uptake percentage of ∆stv was significantly higher as compared to wild type (WT) (Figure 4a). However, the survival of the mutant strain was marginally less relative to wild-type (Figure 4b). Confocal images of ∆stv strain bearing green fluorescent protein-expressing plasmid validated its higher uptake and marginally reduced survival relative to WT within macrophages (Figure 4c,d). Further to understand the role of stv in pathogen survival, the murine macrophages were infected with WT to determine the time-dependent expression profile of STV in vitro. Confocal imaging demonstrated STV expression to be slightly induced at 12 hr and majorly at 24 hr postinfection in macrophages. The 48 hr post infection stage did not show any signs of STV expression (Figure 4e). The macrophages infected with stv mutants were taken as negative control (Figure S2). It was observed that the intracellular macrophage environment induced the expression of STV in a time-dependent manner which possibly correlates with the low survivability of STM ∆stv strains within murine cells. These data is in line with the study where the SPI-6 genes were known to play interactive roles within macrophages and transcriptional profiling had shown these genes of S. Typhimurium to be induced at later stages of infection.37 Similarly, quantitative real time polymerase chain reaction (qRT-PCR) analysis also showed stv to be two-fold upregulated under conditions similar to the acidic compartment of macrophages (Figure S3). As a reference gene, gmk was used after ensuring that its level did not fluctuate under the altered pH conditions.
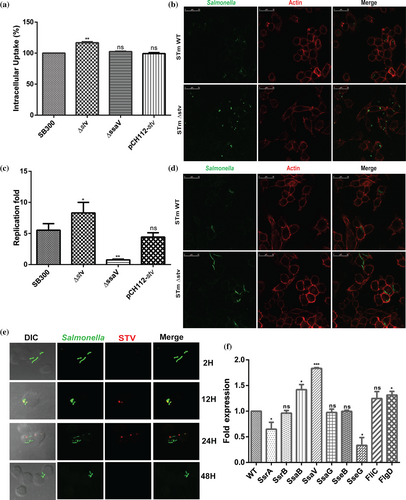
SPI-2 effectors are triggered during bacterial internalization by macrophages to facilitate Salmonella replication in its intracellular environment. Hence, SPI-2 gene expression profile was determined in the stv mutant to corroborate its reduced survival ratios in macrophages. STM ∆stv displayed approximately ⁓1.5-fold reduced expression of SPI-2 effectors (sseB, sseG, sseJ) relative to WT (Figure 4f). Additionally, the regulator ssrA showed significant 1.4-fold downregulation with stv deletion contributing to the replication fold differences between WT and ∆stv strains. As mentioned earlier, SPI-6–encoded T6SS genes are also involved in infection persistence within macrophages and mice by being induced at later stages of infection as stated before. Therefore, their contribution for stv mutant survival within murine cells was further examined. The qRT-PCR analysis showed that the core component clpV displayed sixfold reduced expression in stv mutant relative to WT (Figure 4f). Similarly, the Type VI secreted proteins like hcp and vgrG displayed threefold and fivefold downregulation in ∆stv as compared to WT (Figure 4f). This might reflect the possible regulatory influence of STV on the expression of T6SS components. With reduced expression of hcp and vgrG, the functional T6SS gets impaired thereby hampering the possible survival of ∆stv strains inside macrophages marginally.
3 DISCUSSION
Nontyphoidal Salmonella infections pose a global threat and it is estimated that up to 129.5 million cases are reported each year.37 S. Typhimurium is an intracellular pathogen with adaptive abilities to invade, colonize, and survive within the host. Its genome comprises of essential virulence determinants responsible for Salmonella pathogenesis. Hence the identification and characterization of key bacterial proteins can enable a better understanding of their entire pathogenic cycle and aid in developing new insights into antibacterial strategies. This study reports the structural and functional role of STV, an uncharacterized noncore protein of SPI-6. STV is present near the T6SS coding genes in SPI-6. SPI-6 varies in length (~35–65 Kb) in different Salmonella spp. and it is acquired through horizontal gene transfer during the course of evolution. A single copy of the stv gene is present in all Salmonella genomes even in serovars lacking a functional T6SS (Salmonella paratyphi B) or in serovars having two sets of T6SS genes (S. enterica serovar Weltevreden).
Structural analysis of the STV protein revealed that it share structural similarity with LTxxQ motif-containing proteins CpxP and Spy from E. coli.38, 39 However, both homologous proteins are also present in the Salmonella genome but STV is additional in Salmonella, which makes it an interesting candidate for further investigation. Despite the shared common protein motif, the structure could not be solved by molecular replacement, hence it was solved by the Iodine-SAD method. The crystal structure of native truncated S. Typhimurium STV, determined to 1.58 Å resolution, established it to form a dimer of interconnected helices, where each monomer comprised of five helices with two LTxxQ motifs forming a structural entity. It has been described previously that the LTxxQ motif-containing proteins are dimeric and contribute to the survival of pathogens by participating in stress response modulation.40 In agreement with earlier reports, LTxxQ motif-containing STV is a dimer and also expressed in the periplasm. Hence, based on structural similarities, it may deduce that STV is likely to play an important role in the survival of pathogens by stress-response adaptation processes. Furthermore, the overall structure of STV shares common structural features and identical core folds with CpxP and Spy with certain differences in the N-terminal region, where STV has a short helix and the other two homologs have an unstructured N-terminal part. The structural alignment of STV monomer showed RMSD of 2.6 Å and 2.7 Å with CpxP (PDB id 3ITF) (over 93 aligned pairs) and Spy (PDB id 3OEO) (over 87 aligned pairs) monomers, respectively. CpxP, Spy, and STV did not show any notable resemblance or differences in their electrostatic comparison. It is interesting to note here that structural homologous LTxxQ family proteins are commonly involved in stress response mechanism but varying functional characteristics such as two LTxxQ motif-containing CpxP and Spy assist in protein folding, while a single LTxxQ motif-containing ZraP contribute in zinc-related stress response.40 Therefore, STV may also contribute to stress response modulation for the survival of the pathogen in macrophages infected with Salmonella. In addition to CpxP and Spy encoding genes, stv is also present in Salmonella genome which may provide additional support during the remarkably complex pathogenesis of Salmonella infection. Despite being structural similarities among LTxxQ motif-containing proteins, low sequence similarities have been observed among these proteins and it may indicate that LTxxQ motif-containing proteins are involved in stress response by various mode of action. On comparison of the crystal structure of STV with homologous proteins, the hydrophobic surfaces on the edges of their respective convex sides were conserved sequentially as well as structurally. The residues involved in hydrophobicity are L35, V98, L99 in STV, I68, I129, L130 in Spy, and L62, L123, L124 in CpxP. The third participating leucine residue from each protein is a part of the C-terminal LTxxQ motif. The conserved leucine residue of the LTxxQ motif is essential for motif divergence.40 Hence any mutational changes in this motif or nearby residues may lead to functional abnormalities.38, 40
Structural homologs of STV, CpxP, and Spy show elevated expression in response to stress signals.40 Additionally, SPI-6 genes are crucial factors for pathogen persistence within macrophages. On the basis of our structural analysis, we attempted to investigate role of STV in survival of bacterial pathogen in macrophages. To this effect, our study established STV (the noncore SPI-6 protein) in modulating bacterial survival within murine macrophages, RAW264.7. The STV mutant displayed reduced replication ability relative to WT within murine cells. SPI-2 genes are recognized factors for intracellular survival and pathogenesis.41 Hence the low transcripts of SPI-2 effectors (sseB, sseG) in STM ∆stv provides a clear picture of the impairment of T3SS-29, 42, 44, 45, 43 which probably led to its decreased bacterial counts in murine macrophages relative to WT. Additionally an upregulation of hilA may further contribute to the repression of SPI-2 genes along with repression of ssrA/B transcripts that directly influences the expression of SPI-2 effectors (sseB/sseG) in STV mutant strains.9 SPI-6–encoded T6SS genes have been shown to play a crucial role in Salmonella enteric infection.46 A study reported that the deletion in ClpV homolog and Rhs element caused 30% reduction in intracellular replication inside macrophages.47 The needle complex of T6SS is composed of Hcp (hemolysin-coregulated protein) together with VgrG (valine glycine repeat). Where Hcp forms a channel-like structure for the translocation of T6SS effector, VgrG acts as the cell-puncturing tip.23 The conserved genes for ATPase activity of T6SS are ClpV and IcmF which are essential for Hcp and VgrG secretion.34 These genes are known to have induced expression within the macrophage environment which further helps in the persistence of Salmonella infection.34 However, the SPI-6 T6SS genes (hcp, vgrG, clpV) displayed decreased folds in ∆stv strains relative to WT which supposedly attributed to its decreased survival percentage within murine cells. Since SPI-2 and SPI-6 form complex network of regulatory virulence factors responsible for pathogenesis, survival, and colonization. In our studies, it is observed that expression of stv alters expression of many genes associated with SPI-2 and SPI-6. At a molecular level, it is accomplished by an intricate protein–protein interactions network where CpxP, a close structural homolog of STV, interacts with various proteins involved in stress response.40 Based on gene expression data, STV, being structural homolog of CpxP, possibly regulates stress response by protein–protein interactions. Moreover, our study showed STV protein induction under macrophage inducing conditions during the systemic phase. Because SPI-6 is majorly required for infection sustenance, STV can play a role in adapting to the host environment and modulating bacterial survival within macrophages. Altogether, in agreement with previous literature, our structural studies, and microbiological assays, we believe that this hypothetical noncore protein of SPI-6, STV plays an essential role in Salmonella pathogenesis by assisting survival of the pathogen within macrophages.
4 MATERIALS AND METHODS
4.1 Bacterial strains, plasmids, and growth conditions
The streptomycin-resistant S. Typhimurium SB300 strain63 was used as wild-type strain in the present study. The bacterial strains and plasmids used in this study are listed in Table 2. Wild-type S. Typhimurium and its mutants were grown in Luria-Bertani (LB) medium (10 g/L tryptone, 5 g/L yeast-extract, 5 g/L NaCl; HiMedia, Mumbai, India), at 37°C, 150 rpm. Antibiotics in growth media were used at the following concentrations: ampicillin (Amp), 100 μg/mL; kanamycin (Km), 50 μg/mL; chloramphenicol (Cm), 20 μg/mL; and streptomycin (Sm), 50 μg/mL.
| Description | References | |
|---|---|---|
| Strains | ||
| SB300 | Salmonella enterica serovar Typhimurium SB300 | 63 |
| ∆ stv | SB300 stv::Km (mutant strain) | This study |
| pCH112-stv | pCH112-stv in SB300 stv::Km (complemented strain) | This study |
| ∆ SsaV | S. Typhimurium ΔssaV; Smr | 62 |
| DH5α | Escherichia coli | |
| Codon plus | Escherichia coli (DE3) codon plus | |
| Plasmids | ||
| pKD4 | Template plasmid; FRT-aphT-FRT (containing kanamycin resistance gene) Bla FRT aph FRT PS1 PS2 oriR6K |
62 |
| pKD46 | Red recombinase expression plasmid Bla PBAD gam bet exo pSC101 oriTS |
62 |
| pCH112 | hilA ORF cloned into PBAD/myc-his; oripBR322 | 62 |
| pCH112-stv | stv expressing vector; stv cloned with its 1,000 bp upstream region by replacing hilA in oripBR322 | This study |
| pCJLA-GFP | GFP-plasmid used to tag strains | 62 |
| pET28a | Expression vector with N-terminal his tag for cloning stv without its signal peptide sequence | |
The pH-dependent experiments for S. Typhimurium were performed in M9 minimal media containing magnesium sulfate, calcium chloride, and 0.2% of 5× M9 salt comprising of disodium hydrogen phosphate heptahydrate (64 g), potassium dihydrogen phosphate (15 g), sodium chloride (2.5 g), ammonium chloride (5 g) in IL of Milli-Q water. This media required glucose and casein hydrolysate supplementation (0.22 μm filter sterilized) at a concentration of 0.1 and 0.4%, respectively, for S. Typhimurium. Balanced growth conditions were maintained by proper aeration and temperature conditions, 150 rpm at 37°C after being subcultured (1:100 ratio) from overnight grown cultures.
4.2 Cloning, expression, and purification
The mature form of STV encoding sequence of stv gene was PCR amplified from S. Typhimurium chromosomal DNA using the designed primers mentioned in Table S1. The pET28a expression vector and insert were cleanly double digested with NdeI and XhoI restriction enzymes followed by ligation. The ligation mixture was transformed in E. coli DH5α. Recombinant stv construct was then transformed in expression strain E. coli BL21 (DE3) CodonPlus. A single colony grown overnight at 37°C was subcultured into 2 L of LB medium till the log phase (A600 ~ 0.7), where the bacterial cells were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubated at 18°C for 12 hr. The cells were harvested by centrifugation at 5000g for 20 min.
The harvested cells were resuspended in lysis buffer (50 mM phosphate buffer, pH 8.0, with 500 mM NaCl and 10 mM imidazole) containing complete protease inhibitor cocktail tablet (Roche). Cells were lysed by sonication. The lysate was centrifuged at 20,000g for 60 min to remove cell debris. Lysate was loaded to Ni-NTA agarose resin (GE Healthcare). After binding, resin was washed with lysis buffer followed by protein elution with increasing concentration of imidazole in step gradient (50, 100, 250, and 500 mM). Obtained fractions were run on a 15% sodium dodecyl sulphate–poly acrylamide gel electrophoresis to analyze the purity of protein. Fractions containing pure STV were pooled and concentrated and buffer exchanged in phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4 pH 7.4) using 10 kDa molecular weight cut-off (MWCO) centrifugal filters (Millipore) for thrombin digestion. Concentrated protein was then incubated with thrombin for 16 hr at 22°C to cleave the 6X his-tag. STV was further purified using a Hiload 16/60 Superdex 75 prep grade column (GE Healthcare) in size exclusion buffer (20 mM Tris-Cl, pH 8.0 and 20 mM NaCl) to remove thrombin and to estimate stoichiometry. Pure fractions were pooled and concentrated to ~40 mg/mL in buffer containing 20 mM Tris-Cl, pH 8.0 and 20 mM NaCl for crystallization experiments.
4.3 Crystallization, data collection, and processing
Diffraction quality crystals were obtained in the SaltRx screen (Hampton Research) in a solution containing 1.8 M sodium phosphate monobasic monohydrate and potassium phosphate dibasic, pH 5 at 291 K. For experimental phasing, crystal was soaked in 0.1 M NaI for 5 min before flash-freezing. A single crystal was flash-frozen and used for data collection at BM14 beamline at ESRF, Grenoble, France. The dataset was processed and scaled in the space group P21212 using the HKL2000 package.48
4.4 Structure determination and refinement
The structure of STV was solved by the SAD method using the iodine derivative data sets processed to 2.19 Å resolution. Twenty iodine atoms in the asymmetric unit were predicted in Hybrid Substructure Search and used to obtain initial phases in Phaser-EP.49 Initial phases were density modified by RESOLVE and automatic model building was performed by the program AUTOBUILD.50, 51 The partial model obtained from automatic building were manually rebuilt in COOT using both 2Fo-Fc and Fo-Fc (where Fo and Fc are observed and calculated structure factor amplitudes, respectively).52 The starting model was completed by iterative rounds of model building and refinement using Phenix.refine.53 Nine translation-libration-screw (TLS) group was defined by automatic find TLS group in phenix.refine and these were used for refinement in the later stages.54 As STV is a putative dimer and two molecules were present in asymmetric unit noncrystallographic symmetry (NCS) was applied in later stage of refinement. The seven N-terminal residues of chain A and chain B were not observed in the electron density map. The Rwork and Rfree of the final model were 20.4 and 24.5%, respectively, for SAD data while for native data, the Rwork and Rfree of the final model were observed as 20.3 and 21.3%, respectively. Analysis of the final model with PROCHECK revealed all the residues are in favored and allowed region of the Ramachandran plot.55, 56 Figures were generated using CHIMERA and PyMol.57, 58
4.5 Solution-state NMR spectroscopy
Spectra were acquired on 500 MHz Bruker Avance III spectrometers equipped with a cryogenic triple-resonance triple-resonance cryogenic inverse probe. For resonance assignments, a set of standard double and triple-resonance spectra, including59 2D [15N,1H]-HSQC, 3D HNCA, 3D CBCAcoNH, 3D HNCACB, 3D HNCO, 3D hCccoNH, 3D HcccoNH and 2D [13C,1H]-HSQCs (aliphatic [−5 to 85 ppm 13Cali] and aromatic [110–138 ppm 13Caro]), were measured at 313 K. Steady-state 15N-{1H}-nOes were measured with a saturation period of 4 s.60 Topspin 2.1 and 3.5 (Bruker AG) software was used for acquisition, Fourier transformation, and processing of time-domain data. Backbone and side-chain resonances were assigned manually using Computer Aided Resonance Assignment (CARA) software61 with 1H, 13C, and 15N shifts referenced indirectly to the sodium trimethylsilylpropanesulfonate methyl proton resonance at 0 ppm in all spectra.
4.6 Mutant construction and plasmid-based complementation
Genomic deletion of stv gene (SL1344_0303) was done by using the basic one-step inactivation protocol of lambda-red recombinase system62 with pKD4 as template plasmid. The primers for PCR amplification of Tn5 neomycin phosphotransferase gene in pKD4 template plasmid conferring kanamycin resistance are listed in Table S1. The deletion of the required gene was done without affecting the coding sequences of the upstream or downstream genes. Gene knockout was confirmed by PCR using kanamycin internal primer (ConfKt) and upstream gene-specific primers listed in Table S1.
The stv gene was complemented by using a modified form of pCH112 plasmid; with pBAD backbone, arabinose inducer and pre-cloned segment of hilA with NcoI and XbaI end cut sites.63 The stv gene along with its 1,000-bp upstream sequence was PCR amplified using primers listed in Table S1. The resulting amplicon was digested with restriction enzymes NcoI and XbaI to replace the hilA gene with stv cassette along with the 1,000 bp upstream promoter region. This cloned construct was transformed in ΔSTV (STM_STV::aphT) strain. The inherent pBAD plasmid promoter remained inactive without arabinose; however, the gene's expression could be controlled under its native cloned promoter.
4.7 Uptake and intracellular survival assay
Intracellular survival assay was performed in mouse macrophage cell line RAW264.7 as described previously64, 65 which was cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. 2 × 105 cells/well were seeded on a 24-well plate and grown for 16–18 h. The seeded macrophage cells were infected with overnight grown cultures of SB300 WT, STM ∆stv, and complemented (pCH112-STV in STM ∆stv) strains at an MOI 10. The uptake and intracellular survival assay were determined by the lysis of the infected macrophage cells at varied time-points. For uptake assay, after an infection time-point of 50 min, gentamicin-supplemented DMEM (100 μg/mL) was added as mentioned before and incubated for 2 h postinfection at 37°C, 5% CO2. The lysis of the infected macrophages by 0.1% Triton X-100 in 2 h time-point followed by serial dilution plating denotes the uptake percent of the bacterial cells. With similar 50 min of infection procedures, the lysis of the infected macrophages at 24 h time-point will determine the bacterial survival within the phagocytic cells. The intracellular survival was expressed as fold increase in the number of bacteria (colony forming unit) obtained at 24 versus 2 h. These experiments were conducted thrice in biological triplicates.
4.8 Confocal immunofluorescence microscopy
For confocal imaging of internalized bacteria within phagocytic and nonphagocytic cells, both murine macrophages (RAW264.7) was seeded on sterile coverslips on a 24-well plate and kept for 16 h at 37°C, 5% CO2. The cells were infected with S. Typhimurium WT and ∆stv strains as mentioned above with MOI 100 for microscopy. Following the 2 h gentamicin treatment after infection, the cells were fixed with 4% PFA (v/v) in paraformaldehyde (PBS), pH 7.4 for 15 min, washed (1× PBS), and then permeabilized with 0.1% Triton X-100 (v/v) containing 1% bovine serum albumin (BSA) (w/v) in PBS for 5 min, followed by 20 min incubation with Alexa546 Phalloidin (3.5 μg/mL, Invitrogen) for actin cytoskeletal staining. For visualization, the bacteria are GFP tagged with pCJLA plasmid. The coverslips were mounted onto glass slides with Antifade reagent (Invitrogen Molecular Probes). Confocal microscopy and image acquisition were carried out with Leica TCSSP5 inverted microscope using a 63× oil immersion objective, Central Instrumentation Facility, Institute of Life Sciences, Odisha. Similar procedures were followed for the imaging of the engulfed and surviving bacteria within RAW264.7 at 2 and 24 h time-point, respectively, for WT and mutant strains.
For expression studies, the murine macrophages RAW264.7 were infected with S. Typhimurium WT strain to monitor the expression of Salmonella VirG-like protein (STV) under a stretch of different time-points. The MOI was kept as 10 and infection time as 50 min. After gentamicin treatment, that is, within 1 hr of postinfection, the cells were washed and incubated with lower gentamicin (10 μg/mL) supplemented medium for longer incubation periods. At the respective time-points, the cells were extensively washed with PBS, fixed with 4% PFA for 15 min, and permeabilized in 0.1% Triton X-100 (v/v) for 15 min. Blocking agent used was 3% BSA (w/v) and 0.5% Tween20 (v/v) in 1× PBS. The coverslips were washed (2× PBS) and incubated with primary antibodies for 1 hr, followed by washing (3× PBS) and incubation with secondary antibodies for 1 hr. The primary antibodies used in the study are anti-STV (AbgeneX Pvt. Ltd, India). S. Typhimurium strains were GFP tagged. Antibody dilutions of STV 1:250 was used in blocking solution as per the supplier's protocol. The coverslips were washed thoroughly and mounted onto glass slides with Antifade reagent (Invitrogen Molecular Probes). Image acquisition from the set of stained cells was done with a Nikon A1R laser scanning confocal microscope equipped with a 60X/1.4NA Plan Apochromat DIC objective. Images were acquired using the software EZ-C1 3.80 and NIS-Elements. Image analysis and processing were done using the software Imaris version 7.2 (BitPlane) and further imported and amassed in Adobe Illustrator.
4.9 Quantitative real-time PCR assays
RNA was extracted from both S. Typhimurium wild-type and mutant cultures by Trizol Reagent (Ambion, USA) to check the comparative profile of effectors in vitro in both the strains. For the study of conditional expression, stv transcripts in media were checked under various osmolarity, pH, and temperature profiles, from which RNA extraction was carried out. The pH-dependent experiments for S. Typhimurium were performed in M9 minimal media with glucose and casein hydrolysate supplementation (0.22 μm filter sterilized) at a concentration of 0.1 and 0.4%, respectively for S. Typhimurium as mentioned earlier. The cDNA synthesis was done using Revert-aid cDNA Synthesis Kit (Thermoscientific, India), where the real time enhancer prevents the additional DNase treatment in the cDNA reaction mixture. qPCR was performed in triplicate using Kapa Sybr Fast qPCR Master Mix (2×) (KAPA Biosystems, USA) with normalized cDNA templates. The 16s rRNA gene was used as an internal control. For SPI-2 gene expression, acidic minimal media (pH 5.4) with 0.1% casamino acids was used. Successive RNA extraction and cDNA synthesis followed by qRT-PCR were performed. The mean fold change in the transcript levels were in biological triplicates of three independent experiments.
4.10 Statistical analysis
All data set represents independent triplicate experiments with mean ± standard deviation. One-way analysis of variance (ANOVA) and t tests were used to analyze significant differences during treatments. GraphPad Prism version 6.066 was used for all analyses.
ACKNOWLEDGEMENTS
This work was supported by the research grant from KIIT, Bhubaneswar and ICGEB, New Delhi core funds. We thank Department of Biotechnology, Government of India for access to the synchrotron X-ray beam line (BM14) of the European Synchrotron Radiation Facility at Grenoble, France, in partnership with EMBL and ESRF and for providing financial support for the High Field NMR spectrometers at the ICGEB, New Delhi and NII, New Delhi. We are also grateful to Mr. Bhabani Sankar Sahoo, Confocal In-charge, Institute of Life Sciences, Bhubaneswar, and Ms. Purnima Kumar, Confocal Instrumentation facility, ICGEB, New Delhi, for their valuable help and support. We acknowledge infrastructure support available through DBT-BUILDER program (BT/INF/22/SP42155/2021) at KIIT, Bhubaneswar. N.K.P. and N.V. were supported by Council of Scientific and Industrial Research (CSIR) Junior Research Fellowship (JRF) and Senior Research Fellowship (SRF). G.S.K. was supported by grant no. BT/IC-06/003/91, Flagship proposal, from the Department of Biotechnology, Government of India.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Shilpa Ray: Data curation (supporting); formal analysis (equal); investigation (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Nishant Kumar Pandey: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal). Gajraj Singh Kushwaha: Data curation (supporting); formal analysis (supporting); software (supporting); validation (supporting); writing – review and editing (supporting). Susmita Das: Investigation (supporting); resources (supporting); visualization (supporting). Akshay Kumar Ganguly: Data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); software (equal); validation (supporting); visualization (supporting). Nimi Vashi: Formal analysis (supporting); investigation (supporting); methodology (supporting); validation (supporting); writing – original draft (supporting). Dhiraj Kumar: Data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); resources (supporting); supervision (supporting); visualization (supporting); writing – original draft (supporting). Mrutyunjay Suar: Conceptualization (lead); data curation (equal); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (equal); project administration (lead); resources (lead); supervision (lead); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (lead). Neel Sarovar Bhavesh: Conceptualization (lead); data curation (lead); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (lead); project administration (lead); resources (lead); software (supporting); supervision (lead); validation (equal); visualization (supporting); writing – original draft (equal); writing – review and editing (lead).




