Phylogenetic convergence of phase separation and mitotic function in the disordered protein BuGZ
Funding information: National Institutes of Health, Grant/Award Numbers: 2R01GM063747, R01GM126130
Abstract
Intrinsically disordered proteins (IDPs) effect biological function despite their sequence-encoded lack of preference for stable three-dimensional structure. Among their many functions, IDPs form membraneless cellular compartments through liquid–liquid phase separation (LLPS), also termed biomolecular condensation. The extent to which LLPS has been evolutionarily selected remains largely unknown, as the complexities of IDP evolution hamper progress. Unlike structured proteins, rapid sequence divergence typical of IDPs confounds inference of their biophysical or biological functions from comparative sequence analyses. Here, we leverage mitosis as a universal eukaryotic feature to interrogate condensate evolutionary history. We observe that evolution has conserved the ability for six homologs of the mitotic IDP BuGZ to undergo LLPS and to serve the same mitotic function, despite low sequence conservation. We also observe that cellular context may tune LLPS. The phylogenetic correlation of LLPS and mitotic function in one protein raises the possibility of an ancient evolutionary interplay between LLPS and biological function, dating back at least 1.6 billion years to the last common ancestor of plants and animals.
1 INTRODUCTION
Although disordered protein regions, defined by their lack of a fixed three-dimensional structure, have been long known to imbue proteins with biological function, the last decade has revealed that the functional palette of intrinsically disordered proteins (IDPs) is tremendously richer than initially appreciated.1-3 Among the most surprising novel roles includes participation in an emerging cellular organization paradigm that invokes IDPs as key components of biomolecular condensates, which primarily refers to subcellular liquid–liquid phase separation (LLPS), wherein ribonucleoprotein droplets can spatially and temporally organize the cytoplasm and nucleoplasm.4 Despite the rapidly growing biophysical and cell biological understanding of IDP LLPS, evolutionary understanding of IDP LLPS lags far behind. In contrast, studies of structured proteins enjoy a molecular evolutionary experimental and theoretical synthesis that enables sophisticated structure-based insight and design.5, 6 Nonetheless, condensate evolution has been recognized as a critically understudied topic where insight will be vital to driving advances in condensate biology and engineering.7
Few studies have experimentally examined either the relationship between IDP evolution and biological function, or IDP evolution and LLPS.8-12 As a result, foundational condensate evolutionary relationships have been posited but have yet to be demonstrated. For example, phylogenetic connections between condensate-forming proteins or protein families in fungal and metazoan clades suggest shared evolutionary origins, but these observations have yet to be functionally tested in vivo.13 Mounting appreciation of functional LLPS in plants has motivated hypotheses of LLPS as an ancient and conserved trait, but to date lacks phylogenetic investigation.14 Theoretical identification of selective signatures in disordered regions of condensate-forming proteins, as well as in IDPs in general, have been offered but yet to be experimentally verified.15-17 To our knowledge, no studies have experimentally and systematically linked natural IDP evolution, LLPS, and biological function in a living model. One barrier to such study is the relative difficulty of identifying a group of putative IDP homologs for interrogation, as high IDP primary sequence divergence rates impede de-novo sequence alignment.18
We reasoned that studying an IDP intimately involved in a common process conserved from primitive eukaryotes could facilitate comparison. Mitosis is one such universal process, fundamental to cellular division and proliferation of all extant nucleated cells. As such, we have chosen the disordered protein BuGZ as a vehicle to interrogate the conservation of biomolecular phase separation and biological function. The BuGZ protein promotes mitotic spindle assembly. Prior work has found that Xenopus laevis BuGZ condensates drip onto the surface of and coat microtubules, coinciding with the protein's ability to undergo LLPS.19 These experiments also showed that the disordered domain of the protein is necessary for LLPS, and that LLPS is necessary for mitotic function.
The mitotic function of BuGZ further requires two additional features: a microtubule-binding N-terminal C2-H2 zinc finger domain, and a 17-residue helix–loop–helix Gle2-binding-sequence (GLEBS) motif to bind and stabilize a partner protein called Bub3.20, 21 Together these features allow BuGZ to establish a localized microtubule-organizing environment to drive microtubule–kinetochore interactions, ultimately promoting proper chromosome alignment and mitotic progression. Interestingly, the BuGZ disordered domain displays a degree of multifunctionality, in that chimeric fusion to other proteins that drive LLPS in the nucleus can recapitulate their functions, including a BuGZ binding partner that also condenses on microtubules to accelerate spindle nucleation.22-25 Experimental chimeras represent one extreme of apparent allowable sequence substitution. To what extent has nature explored the phase separating and functional sequence variation of the BuGZ disordered domain?
In this work, we deploy a comparative biophysical and cell biological experimental approach to ask first, whether the ability of the disordered protein BuGZ to phase separate into a biomolecular condensate is conserved across natural homologs in vitro, and second, whether the mitotic function of BuGZ is conserved across natural homologs in an in vivo cell culture system. Our findings reveal a phylogenetic correlation between phase separation capacity and mitotic functional capacity. Unexpectedly, we also observe that the material properties of phase-separated BuGZ may be tuned by cellular context.
2 RESULTS
Mitosis is common to all eukaryotes. Therefore, we searched for candidate BuGZ homologs in a broad scope of eukaryotic model organisms which together shared a last common ancestor 1.6 billion years ago.26 These organisms included X. laevis (African clawed frog), Caenorhabditis elegans (nematode worm), Homo sapiens (human), Mus musculus (house mouse), Drosophila melanogaster (fruit fly), and Arabidopsis thaliana (thale cress). We identified specific BuGZ/ZNF207/Suf4 (all referred to in this study as BuGZ) isoforms linked by orthologous features: first, a shared domain organization of the short N-terminal zinc finger and a long C-terminal intrinsically disordered domain (Figure 1a,b). Second, a conserved and Bub3-binding-competent GLEBS motif punctuating the disordered domain (Table S1, Figure S1). The primary sequences of the structured zinc finger domains are well conserved (77% mean identity) and therefore likely homologous.
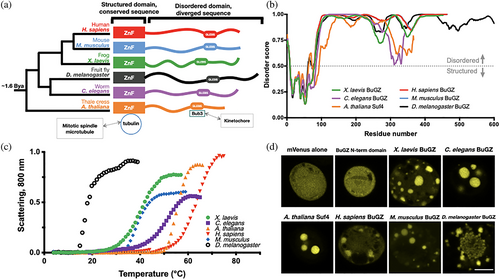
In contrast, pairwise sequence identity between aligned C-terminal disordered domains revealed low primary sequence conservation, (29% mean identity), similar to pairwise identity between BuGZ disordered domains of identical composition but randomly permuted in place (23% mean identity) (Figure S2, Table S2). Because aromatic amino acids are found in each homolog's disordered domain, and aromatic-to-serine point mutations have previously been shown to inhibit BuGZ LLPS and mitotic spindle assembly, we asked whether the disordered domain contained conserved phenylalanines, tyrosines, or tryptophans.19 Though we observed that the BuGZ homologs shared similar proportions of proline and glycine enrichment, we noted considerable variation between homologs in aromatic residue quantities, positions, and spacing as well as differences in physicochemical linear patterning, overall suggesting lack of conservation of simple linear features (Figures S3–S6).
Rapid primary sequence evolution in disordered regions is expected.18, 27 Nonetheless, stabilizing selection may maintain an underlying, cryptic, functional homology between BuGZ disordered domains, despite their sequence divergence. We reasoned that may be the case given the common BuGZ domain organization, conserved GLEBS-Bub3 binding interaction, and known functional importance of the disordered domain's LLPS.19 We, therefore, asked whether all six extant BuGZ homologs first, share an ability to phase separate in vitro, and second, share an ability to recapitulate each other's mitotic function in vivo.
We interrogated the relative ability of the BuGZ homologs to form phase-separated condensates in vitro by expressing and purifying BuGZ proteins from Sf9 insect cells. Purified, dilute (10 μM) BuGZ homologs underwent temperature-sensitive phase separation requiring the C-terminal disordered domain, as observed by changes in scattering caused by micrometer-scale solution inhomogeneity (Figure 1c, Figure S7). mVenus-labeled BuGZ fusion homologs formed visible, micrometer-scale condensates in Sf9 cells following baculovirus-induced protein expression, while the mVenus protein alone or the N-terminal zinc finger lacking the disordered domain did not (Figure 1d).
We further probed the material state of the Sf9 condensates, beginning with fluorescence recovery after photobleaching (FRAP), in which the fast internal molecular rearrangements corresponding to a liquid droplet can be revealed by quick FRAP recovery (Figure 2a,b). BuGZ homologs except the D. melanogaster BuGZ all displayed robust and rapid FRAP recovery, consistent with an LLPS process forming a droplet biomolecular condensate. The D. melanogaster BuGZ FRAP recovery to approximately 20% of its original intensity was diagnostic of a significant immobile fraction, suggesting instead a partially solidified, or gel-like, material.
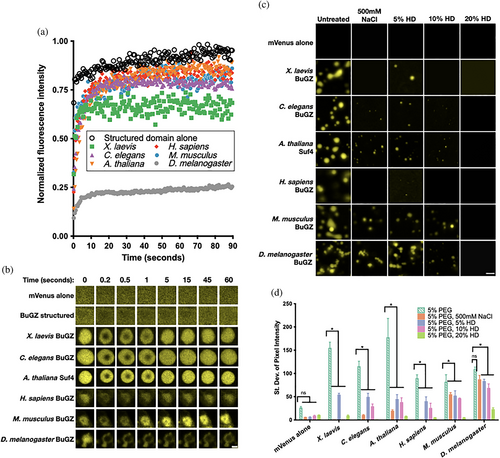
In vitro microscopy to directly visualize micrometer-scale assemblies of purified mVenus-BuGZ corroborated the FRAP measurements, and overall suggested that X. laevis, C. elegans, A. thaliana, H. sapiens, and M. musculus BuGZ homologs share the capacity to condense into liquid droplets in cells and in vitro. Phase-separated BuGZ homologs appeared largely spherical, wetting the surface of the slide, as well as merging into larger droplets, as expected of liquids (Figure 2c). However, D. melanogaster BuGZ uniquely formed a complex microarchitecture of polydisperse, linear, branching sphere assemblies. D. melanogaster BuGZ micrometer-scale condensates also resisted dissolution by 500 mM NaCl, which screens electrostatic interactions, or by increasing concentrations of the aliphatic alcohol 1,6-hexanediol, which is thought to disrupt weak hydrophobic interactions28 (Figure 2d). The D. melanogaster BuGZ microarchitecture and reduced environmental sensitivity suggest it may initially phase separate as spherical liquid droplets, then subsequently mature into a solid-like or gel-like state through an arrested Ostwald ripening and fusion process.9, 12, 29-31 Together, these observations suggest that while X. laevis, C. elegans, A. thaliana, H. sapiens, and M. musculus BuGZ homologs readily undergo LLPS, all BuGZ homologs including D. melanogaster BuGZ in fact conserve some underlying capacity to phase separate into liquid-like droplets in vitro.
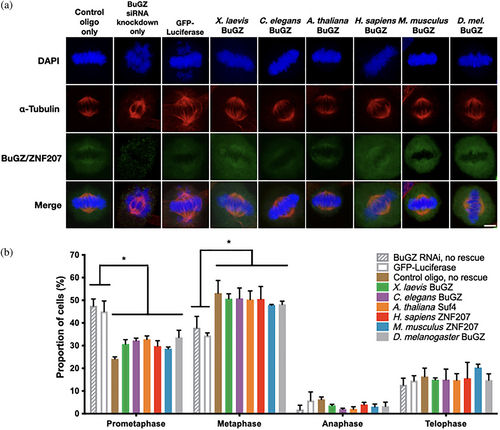
We subsequently investigated whether the mitotic function of BuGZ is conserved by asking whether extant BuGZ homologs could each perform similar mitotic functions in an in vivo cell culture system. To accomplish this, we expressed BuGZ homologs in HeLa cells first treated with BuGZ RNAi that knocked-down endogenous BuGZ. Since BuGZ is known to be necessary for chromosome alignment in mitosis, we observed, as expected, that BuGZ RNAi resulted in an increased percentage of mitotic cells arrested in prometaphase and a reduction in the percentage of cells in metaphase (Figure 3a,b, Figure S8).19-21 This was accompanied by expected chromosome misalignment and BuGZ depletion from the mitotic spindle. We subsequently tested if following RNAi knockdown with transfection of various GFP-fusion BuGZ homolog expression plasmids would rescue mitotic function. Quantification in immunostained cells showed that diverse BuGZ homologs all significantly rescued the mitotic phenotype by restoring progression to metaphase, chromosome alignment, and BuGZ enrichment on the mitotic spindle (Figure 3a,b, Figures S9 and S10). Therefore, diverged BuGZ homologs conserve mitotic functionality in vivo.
Finally, we scrutinized the outlier material behavior of the D. melanogaster BuGZ, which appeared to retain a latent propensity for LLPS. Qualitatively similar clustering morphology was observed between purified D. melanogaster BuGZ and expressed protein in Sf9 cells (Figures 1d, 2c, and 4a). Close inspection of individual in vitro spheres revealed an inhomogeneous, porous microstructure (Figure 4b).
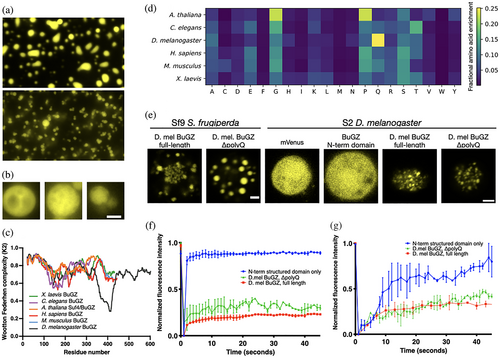
We identified within the D. melanogaster BuGZ disordered domain a unique, 76 residue, low-complexity region containing a polyglutamine tract that distinguished it from the other homologs (Figure 4c). Overall glutamine residue enrichment in the D. melanogaster disordered proteome mirrored this, consistent with prior findings of proteomically enriched D. melanogaster polyglutamine tracts (Figure 4d).32 Deletion of the low-complexity polyglutamine region restored liquid-droplet-like morphology of D. melanogaster BuGZ in Sf9 cells and modestly improved FRAP recovery (Figure 4e). We also observed a limited capability of D. melanogaster S2 cells to modulate full-length D. melanogaster BuGZ FRAP kinetics (Figure 4f,g). While differences in droplet size and relative bleach area could also account for a proportion of the observed kinetic variance, we suggest this modulation implies a degree of cell-type-specific adaptation for homeostasis of polyglutamine-mediated material states.33 The tunable material properties of the D. melanogaster BuGZ reinforce the notion that, like the other BuGZ homologs, D. melanogaster BuGZ's disordered domain features an intrinsic sequence-encoded capacity for LLPS, which in its case can be modified by its glutamine-rich region or by cellular context.
3 DISCUSSION
The comparative experimental strategy we deployed here correlates the phylogenetic tree of BuGZ in vitro phase separation to BuGZ in vivo mitotic function. The convergent tree topologies of these separate traits suggest, by maximum parsimony inference, that both phase separation and mitotic function were ancient, conserved traits, at least dating back to the last common ancestor of plants and animals, approximately 1.6 billion years ago. The full nature and extent of the interdependence between phase separation and function across BuGZ homologs remain to be determined.
Nonetheless, our observations give weight to outstanding hypotheses in the field: first, that disordered protein phase separation is a naturally selected trait that may confer selective advantage, and second, that eukaryotes have long exploited disordered protein phase separation as a primordial biological strategy to organize cellular material. Studies of other LLPS proteins with similar temperature dependence are generally consistent with these possibilities, but BuGZ is so far distinguished in that it implicates its disordered domain as necessary for phase separation.9, 12, 19 Interestingly, no BuGZ homolog is readily identifiable in the model yeast Saccharomyces cerevisae, which, unlike the organisms represented in this study, undergoes closed mitosis instead of open or semi-open mitosis.34 This conspicuous absence raises the possibility of an ancient coevolutionary interaction between LLPS-capable mitotic spindle proteins and the nuclear envelope superstructure.
By relying on the naturally occurring BuGZ primary sequences instead of tests based on rationally selected mutations, our observations are robust to and independent of any specific model of sequence evolution or biophysical sequence-to-function prediction. A quantitative, model-driven accounting of how site-specific physicochemical variation among condensate proteins can lead to evolutionary fixation remains a subject of intense investigation. Increasing temperature drives BuGZ phase separation. In other words, BuGZ features lower critical solution temperature (LCST) behavior. Among other factors, an entropic, temperature-dependent release of ordered water surrounding hydrophobic moieties, including aromatic amino acids in the LCST context, can be thought to promote BuGZ LCST demixing.35, 36 However, the molecular-polymeric details explaining a growing variety of other LLPS archetypes suggest that this mechanism alone is unlikely to exclusively account for the totality of BuGZ LLPS, or how BuGZ LLPS could promote function.4, 37-39 For example, as we noted, the ability of salt to influence the extent of BuGZ LLPS may reflect a complementary degree of dependence on charge, cation-pi, or dipole interactions.40
Overall, the high rate of primary sequence substitution in condensate IDPs may misleadingly imply that these protein sequences lack functional constraint, in contrast to structured proteins wherein a single amino acid substitution can yield outsize adaptive advantage.41 As has been previously shown, significant biological information may be embedded within highly variable sequence, despite superficial sequence divergence.42 Our work here hints at the existence of an underlying molecular evolutionarily logic simultaneously guiding LLPS and biological function.
Future dissection of this logic will have to account for environmental modifiers affecting material states and function alike. As we observe, some living cells appear to be equipped with machinery that can interact with D. melanogaster BuGZ, fluidizing condensates in certain cellular contexts. This is noteworthy considering that, among the homologs studied here, the D. melanogaster BuGZ uniquely contains extended polyglutamine tracts, and, among the species studied here, the D. melanogaster proteome is uniquely enriched in glutamine residues. Glutamine-rich amino acid sequences have an established ability to drive protein self-assembly.43, 44 We speculate that, as observed for other proteins, the mechanisms to achieve fluidization could include proteostatic maintenance machinery, or translational regulation at a predicted D. melanogaster BuGZ C-terminal domain stop codon read-through site modifying polyglutamine-driven association (Table S1).45-47 These mechanisms might tune condensate internal rearrangement and relaxation, resulting in altered liquidity.38 Alternatively, considering the modest degree of fluidization observed, these mechanisms may act on condensate morphology more than fluidity. This would be consistent with recent observations in other proteins of glutamine-rich sequences modulating condensate morphology independently of material state.48 Whichever the case, the existence of an interface between cellular context, the glutamine enriched D. melanogaster proteome, the D. melanogaster BuGZ polyglutamine tract, and D. melanogaster BuGZ condensate attributes highlights a potential coevolutionary relationship between condensate-tuning amino acid sequences and cognate cellular regulatory mechanisms. If present, could such mechanisms permit evolutionary exploration of increased sequence space, expanding the material and functional breadth of condensate-forming IDPs?
While at first glance seemingly at odds with a prevailing narrative that IDP condensation into a solid material drives dysfunction, the D. melanogaster BuGZ may instead be a candidate to join a growing set of phase separating proteins whose solid-like or gel-like states can enable, rather than impair biological function.49-51 Various examples can serve to highlight both biological function and micron-scale complexity that may emerge in non-fluid states specifically from BuGZ-like protein sequences, which at a first order approximation, can be described as proline–glycine enriched (Figure S6). Elastins and collagens are proline–glycine-rich, LCST-type, gel-forming proteins that have biological activity. Engineered microparticles based on proline–glycine-rich IDPs bear striking resemblance to microstructures in D. melanogaster BuGZ arrested microspheres.52 We emphasize that comprehensive analysis of the BuGZ sequence-to-fitness landscape will likely have to contend with addressing tunable, multiphase material behavior at the micron scale.
4 MATERIALS AND METHODS
4.1 Cloning
Plasmids for Sf9 insect cell expression were based on the pFastBac vector backbone (ThermoFisher 10360014) with inserts constructed from two sources: first, PCR was used to amplify the mVenus gene (A206K variant) from the Addgene plasmid mVenus C1 (mVenus C1 was a gift from Steven Vogel, Addgene plasmid 27794).53 Second, double-stranded DNA encoding insect cell codon-optimized versions of the various BuGZ/ZNF207 genes were commercially purchased (Integrated DNA Technologies gblocks). These segments were combined with the linear plasmid backbone via Gibson assembly. Plasmids for S2 insect cell expression was based on the pAC5.1 vector backbone (ThermoFisher V411020). Inserts for pAC5.1 were subcloned from the pFastBac vectors. The choice of the mVenus A206K variant fluorescent protein fusion partner was guided by its exceptionally monomeric character, so as to not interfere with associative processes in phase separation experiments.54
Plasmids for mammalian cell experiments were constructed using the pEGFP-C1 vector backbone (Clontech 6084-1, GenBank Accession U55763). Inserts were subcloned from the insect cell expression vectors by PCR specific to the gene downstream of mVenus, amplifying only the BuGZ/ZNF207 sequences, then combined with the linear plasmid backbone via Gibson assembly.
4.2 Protein expression
Purified pFastBac plasmids cloned as described were transformed into MAX Efficiency DH10Bac Competent Cells (ThermoFisher 10361012) and grown for 48 hr at 37°C on 50 μg/ml kanamycin, 7 μg/ml gentamicin, 10 μg/ml tetracycline, 100 μg/ml Bluo-gal (CAS 97753-82-7), and 40 μg/ml IPTG agar LB plates. Single white colonies, containing recombinant bacmid, were picked then re-streaked to clonal isolates and grown at 37°C for 24 hr to confirm the white recombinant phenotype. A total of 15 ml of LB liquid culture grown overnight at 37°C, 250 rpm was purified using a PureLink HiPure Plasmid Miniprep kit (ThermoFisher K210002). The correct presence and size of the insert were confirmed by PCR with the pUC/M13 forward 5′-CCCAGTCACGACGTTGTAAAACG-3′) and pUC/M13 reverse 5′-AGCGGATAACAATTTCACACAGG-3′) primers.
Low passage (fewer than 30) Sf9 (Spodoptera frugiperda) insect cells were grown in Sf900 II SFM media (ThermoFisher 10902104) in spinner flasks in a 28°C un-humidified incubator. Once viability was greater than 90% and the doubling time 24–48 hr, cells were transfected with the recombinant purified bacmid using Cellfectin II transfection reagent (ThermoFisher 10362100) on the Sf9 cells in six-well tissue culture plates. Cells were incubated at 28°C for 72 hr. The supernatant containing the P1 virus was collected and used to infect a T-75 vented tissue culture flask seeded with 5 × 104 Sf9 cells/cm2. After the cells had grown for 48 hr, the supernatant containing the P2 virus was used to infect 250–400 ml of Sf9 cells in Sf900 II SFM at 28°C in a spinner flask at 2 × 106 cells/ml. After 48 hr, the cells were centrifuged and the supernatant containing the P3 virus was mixed with FBS to a final concentration of 2%, sterile filtered, and stored at 4°C. The virus was titered using an end-point dilution assay.55
A 300–450 ml spinner flask culture of Sf9 cells in Sf900 II SFM with a doubling time of 24–48 hr was infected with P3 virus with a multiplicity of infection (MOI) of 1, at a density of 1.5 × 106 to 2 × 106 cells/ml. After the infected cells were grown at 28°C for 48 hr, a small aliquot of the cell suspension was examined under an inverted epifluorescent microscope for mVenus fluorescence to confirm proper protein expression. Once expression was confirmed, the cells were collected by centrifugation at 4,000g for 30 min at 5°C. The cell pellet was immediately frozen at −80°C.
4.3 Protein purification
Frozen Sf9 cell pellets were thawed to room temperature in a 50 ml conical tube filled with cold Lysis Buffer (50 mM Tris Base, 500 mM NaCl, 40 mM Imidazole, 5 mM TCEP, 5% Glycerol [w/v], pH 7.4 adjusted with NaOH/HCl) containing EDTA-free SigmaFast Protease Inhibitor Cocktail Tablet (Sigma S8830). Keeping the pellet chilled, the pellet was resuspended for 15–30 min in a 4°C cold room by stir bar mixing in a beaker. Once resuspended, the pellet suspension was placed on ice, spiked with Benzonase nuclease (CAS 9025-65-4), and sonicated with a Branson Sonifier 450 at power 6, duty cycle 25%, in 30-s cycles punctuated by 2-min rests with light stirring on ice to maintain a cold temperature. After sonication, the lysate was transferred to a pre-chilled Oak Ridge tube and centrifuged at 15,000 rpm (SA 600 rotor), 30 min, 4°C.
The cleared lysate supernatant was then filtered through a 0.22 μm polyethersulfone syringe filter unit and loaded in a 4°C cold room to a gravity-flow column packed with nickel-agarose resin pre-equilibrated with Lysis Buffer (ThermoSci R90110). The resin was washed with 5 column volumes of Lysis Buffer twice, then eluted with 3 column volumes of Elution Buffer (50 mM Tris Base, 500 mM NaCl, 500 mM Imidazole, 5 mM TCEP, 5% Glycerol, pH 7.4 adjusted with NaOH/HCl).
In the case of full-length BuGZ/ZNF207 proteins, the eluate was then concentrated using a centrifugal concentrator unit (EMD Millipore UFC901008, Amicon Ultra-15, pore size NMWL 10 kDa) 4°C, buffer exchanged once in a 50% dilution with SEC buffer (20 mM HEPES, 225 mM NaCl, 1 mM TCEP, pH 7.4 adjusted with NaOH/HCL), then injected on to a HiLoad 16/600 Superdex 200 pg Size Exclusion Column (GE Healthcare Life Sciences 28989335) on an AKTA Pure 25 L (GE Healthcare Life Sciences 29018224) FPLC system in the 4°C cold room, equilibrated with SEC Buffer. All BuGZ full-length proteins eluted in SEC buffer at 0.35 ml/min in 1.65 ml fraction sizes after approximately 0.3 column volumes.
In the case of the mVenus truncation variant, the eluate was diluted 20-fold in 20 mM PIPES 50 mM NaCl pH 7.2 and loaded on to a HiTrap Q HP anion exchange column (GE Healthcare Life Sciences 17-1153-01) and eluted over a 15 column volume gradient with a 20 mM PIPES 1 M NaCl pH 7.2 buffer at 4°C.
Eluates were concentrated with a pre-chilled centrifugal concentrator (EMD Millipore UFC901008, Amicon Ultra-15, pore size NMWL 10 kDa), then buffer exchanged once in a 50% dilution with SEC buffer. SDS-PAGE was used to inform fraction pooling, after which pure fractions were further concentrated (EMD Millipore UFC801008, Amicon Ultra-4, pore size NMWL 10 kDa), aliquotted to protein lo-bind tubes (Eppendorf 022431081) in 25-50 μl aliquots, snap-frozen with liquid nitrogen, and immediately stored at −80°C.
4.4 Phase separation of in vitro droplets
To conduct scattering measurements, 250–300 μl of protein solutions were prepared on ice then loaded into 700-μl semi-micro quartz cuvettes (Thor Labs, Newton, NJ). The cuvettes were then quickly loaded at beam-height to the temperature-controlled rotor of an Aviv model 14 spectrophotometer (Aviv Biomedical, Lakewood, NJ), pre-equilibrated to 4°C. Temperature scans were conducted at 800 nm with a bandwith of 2 nm, 1° temperature steps, 0.1°C temperature deadband, 0.1 min equilibration time, averaging time of 1.5 s, and 2.5°C/min scan speed. Apparent absorption intensity at 800 nm was used as a proxy for the extent of Rayleigh and Mie scattering of phase-separated assemblies.
To image droplets under salt and hexanediol conditions, 96-well glass-bottomed plates (MatTek P96G-0-5-F) were treated with a 10-min room temperature incubation of hydrophobic surfacing agent (Sigmacote SL2 Sigma), rinsed with deionized water, and left to dry at room temperature. Protein stocks were thawed on ice, mixed gently, then spectrophotometrically measured to determine their concentration (ThermoScientific Nanodrop 2000, Extinction coefficient at 515 nm = 92,000 M−1 cm−156). Stock solutions of 20% PEG 8000 (CAS 25322-68-3), and 40% 1–6 Hexanediol (CAS 629-11-8) in 20 mM HEPES pH 7.4 were used to prepare mixtures, which were then immediately imaged. Unless indicated, tested conditions included 5 μM BuGZ protein, 5% PEG, 20 mM HEPES, 150 mM NaCl, pH 7.4. Images were captured at room temperature (approximately 25°C) using a 60× apochromat oil immersion objective on a Nikon Eclipse TE200 inverted microscope under Hoffmann modulation contrast. Standard deviation of pixel intensity in a 50 × 50 μm area of an image was used to quantify the extent of the phase separation.
4.5 Fluorescence recovery after photobleaching
FRAP experiments were performed at room temperature on a Leica TCS SP8 confocal microscope using a 63×/1.40 oil immersion HCX Plan Apochromat Lambda Blue objective. Imaging used 514 nm excitation at 1.5% laser power. Detection was performed with a photomultipler spectral detector from 515 to 575 nm, 12 bit depth, in a 1,024 × 200 format, 800 Hz scan speed, bidirectional scan with no averaging. Droplets approximately 2 μm in diameter were chosen for bleaching. Bleaching was performed with a supercontinuum white light laser selected to expose the region of interest to 514 and 506 nm light for 0.1 s simultaneously, at 100 and 75% power, respectively. The data from a given set of bleaches were normalized using the fluorescence intensity immediately prior and directly after bleaching.
4.6 Cell culture and transfection
HeLa cells (ATCC CCL-2) and HEK293T cells (ATCC CRL-3216) were maintained in DMEM media (ThermoFisher 11995065) supplemented with 10% Fetal Bovine Serum (ThermoFisher 26140087) and Penicillin–Streptomycin (ThermoFisher 15140122). For immunoprecipitation experiments, 10 μg of pEGFP-BuGZ and 10 μg pcDNA3.1-N-FLAG-Bub3 (Coding for a FLAG-tagged amino acid sequence of UniProt Q9WVA3-1) were co-transfected with calcium phosphate in HEK293T cells to achieve heterologous expression, then harvested for 48 hr later for analysis. For knockdown-rescue experiments, HeLa cells grown to 90% confluency on sterilized polylysine-coated coverslips were first transfected with siRNA for 4 hr at 37°C using Lipofectamine RNAiMAX (ThermoFisher 13778075), recovered in 1 ml of fresh DMEM media with antibiotic for 2 hr, then transfected with pEGFP-BuGZ expression plasmids using Lipofectamine 2000 for 4 hr at 37°C. Finally, the cells were then exchanged back into 1 ml of fresh DMEM media with antibiotic, then 60 hr after transfection, the coverslips with cells were processed for immunostain analysis.
The Lipofectamine RNAiMAX transfection mixture for one well of a 12-well plate was prepared by combining 2 μl of 20 μM double-stranded RNAi oligos with 150 μl of OptiMEM media (ThermoFisher 31985062) and 3 μl RNAiMAX reagent, then incubating for 15 min.19 While waiting, media on the covering the coverslip of cells in each well of the 12-well plate was exchanged with fresh 350 μl antibiotic-free DMEM containing 10% FBS. Finally, 150 μl of the RNAiMAX mixture was added per well and gently rocked to mix. RNAi oligos used included an off-target control oligo (ThermoFisher 12935300) or an RNAi oligo antisense to human BuGZ protein, 5′-GCCUGCUAC ACUUACAAC AACUAGU-3′ (purchased synthesized by LifeTechnologies).
The Lipofectamine 2000 mixture for one well was prepared by adding 125 μl of OptiMEM media to 3 μl lipofectamine 2000 reagent, combining that with a separately prepared mixture of 125 μl OptiMEM media with 2 μg of plasmid, and incubating for 15 min. After exchanging the well media with 350 μl of antibiotic-free DMEM media with 10% FBS, all 250 μl of the lipofectamine 2000 mixture was added to the well and gently rocked to mix.
For S2 cell experiments, Schneider 2 (S2) cells were cultured in vented T75-flasks at 26°C using Schneider's Drosophila Media (SDM) (ThermoFisher 21720-024) and 10% heat-inactivated FBS (v/v) with normocin antibiotic-antimycotic (InvivoGen ant-nr-1). To prepare for transfection, cells were seeded in a six-well culture dish at a density of 3 × 106 cells per well and incubated at 26°C for 10–14 hr. Media was then replaced with 2 ml/well of serum-free, antibiotic-free SDM. To transfect one well of cells, 8 μl Cellfectin II (ThermoFisher 10362100) was mixed with 100 μl SDM, 1,500 ng DNA was mixed with 100 μl SDM, then the two solutions were mixed and incubated for 15 min at room temperature. The combined mixture was added dropwise to cells, gently mixed by tilting, then after 4 hr of incubation at 26°C was replaced with fresh SDM containing normocin and 10% FBS. S2 cells were returned to 26°C incubation, then imaged after 72 hr.
4.7 Co-immunoprecipitation
One plate of HEK293T cells were lysed with 20 mM Tris, 100 mM KCl, 0.1% NP-40, 1 mM EDTA, 10% Glycerol, 10 mM sodium pyrophosphate, 3 mM DTT, pH 7.5, with protease inhibitor cocktail. Immediately after lysis, cells were spun at 13,000 rpm for 10 min at 4°C. The supernatant was incubated with 40 ml of a 50% slurry of anti-FLAG beads (Sigma A2220) for 4 hr at 4°C with gentle mixing. The beads were then washed six times by gentle resuspension using ice-cold 20 mM Tris, 150 mM KCl, 0.5% NP-40, 1 mM EDTA, 10% Glycerol, 10 mM sodium pyrophosphate, 3 mM DTT, pH 7.5, with protease inhibitor cocktail. Finally, 50 μl of 100 μg/ml 3X-FLAG peptide solution (Sigma F4799) was used to elute the co-immunoprecipitating complexes.
4.8 Western blotting
Heterologously expressed GFP-BuGZ fusion proteins for western blot were probed with the following antibodies: rabbit Anti-GFP (Invitrogen A-6455) 1:3,000 dilution, rabbit Anti-BuGZ (Sigma HPA017013) 1:2,000 dilution, rabbit Anti-GAPDH (ProteinTech 10494-1-AP) 1:50,000 dilution, rabbit Anti-FLAG 1:400 dilution (Sigma F7425), and goat-Anti-Rabbit HRP (GE Amersham NA934-1ML).
4.9 Immunostaining and mitotic phenotype imaging
Cultured HeLa cells were gently washed with PBS then fixed directly on their coverslips with 4% paraformaldehyde in PBS for 15 min. After another brief PBS wash, cells were permeabilized with PBS containing 0.5% Triton X-100 for 15 min, subsequently washed again with PBS, then blocked with blocking buffer (4% BSA in TBST) for 60 min at room temperature. The primary antibody was diluted in blocking buffer, then incubated with the slide overnight at 4°C. After washing in blocking buffer three times, the secondary antibody also in blocking buffer was incubated with the sample for 60 min at room temperature. Then, the slides were washed in TBST three times for 5 min each. Finally, after a 3-min incubation with DAPI then washing in TBST three times, the coverslips were mounted to slides with Immumount (ThermoScientific 9990402). DAPI (4′,6-diamidino-2-phenylindole), was used at a 1:20,000 dilution of a 1 mg/ml stock solution. The primary antibody used to detect tubulin was mouse anti-alpha-tubulin clone DM1A (Sigma T9026) used at a 1:500 dilution. Intrinsic GFP fluorescence was used to detect GFP-fusion proteins. For non-GFP-fusion conditions, the primary antibody used to detect BuGZ/ZNF207 was rabbit Anti-ZNF207 (Sigma HPA017013), used at a 1:200 dilution.
A Leica SP5 confocal microscope with a 63×/1.4–0.6 oil Plan Apochromat oil immersion objective was used to image mitotic phenotypes. Optical sections were imaged at 0.5 μm intervals, and z-stack images were obtained with maximum intensity projections.
A Nikon Eclipse E800 epifluorescent microscope was used to manually quantify mitotic phenotype proportions. Three independent biological transfection-rescue replicates were prepared for each BuGZ/ZNF207 variant and GFP-luciferase control tested, and proportions from each slide were calculated from arithmetic means of 100 to 150 scored cells.
4.10 Protein sequence bioinformatics analysis
BuGZ homolog sequence alignment was performed on the selected orthologs using the T-COFFEE algorithm.57 T-COFFEE alignments were processed with Jalview 2.11.1.0 to calculate percent pairwise identities, that is, the number of identical residues divided by the total number of aligned residues.58 Average percent identity was calculated as the arithmetic mean of all pairwise comparisons between species, excluding the H. sapiens to M. musculus pair.
Sequence randomization averages were calculated as the grand average of 50 independent trials of sequence permutation in-place. For the ordered-only and disordered-only samples, the input for a single trial of permutation, alignment, and pairwise-ID was the entire ordered or disordered domain, where the disordered domain was defined as all residues C-terminal to the conserved aligned region with a VSL2 disorder score greater than 0.5 (residues C-terminal to residue I85, or residue A93 in the case of Suf4). For the full-length samples, the input to randomly permute was only disordered domains, which were subsequently C-terminally concatenated to the ordered domains before alignment and pairwise-ID calculation.
Disorder prediction was performed using the VSL2b disorder predictor.60
Linear window-averaged physicochemical profiles were constructed by assigning numerical values derived from indicated scales to each residue. Then any given positional value was calculated as the arithmetic mean of those values within a 15 residue window centered at the given position, encompassing seven residues N-terminal and seven residues C-terminal to the center.
Amino acid frequency heat maps were created by calculating the fraction of amino acids of a given residue divided by the total number of amino acids in either that one sequence or that one subsetted proteome. Non-canonical amino acids, such as the single “X” in D. melanogaster BuGZ, were discarded in this analysis.
The following Uniprot reference proteomes (https://www.uniprot.org) were used in this study: UP000006548, UP000001940, UP000000803, UP000005640, UP000000589, UP000186698.
ACKNOWLEDGEMENTS
This work was financially supported by the National Institutes of Health grants 2R01GM063747 and R01GM126130. We thank H. Zhao for his generous technical assistance with mammalian cell culture. We thank T. Schroer for use of her facilities and equipment to grow insect cells. We thank J.O. Wrabl for his scientific suggestions and his careful edits to the manuscript.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Alexander F Chin: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); writing – original draft (lead); writing – review and editing (equal). Yixian Zheng: Formal analysis (supporting); supervision (supporting); writing – review and editing (equal). Vincent J. Hilser: Formal analysis (supporting); funding acquisition (lead); supervision (supporting); writing – review and editing (equal).




