Chemotherapy Immunophenoprofiles in Non-Small-Cell Lung Cancer by Personalized Membrane Proteomics
Abstract
Objectives: No study has addressed how the immune status at the molecular level is affected by first-line pemetrexed and cisplatin (PEM–CIS) combination therapy in patients with non-small-cell lung cancer (NSCLC). Thus, we aimed to identify the immune status from membrane proteome alterations in patients with NSCLC upon PEM–CIS treatment.
Methods: The paired peripheral blood mononuclear cells (PBMCs) were collected from four patients with lung adenocarcinoma before and after the first regimen of PEM–CIS treatment and applied quantitative membrane proteomics analysis.
Result: In the personalized PBMC membrane proteome profiles, 2424 proteins were identified as displaying patient-specific responsive patterns. We discovered an elevated neutrophil activity and a more suppressive T-cell phenotype with the downregulation of cytotoxic T lymphocyte antigen 4 degradation and the upregulation of type 2 T-helper and T-regulatory cells in the patient with the highest progression-free survival (PFS) of 14.5 months. Patients with a PFS of 2 months showed higher expressions of T-cell subsets, MHC class II pathways, and T-cell receptor signaling, which indicated an activated immune status.
Conclusion and Clinical Relevance: Without the additional isolation of specific immune cell populations, our study demonstrated that PEM–CIS chemotherapy altered patients’ immune system in terms of neutrophils, T cells, and antigen presentation pathways.
1 Introduction
Lung cancer is one of the most frequently diagnosed malignancies worldwide. Non-small-cell lung cancer (NSCLC), which includes the subtypes of adenocarcinoma (40%), squamous cell carcinoma (25–30%), and large-cell carcinoma (10–15%), accounts for approximately 85% of cancer incidence. Currently, the first-line treatment for patients with advanced nonsquamous NSCLC carrying the wild-type epidermal growth factor receptor genotype is a combination of pemetrexed (PEM) with the platinum-based agent cisplatin (CIS); compared with other antineoplastic combinations, this combination chemotherapy has proven to be more tolerable and results in considerable improvement in survival rates.1, 2 Despite the tremendous number of studies conducted to improve NSCLC diagnosis and therapeutic approaches, disease detection and the response to therapy are still poor, and the majority of patients are diagnosed at a late stage and exhibit a 5-year survival rate of less than 5% when diagnosed at the metastatic stage.3, 4
The immune system plays critical roles in carcinogenesis and cancer progression. For treatment monitoring, an examination of the immune system through immunophenotyping using cell surface markers, cytokine measurement, and a complete blood cell test on blood samples reflects the host's immune status.5 Despite their known mechanisms to alter DNA synthesis and replication, the anticancer therapeutic drugs PEM and CIS have been separately reported to modulate6, 7 and suppress innate and adaptive immunity,5, 8 which result in different responses to chemotherapy in melanoma and ovarian and pancreatic cancers.9 However, no study has addressed how the immune status at the molecular level is affected by first-line PEM–CIS combination therapy in patients with NSCLC.
Due to advancements in instrumentations and analytical strategies, MS-based proteomics analysis has become one of the most useful methods for the global protein profile screening of patients’ peripheral immune cells and peripheral blood mononuclear cells (PBMCs) and for the identification of protein signatures, thus assisting in the prediction of individuals at a high risk of cardiomyopathy10 or the characterization of inflammation-activated lymphocyte cells.11 Koncarevic et al. used commercially available PBMCs as samples, and using various LC-MS methodologies as well as tandem mass tag labeling, they identified 4129 proteins that were substantially involved in lymphocyte activation, differentiation, and proliferation.12 Although a comprehensive proteome analysis of total proteins is valuable in assessing molecular processes, observing specific cell markers for evaluating the response of different immune cells remains difficult.
Clinical Relevance
In this study, peripheral blood mononuclear cells of patients with advanced lung adenocarcinoma were analyzed using a personalized membrane proteomics approach. Without the additional isolation of specific immune cell populations, we quantitatively compared the membrane proteome profiles among patients with lung adenocarcinoma receiving first-line combination chemotherapy using cisplatin and pemetrexed, and we interpreted the overall immune status from membrane proteome alterations to correlate the immune status with the response to treatment for each patient. We provided novel insights on patients’ immune response following chemotherapy and proposed a potential membrane protein signature to predict the response to treatment and prognosis. With further validation on more patients, we hope to provide useful molecular parameters for constructing patient immunograms that assist in identifying other therapeutic strategies
Given that cell surface membrane markers are routinely used to isolate and recognize different immune cells, we hypothesize that the membrane proteomics profiling of patients’ immune cells upon treatment may directly reveal expression changes in these membrane markers and immune-related pathways, which provides a systematic examination of the immune system response of individual patients. Without the additional isolation of different immune cells, we conducted the individualized membrane proteomics analysis13, 14 of patients’ PBMC to study drug–immune cell interactions and to investigate the immune status of patients with NSCLC after PEM–CIS combination chemotherapy. To our knowledge, this is the first study that determined the overall immune status of patients with NSCLC in response to PEM–CIS combination therapy by performing the personalized membrane proteome profiling of patients’ PBMC.
2 Experimental Section
2.1 Patients and PBMC Samples
Four patients who visited the pulmonary clinic in Shuang Ho Hospital (New Taipei City, Taiwan) and who consented to an IRB-approved study (approval number: 201505003) were the study patients. The patients comprised two men and two women, with age ranging from 55 to 75 years, and all were diagnosed with advanced lung adenocarcinoma according to histological examinations (Table 1). Patients were administered first-line standard combination chemotherapy using CIS (75 mg m−2) and PEM (500 mg m−2) for four regimens with 500-μg folate supplementation every 9 weeks. Patients’ blood was initially collected prior to the first regimen and just before the second regimen of chemotherapy (on Day 22). In addition to PBMC collection, a complete blood count was also obtained for white blood cells (WBCs), neutrophils, and lymphocytes. To collect PBMCs, 35 mL of peripheral blood was drawn and isolated using Ficoll density gradient separation. The volume of collected PBMC pellets was approximately 10 μL.
| Patient/category | A01 | A02 | A03 | A04 |
|---|---|---|---|---|
| Gender | F | F | M | M |
| Age (year) | 60 | 65 | 55 | 75 |
| Smoking | No | No | No | Yes |
| Staging | T4N2M1a | T2N2M1a | T1N1M1a | T3N1M1a |
| Response | Progressive disease | Progressive disease | Stable disease | Partial response |
| PFS (month) | 2.13 | 2.7 | 4.72 | 14.5 |
| OS (month) | 20.5 | 4.91 | 21 | 16.9 |
| Pretreated WBC | 20100 | 8200 | 5700 | 6000 |
| Pretreated neutrophil (%) | 88 | 80 | 55 | 61 |
| Pretreated lymphocyte (%) | 6 | 10 | 34 | 24 |
| Posttreated WBC | 10700 | 8600 | 6700 | 7700 |
| Posttreated neutrophil (%) | 67 | 78 | 51 | 89 |
| Posttreated lymphocyte (%) | 18 | 12 | 31 | 9.6 |
- PFS, progress-free survival; OS, overall survival.
2.2 Membrane Protein Purification, Gel-Assisted Digestion, and iTRAQ Labeling
An overview of the workflow is presented in Supporting Information, Figure 1, and the detailed experimental procedure is previously reported in refs. 14 and 15. In brief, PBMC pellets were immersed in hypotonic buffer (10 mM HEPES, pH 7.4; 1.5 mM MgCl2; 10 mM KCl; and 1× protease inhibitor) for 15 min, then stroked 50 times in a Dounce homogenizer (Wheaton, NJ, USA), and centrifuged at 1000 × g for 10 min at 4°C to remove the nucleus. Sucrose at a final concentration of 0.25 M was added to the postnuclear supernatant and was centrifuged at 13,500 rpm at 4 °C for 1 h to collect the membrane protein pellet. The pellet was washed with 0.1 M Na2CO3 (pH 11.5) to remove the membrane-associated proteins. The membrane pellet was vacuum-dried and dissolved in 90% formic acid (FA) for a Bradford assay to determine the membrane protein amount.
The membrane proteins were vacuum-dried and resuspended in 6 M urea, 5 mM EDTA, and 2% SDS in 0.1 M triethylammonium bicarbonate (TEABC, pH 8). Moreover, 5 mM tris (2-chloroethyl) phosphate was added for the chemical reduction of protein disulfide bonds, and 2 mM methyl methanethiosulfonate was applied for alkylation at room temperature for 30 min. Proteins were directly incorporated into gels in a microcentrifuge tube by adding acrylamide/bisacrylamide solution (40%, 29:1), 10% ammonium persulfate, and 100% Tetramethylethilenediamine. The gel was cut into small pieces, washed several times with TEABC containing 50% ACN, and further dehydrated with 100% ACN before drying with SpeedVac (Thermo Fisher Scientific, MA, USA). Proteolytic digestion was then performed with trypsin (Promega, WI, USA; protein/trypsin = 10:1, g g−1) in 25 mM TEABC and incubated overnight at 37°C. Peptides were extracted from the gel using sequential extraction with TEABC, 0.1% TFA, 0.1% TFA in ACN, and 100% ACN. The solutions were combined and concentrated in a SpeedVac.
The resulting membrane peptides from paired pre- and posttreatment PBMC samples from the same patient were labeled with iTRAQ tags (Applied Biosystems, Foster City, CA, USA). The prechemotherapy peptides of patient A01, A02, and A03 were labeled with iTRAQ114, and their postchemotherapy peptides were labeled with iTRAQ115. The prechemotherapy peptides of patient A04 and their subsequent postchemotherapy peptides were labeled with iTRAQ116 and iTRAQ117, respectively. The labeled samples from each patient were mixed to generate three groups of iTRAQ experiments for C18 RP StageTip fractionation.
2.3 RP Stagetip Fractionation and LC-MS/MS Analysis
A C18 RP StageTip was prepared using a small piece of C8 EmporeTM disk extracted by a 16-gage blunt end needle, which was fit into the top of D200 tips (Gilson, Middletown, USA).16 Five milligram of C18 resin (ReproSil-Pur 120 C18, 5 μm) was dissolved in 100 μL of buffer A (1.62% FA and 3.48% NH4OH, pH 10) and 100 μL of buffer B (100% ACN) and was added to the C8 disk-fitted tips. The RP StageTip was activated by passing methanol and was equilibrated with 80% ACN in buffer A and 20.8% ACN in buffer A sequentially by centrifugation at 1500 × g for 2 min each. After conditioning the tip by soaking with buffer A for 10 min, 10 μg of iTRAQ-labeled membrane peptides was dissolved in buffer A and loaded onto the tip through centrifugation at 1500 × g for 2 min. The unbound peptides were collected as a flow-through fraction. Peptides were then fractionated through step-wise elution with 11.1, 14.5, 17.4, 20.8, 45, and 80% ACN in buffer A by centrifugation at 1500 × g for 2 min for each step. All collected fractions were vacuum-dried.
Each RP fraction was resuspended in 0.1% FA and analyzed using an LTQ-Orbitrap Velos (Thermo Fisher Scientific) equipped with a nano-ACQUITY UPLC (Waters Corporation, Milord, MA, USA). The samples were loaded and separated on a 75-μm id, 25-cm length C18 BEH column (Waters, Milford, MA, USA) packed with 1.7-μm particles with a pore width of 130 Å, and the samples were separated using a segmented gradient from 5 to 40% solvent B (ACN with 0.1% FA) in 120 min at a flow rate of 300 nL min−1 and a column temperature of 35°C. The MS survey scan range was m/z 350–1600. The mass spectrometer was operated in the data-dependent mode. Briefly, full scan MS spectra were acquired in the orbitrap (m/z 350–1600), with the resolution set to 60K at m/z 400, and the automatic gain control target was 5 × 105. The ten most intense ions were sequentially isolated for higher energy collisional dissociation and detected in the orbitrap with previously selected ions dynamically excluded for 60 s. The MS proteomics data were deposited to the ProteomeXchange Consortium through the PRIDE17 partner repository with the dataset identifier PXD006297 (username: [email protected], password: pMNolLgM).
2.4 Protein Identification, Quantitation, and Functional Analysis
The MS/MS files were applied in database searching against the Swiss-Port human protein sequence database (release 2015_08, 20,204 sequences) using the MASCOT Distiller (v2.5.1, Matrix Science, London, United Kingdom) with the following parameters: tryptic peptides with a maximum of two missed cleavage sites and MS tolerance of 10 ppm and MS/MS tolerance of 0.05 Da. The variable modifications were set to be deamidation (NQ), oxidation (M), methylthio (C), iTRAQ (K), and iTRAQ (N-terminal). All identified peptide and proteins were filtered to 1% false discovery rate (FDR) (p < 0.05). Protein quantitation was performed using Multi-Q.18 Only unique, iTRAQ-labeled peptides with MS/MS spectra containing the isotopic peaks of m/z 114, 115, 116, and 117 with ion counts ≥ 5000 were used for peptide quantitation. Protein ratios were determined by calculating the weighted average of their quantified peptides and were normalized by the mean of the ratios.
The identified proteins were annotated by biological process, molecular function, and cellular component properties against Gene Ontology (GO) using GoMiner19 and the Database for Annotation, Visualization, and Integrated Discovery (DAVID). Moreover, pathway analysis was performed using the IPA and the Kyoto Encyclopedia of Genes and Genomes. Proteome expressions were analyzed using the Pearson correlation coefficient and principle component analysis (PCA) to reveal the profile differences among patients. The proteome expression ratios of each patient were first log2-transformed and analyzed using the Kolmogorov–Smirnov test to determine whether the data are normally distributed. The unquantified proteins in any patient were omitted from the statistical analysis. Immune cell protein expressions were selected based on the functional and pathway analyses of roles in immune-related mechanisms by using DAVID and were subsequently clustered for lineage and cell specificities. Distinct immune cell markers were evaluated based on the study of Maecker et al.20 and the Human and Mouse CD Marker Handbook provided by BD Bioscience (San Jose, CA, USA).
3 Results
3.1 Quantitative Analysis of Membrane Proteome Profiles of Paired Pre- and Postchemotherapy PBMCs from Patients with NSCLC
To investigate patients’ immune response to PEM–CIS chemotherapy, we conducted a quantitative analysis of the membrane proteome of pre- and posttreated PBMCs for individual patients with NSCLC. Blood samples were collected from four patients with lung adenocarcinoma, whose progression-free survival (PFS) ranged from 2.13 to 14.5 months, before and after the first regimen of PEM–CIS chemotherapy. For all blood samples, a complete blood count was obtained for WBCs, lymphocytes, and neutrophils. Patient A01 exhibited a notably higher WBC count before treatment initiation and demonstrated a marked decrease after the first regimen of PEM–CIS chemotherapy. Pre- and posttreatment WBC counts of patient A02, A03, and A04 were within the normal range, and patient A04 exhibited a noted increase in the neutrophil count percentage (Table 1).
PBMCs were further purified from blood using Ficoll density gradient separation, and the individualized iTRAQ-based quantitative analysis of membrane proteome profiles was performed, with high accuracy and precision.14, 15, 21 In brief, membrane proteins were purified from PBMC samples, subjected to gel-assisted digestion, and iTRAQ labeled individually. Prechemotherapy peptides were labeled with iTRAQ114 (or iTRAQ116), and postchemotherapy peptides were labeled with iTRAQ115 (or iTRAQ117). iTRAQ-labeled peptides from paired pre- and postchemotherapy PBMCs were pooled, fractionated using C18 RP StageTip, and analyzed in duplicate using LC-MS/MS (Supporting Information, Figure 1).
A total of 2093, 1850, 2166, and 2166 proteins were identified from the PBMCs of patients A01, A02, A03, and A04, respectively (FDR < 1%). The GO analysis annotated an average of 56% of membrane proteins in three sets of experiments (Supporting Information, Table 1). Among these, we identified various immune-related proteins, including 50 immune cell marker proteins, 47 CD molecules (where CD is cluster of differentiation), 10 S100 family proteins, 24 integrins, 16 chemokines and chemokine receptors, and 39 HLA molecules (Supporting Information Tables 2–5). Of all identified proteins, the expression levels of 1903, 1442, 1965, and 1945 proteins from the pre- and postchemotherapy PBMCs of the four patients were determined using Multi-Q. Based on the technical replicate experiments (Supporting Information, Figure 2), we defined the significantly differential expression for each sample by a 2 SD rule (95% confidence interval) in abundance (thresholds in Supporting Information, Table 1). Under this criterion, 782, 630, 723, and 522 differentially expressed proteins were detected in patients A01, A02, A03, and A04, respectively. Proteins identified to be upregulated ranged from 265 (patient A04) to 428 proteins (patient A01), and the number of downregulated proteins ranged from 330 (patient A04) to 468 (patient A03; Supporting Information, Table 1). The overlapping of up- and downregulated proteins in the four patients is presented in Supporting Information, Figure 3.
3.2 Differential Membrane Proteome Expressions in Patients with NSCLC
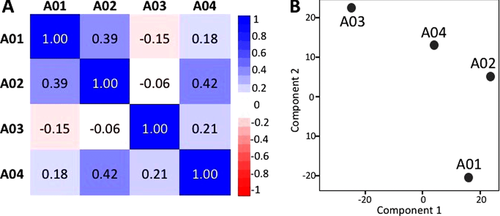
To explore the expression differences among patients, we compared the membrane proteome profiles of the four patients using the Pearson correlation analysis and PCA. As illustrated in Figure 1A, the Pearson correlation analysis revealed a low degree of correlation among patients, with the correlation coefficient ranging from −0.15 to 0.42. The highest correlation coefficient was 0.42 for patients A02 and A04. Patient A03 exhibited the most distinct profiles compared with other patients; negative correlations were observed for patients A01 and A02, and a correlation coefficient of 0.21 was observed for patient A04. PCA revealed similar results (Figure 1B), suggesting a diverse and patient-specific membrane proteome pattern resulting from patient heterogeneity and their differential responses to chemotherapy.
Among the differentially expressed proteins identified in each patient, 16 proteins were found to exhibit common expressions after chemotherapy (Table 2). Nine proteins were commonly upregulated in the four patients, of which autophagy-related protein 9A and NADH-cytochrome b5 reductase 3 (CYB5R3) were reported to be upregulated in a PFS-dependent manner. Three other upregulated proteins, CD9, CD36, and platelet glycoprotein Ib alpha-chain (GP1BA), are involved in platelet differentiation from hematopoietic cell lineage. We then analyzed proteins exhibiting inconsistent expressions in the patient with the highest PFS. As revealed in Table 3, 16 proteins were differently expressed in patient A04. Interestingly, the expressions of neutrophil elastase (ELANE) and myeloperoxidase (MPO) were decreased in patients A01, A02, and A03, but were increased in patient A04. Both proteins are associated with neutrophil activity, and the higher expressions of ELANE and MPO suggested that neutrophils were activated after PEM–CIS chemotherapy in the patient with the highest PFS.
| No. | Gene name | Protein name | A01 | A02 | A03 | A04 |
|---|---|---|---|---|---|---|
| Upregulation in four patients | ||||||
| 1 | CMTM5 | CKLF-like MARVEL transmembrane domain-containing protein 5 | 1.84 | 2.65 | 3.16 | 1.56 |
| 2 | CD36 | Platelet glycoprotein 4 | 1.93 | 2.54 | 2.35 | 1.98 |
| 3 | RDH11 | Retinol dehydrogenase 11 | 1.65 | 2.48 | 1.67 | 1.78 |
| 4 | CD9 | CD9 antigen | 1.64 | 2.37 | 2.43 | 1.45 |
| 5 | GP1BA | Platelet glycoprotein Ib alpha chain | 2.79 | 2.07 | 2.53 | 2.32 |
| 6 | ATG9A | Autophagy-related protein 9A | 2.08 | 1.87 | 1.48 | 1.42 |
| 7 | SAR1A | GTP-binding protein SAR1a | 2 | 1.84 | 1.45 | 1.53 |
| 8 | SLC4A1 | Band 3 anion transport protein | 3.39 | 1.79 | 8.36 | 2.16 |
| 9 | CYB5R3 | NADH-cytochrome b5 reductase 3 | 1.79 | 1.75 | 1.52 | 1.47 |
| Downregulation in four patients | ||||||
| 1 | TGM3 | Protein-glutamine gamma-glutamyltransferase E | 0.59 | 0.72 | 0.45 | 0.62 |
| 2 | CPT2 | Carnitine O-palmitoyltransferase 2 | 0.56 | 0.67 | 0.64 | 0.38 |
| 3 | CELF2 | CUGBP Elav-like family member 2 | 0.58 | 0.61 | 0.57 | 0.52 |
| 4 | KPRP | Keratinocyte proline-rich protein | 0.75 | 0.57 | 0.51 | 0.53 |
| 5 | NOP58 | Nucleolar protein 58 | 0.6 | 0.57 | 0.56 | 0.58 |
| 6 | XP32 | Skin-specific protein 32 | 0.48 | 0.47 | 0.37 | 0.47 |
| 7 | SNRPB | Small nuclear ribonucleoprotein-associated proteins B and B′ | 0.57 | 0.37 | 0.56 | 0.32 |
- All the ratios represent the protein expression changes of postchemotherapy versus prechemotherapy.
| No. | Gene name | Protein name | A01 | A02 | A03 | A04 |
|---|---|---|---|---|---|---|
| Downregulation in A01–A03, Upregulation in A04 | ||||||
| 1 | ELANE | Neutrophil elastase | 0.24 | 0.71 | 0.43 | 2.47 |
| 2 | MPO | Myeloperoxidase | 0.31 | 0.67 | 0.36 | 2.77 |
| Downregulation in A01–A03, unchanged in A04 | ||||||
| 3 | DSC1 | Desmocollin-1 | 0.70 | 0.66 | 0.63 | 0.71 |
| 4 | FLG | Filaggrin | 0.64 | 0.59 | 0.42 | 1.15 |
| 5 | NARS | Asparagine–tRNA ligase | 0.68 | 0.54 | 0.53 | 0.89 |
| 6 | CTSG | Cathepsin G | 0.20 | 0.50 | 0.64 | 1.32 |
| Upregulation in A01–A03, unchanged in A04 | ||||||
| 7 | CMTM6 | CKLF-like MARVEL transmembrane domain-containing protein 6 | 1.87 | 3.14 | 1.79 | 1.32 |
| 8 | CD47 | Leukocyte surface antigen CD47 | 1.65 | 2.33 | 1.76 | 1.29 |
| 9 | NNT | NAD(P) transhydrogenase | 1.74 | 2.33 | 1.52 | 0.88 |
| 10 | MLEC | Malectin | 2.42 | 2.04 | 1.66 | 1.31 |
| 11 | CLDN3 | Claudin-3 | 2.23 | 1.96 | 1.57 | 0.76 |
| 12 | ATAD3A | ATPase family AAA domain-containing protein 3A | 1.75 | 1.93 | 1.58 | 1.32 |
| 13 | STX11 | Syntaxin-11 | 2.27 | 1.69 | 2.65 | 1.23 |
| 14 | FCER1G | High affinity immunoglobulin epsilon receptor subunit gamma | 1.99 | 1.64 | 1.49 | 1.10 |
| 15 | TMEM63A | CSC1-like protein 1 | 1.74 | 1.63 | 1.75 | 1.26 |
| 16 | FAM162A | Protein FAM162A | 2.02 | 1.57 | 1.58 | 1.24 |
- All the ratios represent the protein expression changes of postchemotherapy versus prechemotherapy.
3.3 Signature Profiles of Peripheral Immune Cells
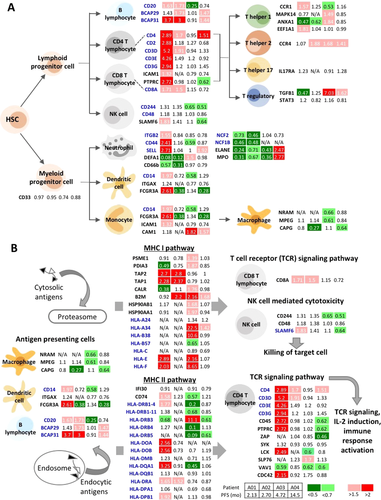
Based on the well-studied cell surface markers and immune-related pathways of different immune cells, we further extracted the expression profiles of proteins expressed by various immune cells with lymphoid and myeloid lineages, as well as antigen presentation pathways. Due to limitations in the detection of negative markers, we aimed to identify the positive markers of each immune cell population. Upon chemotherapy, the expression changes (ratio, R = posttreated/pretreated) of identified marker proteins and proteins involved in the downstream signaling of antigen presentation and T-cell signaling pathways from the four patients are illustrated in Figure 2A, B.
3.3.1 Lymphoid Cells
Following PEM–CIS treatment, overall T-cell subset and natural killer (NK) cell populations (Figure 2A), which are represented using the CD2+ marker, were reported to be increased in patient A01 (R: 2.88), whereas they remained unchanged in patients A02, A03, and A04 (R: 1.18, 1.27, and 0.68, respectively). In addition, the CD3 complex (CD3D, CD3E, and CD3G) was upregulated in patient A01 (R: 5.2, 4.26, and 2.94, respectively), and CD3D was upregulated in patient A02 (R: 1.91). The specific CD4 marker of T-helper (Th) cells was upregulated in patients A01, A02, and A04 (R: 2.89, 1.7, and 1.51, respectively), and CD8, the marker of CTL, was upregulated in patients A01 (R: 1.71) and A02 (R: 1.5). The expressions of several proteins in different subsets of Th cells were also identified. CC chemokine receptor 1 (CCR1) was upregulated in patient A01 (R: 1.57) and was downregulated in patient A03 (R: 0.53), and CCR4 was upregulated in patients A02, A03, and A04 (R: 1.88, 1.68, and 1.41, respectively). The transforming growth factor β1 cytokine was upregulated in patients A03 and A04 (R: 7.03 and 1.62, respectively) and was downregulated in patient A01 (R: 0.47). The expression of interleukin-17 receptor A (IL17A) and signal transducer and activator of transcription 3 (STAT3) remained unchanged in all patients.
CD244, also known as NK cell receptor 2B4 and a marker of NK cells, was downregulated in patients A03 (R: 0.65) and A04 (R: 0.51), and the self-ligand receptor of the signaling lymphocytic activation molecule family 6 (SLAMF6) was downregulated in patient A04 (R: 0.64) but was upregulated in patient A01 (R: 1.83). CD20, a B-cell marker, was upregulated in patients A01 (R: 1.63) and A02 (R: 1.73) and was downregulated in patient A03 (R: 0.25). The expression levels of B-cell receptor associated proteins, BCAP29 and BCAP31, were increased in patients A02 (R: 1.73 and 3, respectively) and A04 (BCAP29: 1.47; BCAP31: 1.44), with an increase in BCAP31 expression in patient A01 (R: 3.7).
3.3.2 Myeloid Cells
As revealed in Figure 2A, the overall expression of monocytic/myeloid lineage cells indicated by the CD33 marker remained unchanged among all patients (R: 0.97, 0.95, 0.74, and 0.88 in patients A01, A02, A03, and A04, respectively). However, the molecular expressions of different myeloid lineage cells displayed opposite profiles among patients. CD14, a marker of both monocytes and dendritic cells (DCs), was increased in patient A01 (R: 1.97). In addition, Fc gamma receptor III-A (FCGR3A, also known as CD16a), an IgG receptor, was upregulated in patient A01 (R: 2.61). In patients A02 and A04, CD14 expression remained unchanged (R: 0.72 and 1.29, respectively), but FCGR3A expression was downregulated (R: 0.38 and 0.28, respectively). By contrast, in patient A03, CD14 expression decreased (R: 0.58), but FCGR3A expression remained unchanged (R: 1.34). There results suggested a higher monocyte/DC level in patient A01, but a lower level in patients A02, A03, and A04. Regarding differentiated macrophages, two markers, macrophage-expressed gene protein (MPEG) and natural resistance–associated macrophage protein 1 (NRAM1), were downregulated in patient A03 (R: 0.66 for NRAM1; R: 0.61 for MPEG), but remained unchanged in other patients.
Neutrophil cell surface molecules, including integrin beta 2 (ITGB2, also known as CD18), CD44, and l-selectin (SELL), were upregulated in patient A01 (R: 1.55, 2.41, and 2.71, respectively), with the upregulation of SELL in patient A04 (R: 1.92) and the downregulation of CD44 in patient A03 (R: 0.59). However, proteins involved in neutrophil activity were mainly downregulated. CD66b and neutrophil defensing 1 (DEFA1), which participate in the neutrophil degranulation pathway, were downregulated in patients A01 (R: 0.57 and 0.08, respectively) and A02 (R: 0.31 and 0.12, respectively), but DEFA1 was upregulated in patient A03 (R: 1.5). Neutrophil cytosolic factors (NCF1B and NCF2), which are involved in superoxide generation, were downregulated in patients A01 (R: 0.46 and 0.73, respectively) and A02 (R: 0.48 and 0.46, respectively). Moreover, the neutrophil activity proteins ELANE and MPO were downregulated in patients A01, A02, and A03, but were upregulated in patient A04.
3.3.3 Antigen-Processing and Presentation Pathways
MHC I and II complexes involved in antigen presentation pathways were identified in all four patients (Figure 2B, Supporting Information, Table 5). Patient A03 exhibited enriched proteins in the MHC class I pathway, with marked increases in classical human leukocyte antigen A (HLA-A34, R: 22.5) and HLA-B38 (R: 10.6) and in nonclassical HLA-E (R: 2.16) and HLA-F (R: 8.65). Additionally, proteasome activator complex subunit 1 (PSME1), protein disulfide isomerase A3 (PDIA3), calreticulin (CALR), beta-2-microglobulin (B2M), and heat shock protein 90 alpha and beta (HSP90AA1 and HSP90AB1) were also upregulated in patient A03 (R: 1.39, 1.87, 1.76, 2.16, 1.68, and 1.97, respectively). However, the expressions of proteins involved in the MHC class II pathway, notably CD74 (R: 0.57), HLA-DRB1 (HLA-DRB1-4: 0.2; HLA-DRB1-11: 0.68), HLA-DRB4 (R: 0.1), HLA-DRB5 (R: 0.09), and HLA-DQA1 (R: 0.45), were decreased in patient A03.
Patient A01, who had the shortest PFS outcome, exhibited increased expressions of proteins involved in both MHC class I and II antigen presentation pathways. The upregulation of antigen peptide transporters 1 and 2 (TAP1 and TAP2; R: 2.28 and 2.7, respectively), HLA-E (R: 2.89), and HLA-F (R: 2.03) and the downregulation of PDIA3 (R: 0.49) and CALR (R: 0.38) were identified in the MHC class I pathway after chemotherapy. Molecules, including CD74 (R: 1.58), HLA-DRB1-4 (R: 1.77), HLA-DOA (R: 2.56), HLA-DOB (R: 2.56), HLA-DQA1 (R: 3.25), HLA-DRA (R: 1.65), and HLA-DPB1 (R: 1.92), were also upregulated in the MHC class II pathway, with only one molecule downregulated, namely HLA-DRB3 (R: 0.68). In contrast to patients A03 and A01, patient A04 exhibited no expression changes in molecules involved in both antigen presentation pathways. Only a slight increase was observed in B2M (R: 1.68) and HLA-A34 (R: 1.42) in the MHC class I pathway, and a decrease was observed in in HLA-DRB3 (R: 0.61) and HLA-DRB5 (R: 0.61) in the MHC class II pathway. In patient A02, we only detected the upregulation of TAP1 (R: 2.8), TAP2 (R: 2.37), and B2M (R: 2.2) in the MHC class I pathway and HLA-DRA (R: 1.52) in the MHC class II pathway.
3.3.4 Immune Checkpoints
In addition to immune cell markers, we detected the differential expressions of several immune checkpoint proteins (Supporting Information, Table 2). Programmed cell death 1 ligand 1 was upregulated in patient A03 (R: 2.42). Programmed cell death protein 6 (PDCD6) was downregulated in patient A01 (R: 0.3). However, in patients A02, A03, and A04, PDCD6, and PDCD6IP expression remained unchanged (R: 0.79, 0.97, and 0.73 for PDCD6; R: 0.72, 1.17, and 0.87 for PDCD6IP). Furthermore, activator protein (AP) complexes were downregulated, which mediate the lysosomal degradation of cytotoxic T lymphocyte antigen 4 (CTLA4) (Supporting Information, Figure 4). Three AP1 proteins (AP1B1, AP1G1, and AP1M1; R: 0.58, 0.67, and 0.64, respectively) and three AP2 proteins (AP2A1, AP2B1, and AP2M1; R: 0.58, 0.57, and 0.56, respectively) were downregulated in patient A04. Additionally, two AP2 complexes (AP2A1 and AP2M1; R: 058 and 0.72, respectively) were downregulated in patient A01. The downregulation of AP complexes suggested the decreased degradation of CTLA4 by lysosomes and the higher expression level of CTLA4 on the cell surface for immune signaling suppression in the patient with the highest PFS.
4 Discussion
A growing body of evidence indicates that cytotoxic chemotherapies not only induce the direct killing of cancer cells, but also trigger off-target effects on innate and adaptive immunity. Coordination between chemotherapeutic drugs and the host immune system may promote maximum cytotoxic efficacy against tumor cells and may aid in identifying optimal combinations with other chemo- or immuno-therapies.5, 22 To assess the global immune response, PBMCs, which comprise lymphocytes, monocytes, and several minor immune cell types, are easily collected to investigate the population change and the immune status during treatment. In this study, we conducted an individualized membrane proteomics analysis of PBMCs from patients with NSCLC to determine the effect of PEM–CIS combination chemotherapy on the immune status of patients, which has not yet been studied extensively. Two thousand eight hundred fifteen proteins were confidently identified in purified membrane protein fractions (8–20 μg) from a limited number of patients’ PBMCs.
No study has reported the proteomics screening of the PBMCs of patients receiving PEM–CIS treatment. However, several research groups have studied the PBMC proteome to elucidate the induced molecular mechanisms or to identify the markers of immune responses in various diseases. For example, Garg et al. analyzed the PBMCs of Trypanosoma cruzi infected patients and healthy controls using BODIPY FL-maleimide and 2-DE analysis. They identified pathomechanisms including the dysregulation of cytoskeletal assembly, recruitment/activation and migration of immune cells, hypoxia, and oxidative/inflammatory stress in disease progression and proposed a panel of proteins to assist in the prediction of seropositive individuals at a high risk of cardiomyopathy, with a success rate of >93%.10 Haudek-Prinz et al. applied both 2-DE and shotgun LC-MS/MS approaches to characterize NAMPT and PAI2 as markers of PBMC activation and IRF4, GBP1, and GBP5 as markers of specific T-cell inflammation.11 Up to now, the largest PBMC proteome dataset was provided by Zucht's research group; they utilized tandem mass tag and strong cation exchange fractionated proteomics strategies for the comprehensive analysis of PBMCs. More than 4000 proteins in PBMCs were identified to participate in various cellular functions in the immune system and in related diseases. In this dataset, 792 membrane proteins were also identified, among these, 36 were CD proteins.12
To examine the cell surface markers of immune cells, we directly analyzed membrane proteins through gel-assisted digestion, multiplexed iTRAQ tagging, RP StageTip fractionation, and LC-MS/MS analysis for the efficient and precise quantitation of PBMC membrane proteomes in the four patients. Among the 2815 identified proteins, approximately 56% were annotated as membrane proteins, including 50 immune cell markers, 47 CD proteins, 16 chemokine and chemokine receptors, 39 HLA antigens, 10 S100 proteins, 24 integrins, as well as proteins involved in antigen presentation and CD28 and CTLA4 pathways (Figure 2; Supporting Information, Table 2–5; Supporting Information, Figure 4 and 5). Compared with the total proteome analysis of PBMCs and conventional strategies that require the additional isolation of specific immune cell populations for immunophenotyping, this approach provided a reliable overall examination of cell surface markers as well as regulated downstream membrane-associated molecules for identifying the signatures of different immune cells from a limited cell sample. The PBMC membrane proteome profiles obtained from patients not only provided an indicator of immune responses, but also revealed personalized molecular changes for the systematic evaluation of their perturbed immunity under PEM–CIS treatment.
Despite the lack of molecular investigation of the effect of PEM–CIS treatment on patient immunity, the immunological regulations of CIS and PEM have been separately reported in different cancers. CIS was observed to modulate DCs and eliminate myeloid-derived suppressor cells in mice with melanoma.23 Moreover, it was reported to induce CD8 T-cell cytotoxic activity either as a single agent or in combination with paclitaxel and vinorelbine in a lung cancer in vitro model.24 CIS treatment enhanced antitumor immunity and prolonged survival in ovarian cancer-bearing mice with CD4 T-cell responses.25 PEM was reported to increase the activation of NK cells in patients with pancreatic adenocarcinoma.9 In our data, we highlighted the role of innate immune cells in association with the response to PEM–CIS chemotherapy. Following chemotherapy, the expression levels of neutrophil proteins (NCF1B and 2, DEFA1, and CD66b) were downregulated in patients A01 and A02. ITGB2, CD44, and SELL, which have neutrophil degranulation and adhesion functions, were upregulated in patient A01, indicating that although the neutrophil count was decreased, neutrophils were not defective.26 More interestingly, the neutrophil activity proteins (ELANE and MPO) were downregulated in patients A01, A02, and A03, but were only upregulated in patient A04. Our observations on circulating mononuclear cells suggested a lower number of neutrophils and lower neutrophil activity in the patient with lower PFS, which was consistent with the decreased count and percentage of neutrophils in patient A01. The patient with the highest PFS (A04) showed increased neutrophil expression (count and percentage) and activity (Figure 2A and Table 1).
Controversial outcomes have been reported for neutrophil infiltration within the tumor microenvironment; it has been observed to promote tumor growth and invasiveness27, 28 and has been implicated in higher CD8+ T-cell recruitment for higher survival in patients with colorectal cancer.29 Studies of ELANE have commonly indicated its negative effect on patients with lung cancer,30 but studies of MPO, a key enzyme of tobacco-induced carcinogenesis, have found both increased and reduced risks of the disease, and this discrepancy may be attributed to allele polymorphism, ethnicity, gender, age, and smoking status.31, 32 In the current study, we observed a positive effect on the number and activity of peripheral blood neutrophils and the response to PEM–CIS treatment in patient A04. The lower circulating neutrophil expression and activity in patients A01–A03 might be a result of PEM toxicity, which has been reported to induce neutropenia as a side effect,33 and the higher expression of peripheral neutrophil activity in the patient with a more favorable prognosis (A04) might be an inflammatory response to acute tumor cell destruction following chemotherapy, even though the high MPO expression in patient A04 might also be associated with smoking history.34, 35 However, whether increased neutrophil activity in peripheral blood is correlated with increased infiltration within the tumor microenvironment remains unknown, and further investigation is required.
Our study revealed that CD244 and SLAMF6, both proteins of NK cells, were negatively correlated with PFS. A similar result was reported in a study evaluating the immunological effect of PEM on advanced pancreatic adenocarcinoma, which revealed a considerable increase in NK cells 8 days after treatment and later a decrease upon combination therapy with gemcitabine.9 CD244 has been reported to enhance the functions of both NK cells and CTL and elicits an inhibitory signal for NK cells, depending on the intensity of cell surface expression and the relative availability of intracellular adaptor molecules.36, 37 Importantly, CD244 signaling that occurs through SLAM-associated protein may induce NK cell activation.38 However, both CD244 and SLAMF6 were downregulated in patient A04, indicating the suppressed number and cytotoxic activity of NK cells in patients with a more favorable response to treatment.
Regarding other innate immune cells, although the specific markers of monocytes, macrophages, and DCs were difficult to determine, the general markers of myeloid subsets, CD14, and CD33 were observed. Although CD33 levels remained unchanged among patients, CD14 expression was upregulated in patient A01 and downregulated in patient A03. In addition, other proteins involved in downstream pathways were downregulated in patients with a more favorable response to treatment, indicating less NK activity in the patient.
In the lymphocytic pool, the overall cell count was increased in the patient with a worse response to treatment, as observed in both the blood count (Table 1) and marker protein expressions (Figure 2A). An observation of specific T-cell subsets revealed that patient A01 exhibited an upregulation of Th1 markers, and patients A02, A03, and A04 had more activated Th2 and T regulatory (Treg) responses. In cancer progression, both Treg and Th17 subsets facilitate a protumoral environment that may favor tumorigenesis and metastasis by the promotion and maintenance of an immunosuppressive state.39 In our study, although the differentiation markers of Treg and Th17 subsets were not exclusive, TGFβ (cytokine released by Tregs that induces further Treg differentiation40, 41) and CCR4 (commonly found on the cell surface of both Th2 and Treg cells) were both upregulated in patients with more favorable responses to treatment, and STAT3 protein (promotes the differentiation of both Th17 and Th2 subsets42, 43) and IL17RA (a receptor for IL17A—a characteristic cytokine expressed by Th17 cells) remained unchanged among all patients, indicating a skew toward Th2 and Treg responses. Moreover, the AP protein complexes were downregulated in patient A04, reflecting a diminished degradation of the CTLA4 checkpoint molecule that binds to CD28 with higher affinity and thus inhibiting IL-2 cytokine induction and the subsequent Th1 response.44 Accordingly, we speculated that this increase in immunosuppressive cell number and activity was a function of the body's homeostatic response to limit the overactivity of immune cells, which may lead to extensive inflammation and an autoimmune state. Furthermore, similar to our result, Davis et al. also reported a strong correlation of FoxP3+ CD8+ Treg cells with overall survival and PFS in patients with pancreatic adenocarcinoma treated with PEM and gemcitabine.9 They proposed that the reduced expression of proinflammatory cells and their released cytokine, IFNγ, is beneficial.
In our data, patient A03 demonstrated the upregulated expression of MHC class I molecules and the downregulated expression of MHC class II molecules in the antigen presentation pathway, whereas patient A01 presented contradictive expressions (Figure 2B). It has been widely reported that most tumor antigens are presented by MHC class I molecules to trigger the activation of CTL and NK cells, which mediate the direct killing of tumor cells. The loss or downregulation of HLA class I antigens in the tumor represents a cancer immune escape mechanism and is correlated with more severe disease and a worse prognosis.45-47 Although increased MHC class I molecule expression was observed in patient A03, no significant increase in the responding cells, CTL and NK cells, was observed, indicating that another mechanism or molecule is involved in CTL and NK cell regulation. In recent years, increased research on the MHC class II pathway and its relationship to cancer has been conducted. MHC class II molecules are involved in presenting the phagocytosed antigen to CD4+ T cells (Th cells), which further activate the cascade of immune responses. Although Th cells can also directly mediate cytotoxicity against tumor cells,48 Th-cell activation generates an increased level of Treg cell differentiation, which may limit the immune response for tumor eradication.49 Classical MHC class II molecules (HLA-DR, HLA-DP, and HLA-DQ) bind to the invariant chain (Ii), which is then degraded in the endosome. The peptide-binding groove of most MHC class II molecules must be released by the action of the nonclassical molecule, HLA-DM. In most resting antigen presenting cells, the function of HLA-DM is negatively regulated by HLA-DO.50 In this study, HLA-DM remained unchanged in patient A01, but expressions of HLA-DOs were increased. Thus, although the overall T-cell population increased, Treg differentiation was not affected by PEM–CIS combination therapy. The higher expressions of MHC class II molecules in the antigen presentation pathway in patient A01 suggested that T-cell receptor signaling was induced (Figure 2B and Supporting Information, Figure 4); the higher expressions increased the activation of the Th cell response. By contrast, in patient A04, CTLA4 expression was higher, indicating that the immunosuppressive environment was more prominent.
Our study provided a personalized molecular profile of changes in patients’ immune status upon PEM–CIS chemotherapy. However, other factors may affect patients’ immune status, and technical limitations hindered our study. Both CIS and PEM have been revealed to induce impaired renal function and myelosuppression. In addition, the presence of concomitant infection or disease may also be involved in the alteration of the immune status upon PEM–CIS chemotherapy. Therefore, monitoring the immune status change during the treatment course may be essential and should be investigated in future studies. The lack of a large sample size hampered drawing a general correlation between the immune status and the response to chemotherapy and identifying a universal protein panel for clinical practice. The limited amount of PBMCs from patients’ blood did not allow for further validations using immunoassays. The technical limitations in the identification of lower abundant specific cell markers for T lymphocyte subtypes also resulted in limited information being obtained on the different subsets of macrophages, Th cells, and NK cells, which may execute either the activation or inhibition of the immune response. Furthermore, more immunological and molecular studies are required to reveal the functional effect and establish more accurate data that can be translated into clinical practice as biomarkers for predicting the response to therapy and prognosis.
Our study utilized personalized membrane proteomics to observe the immune response of patients with advanced NSCLC to first-line combination PEM–CIS chemotherapy. We observed that the patient with a shorter PFS showed relatively more lymphocyte number, probably as a response to the higher expression of MHC class II molecules with antigen-presenting activity. In addition, innate immune cells other than neutrophils were more abundant, but neutrophil expression and activity were lower. The patient with a partial response to treatment and thus a longer PFS exhibited an immunosuppressive state, which was demonstrated by higher expressions of Th2 and Treg markers as well as the inhibition of CTLA4 degradation, reflecting the host's effort to achieve homeostasis. A marked increase in neutrophil number and activity was also observed, although the expression and activity of other innate immune cells were downregulated. Patients with a stable disease upon treatment exhibited an enriched expression of MHC class I molecules, but without a noted increase in CTL and NK cell numbers as a response. Together, these observations revealed an overall immune status characterization of patients receiving PEM–CIS chemotherapy. In the future, more samples should be analyzed to establish more accurate molecular profiles that may contribute to an improvement in the prediction of responses to treatment.
Abbreviations
-
- AP
-
- activator protein
-
- B2M
-
- beta-2-microglobulin
-
- BCAP
-
- B-cell receptor associated proteins
-
- CCR
-
- CC chemokine receptor
-
- CD
-
- cluster of differentiation
-
- CIS
-
- cisplatin
-
- CTLA4
-
- cytotoxic T lymphocyte antigen 4
-
- DC
-
- dendritic cell
-
- DEFA1
-
- neutrophil defensing 1
-
- ELANE
-
- neutrophil elastase
-
- FCGR3A
-
- Fc gamma receptor III-A
-
- HLA
-
- human leukocyte antigen
-
- MPO
-
- myeloperoxidase
-
- NCF
-
- neutrophil cytosolic factors
-
- NK cell
-
- natural killer cell
-
- NSCLC
-
- Non–small-cell lung cancer
-
- PBMC
-
- Peripheral blood mononuclear cell
-
- PDCD6
-
- programmed death protein 6
-
- PEM
-
- pemetrexed
-
- PFS
-
- progression-free survival
-
- SELL
-
- l-selectin
-
- SLAMF6
-
- signaling lymphocytic activation molecule family 6
-
- TAP
-
- antigen peptide transporter
-
- TEABC
-
- triethylammonium bicarbonate
-
- Th cell
-
- T-helper cell
-
- Treg
-
- T regulatory
Acknowledgements
D.U.P. and P.-H.F. contributed equally to this work. This research was financially supported by the Ministry of Science and Technology (MOST105-2113-M-038-002 and MOST106-2113-M-038-004-MY2), Taipei Medical University (TMU103-AE1-B14), and Taipei Medical University-Shuang Ho Hospital (104TMU-SHH-22) in Taiwan. LTQ-Orbitrap data and additional technical assistance were provided by the Academia Sinica Common Mass Spectrometry Facilities located at the Institute of Biological Chemistry.
Conflict of Interest
The authors declare no conflict of interest.




