Shotgun proteomics identifies proteins specific for acute renal transplant rejection
Abstract
Purpose: Acute rejection (AR) remains the primary risk factor for renal transplant outcome; development of non-invasive diagnostic biomarkers for AR is an unmet need.
Experimental design: We used shotgun proteomics applying LC-MS/MS and ELISA to analyze a set of 92 urine samples, from patients with AR, stable grafts (STA), proteinuria (NS), and healthy controls.
Results: A total of 1446 urinary proteins (UP) were identified along with a number of non-specific proteinuria-specific, renal transplantation specific and AR-specific proteins. Relative abundance of identified UP was measured by protein-level spectral counts adopting a weighted fold-change statistic, assigning increased weight for more frequently observed proteins. We have identified alterations in a number of specific UP in AR, primarily relating to MHC antigens, the complement cascade and extra-cellular matrix proteins. A subset of proteins (uromodulin, SERPINF1 and CD44), have been further cross-validated by ELISA in an independent set of urine samples, for significant differences in the abundance of these UP in AR.
Conclusions and clinical relevance: This label-free, semi-quantitative approach for sampling the urinary proteome in normal and disease states provides a robust and sensitive method for detection of UP for serial, non-invasive clinical monitoring for graft rejection after kidney transplantation.
1 Introduction
Publication of human genome data has facilitated high throughput studies, including the large scale analysis of human proteins in different biofluids 1, 2. Biofluids such as blood, urine, nipple aspirate fluid, bronchoalveolar lavage, and cerebrospinal fluid 3-6 have been analyzed by various proteomics techniques, and based on their relevance to the concerned disease; however, detection of low-abundance proteins and the identification of the statistically significant proteins are still positioned as bottlenecks for proteomics analysis.
Recent studies 7-11, including those by Adachi et al. 12 and Gonzales et al. 13 have used gel-based as well as gel-free proteomics approaches to contribute to the atlas of urinary proteins (UP) in normal individuals. Using 1-D SDS-PAGE and a reversed phase HPLC protein separation and fractionation method followed by application of LTQ-FT and LTQ-Orbitrap technology, Adachi et al. analyzed urine samples collected from healthy individuals and identified 1543 proteins 12. Gonzales et al. analyzed urinary exosomes using LC-MS/MS and identified 1132 proteins 13. In this study, we have undertaken a pilot study of ten normal samples, 40 urinary samples from patients with nephrotic syndrome as well as renal transplant patients with stable graft (STA) function and biopsy proven acute rejection (AR). The purpose of the study was to study if phenotype specific differences could be identified in urinary samples from patients with different etiologies of native and transplant-associated renal injury.
The benefit of identifying rejection-specific proteome biomarkers in urine is very relevant. Renal transplantation is the ultimate treatment for patients with end stage kidney disease 14, but there is no current non-invasive means to monitor for acute graft rejection. The renal biopsy, the gold standard for diagnosing rejection, is an invasive procedure that suffers from sampling heterogeneity, has associated complications of pain, sedation, hematuria, arteriovenous fistulae, graft thrombosis, and transfusion risk 15, and correlates poorly with treatment response and prognosis. Because of the ability of urine to reflect both local processes within the kidney as well as a reflection of changes within plasma, urine is particularly useful to diagnose kidney diseases and kidney transplant dysfunction 16. Discovery of a urine biomarker for assessing the rejection status of patients following kidney transplant could significantly improve patient outcomes and decrease the cost of care.
To test the validity of the discovery of AR-specific protein biomarkers by our study approach, we performed ELISA assays on selected protein biomarkers using an independent set of 52 unique patient urines. ELISA results established that the approach taken in this study is a viable way to discover potential biomarkers. The report demonstrates how high-throughput, high-cost, labor-intensive MS-based discovery can eventually be developed into an economical, rapid turn-around, clinically applicable diagnostic assay for transplant patients.
2 Materials and methods
2.1 Materials
The following reagents were used for the proteomics sample preparation: nanopure or Milli-Q quality water (∼18 megohm·cm or better); bicinchoninic acid (BCA) Assay Kit was purchased from Pierce (Rockford, IL, USA); Amicon Ultra centrifugal filtration tubes were obtained from Millipore (Bedford, MA, USA) ammonium bicarbonate, ammonium formate, and formic acid were obtained from Fluka (St.Louis, MO, USA); Tris-HCl, urea, thiourea, DTT, iodoacetamide, calcium chloride, and TFA were obtained from Sigma-Aldrich (St. Louis, MO, USA); HPLC-grade methanol (MeOH) and HPLC-grade ACN were purchased from Fisher Scientific (Fair Lawn, NJ, USA); 2,2,2-trifluoroethanol was obtained from Aldrich Chemical (Milwaukee, WI, USA); and sequencing grade modified trypsin was purchased from Promega (Madison, WI, USA). Pigment epithelium-derived factor (PEDF) (SERPINF1) ELISA kit was purchased from Bioproducts, MD (Middletown, MD, USA).
2.2 Samples
Forty individual and clinically annotated urine samples were included in the study. We used ten renal transplant patients, each with biopsy proven AR, as diagnosed by the standardized Banff criteria 17 and ten renal transplant patients with STA, classified by the absence of any AR or significant chronic injury on a protocol biopsy. All urine samples were collected at the time of a clinically indicated or protocol biopsy, prior to any treatment intensification. Our controls included ten non-specific proteinuria (NS) patients, from minimal change disease, not currently on any immunosuppression treatment, and ten age-matching healthy children as healthy controls (HC). As some element of proteinuria from injury in AR can be seen, the NS patients provided controls for the presence or absence of proteinuria. Patient demographics were matched (Table 1). The samples were collected between January 2005 and June 2007 and were obtained as part of an ongoing IRB-approved study at Stanford University. Approval for the conduct of this research was obtained from the Institutional Review boards at Stanford University and Pacific Northwest National Laboratory (PNNL) in accordance with federal regulations.
| AR) (n=30) | STA function (n=30) | p Value | |
|---|---|---|---|
| Mean age | 12±5 | 14±5 | 0.21 |
| Age range | 3–19 | 6–21 | |
| Immunosuppression, %SFa) | 66% | 50% | 0.19 |
| Raceb) | 63,13,0,17 and 7% | 59,7,10,17 and 7% | 0.45 |
| Donor, % living donor | 40 | 53 | 0.44 |
| Mean glomerular filtration rate (mL/min/1.73m2) | 87.45±38.46 | 124±29.86 | 0.0001 |
- a Patient information on 60 renal transplant patients (30 AR, 30 STA).
- a) a) SF: Steroid-free immunosuppression treatment, consisting of daclizumab induction+mycophenolate mofetil+tacrolimus.
- b) b) Race: 1=Caucasian; 2=Hispanic; 3=Asian; 4=African American; 5=Other.
2.3 Urine collection, initial processing, and storage
Second morning void mid-stream urine samples (50–100 mL) were collected in sterile containers and were centrifuged at 2000×g for 20 min at room temperature within 1 h of collection. The supernatant was separated from the pellet containing any particulate matter including cells and cell debris. The pH of the supernatant was adjusted to 7.0 and stored at −80°C until further analysis. Urine osmolality was measured on all samples.
2.4 Recovering and quantification of UP
UP were isolated by removing small MW peptides and other pigments (<10 kDa) by filtering the supernatant through Amicon Ultra centrifugal filtration tubes (Millipore). The tubes were pre-equilibrated with 10 mL Milli-Q water and centrifuging at 3000×g for 10 min at 10°C using swinging bucket rotors. After equilibration, 10 mL of urine supernatant was centrifuged for 20 min at 3000×g at 10°C. The filtrate was recovered and saved for peptidomic analysis. Urine creatinine was measured using Quantichrom™ Creatinine Assay Kit (DICT-500) (BioAssay Systems, Hayward, CA, USA). The manufacturer's manual was followed for the assay. The retentate was washed twice with 10 mL of 20 mM Tris-HCl (pH 7.5). The final volume of the retentate was brought to 400 μL with 20 mM Tris-HCl (pH 7.5) and was quantified by using BCA protein assay (Pierce). The mean protein to creatinine ratios for AR, STA, HC, and NS were 0.22±0.15, 0.14±0.06, 0.05±0.02, and 0.67±1.40, respectively. After the quantification of individual samples, four pooled samples for each AR, STA, NS, and HC categories were prepared using 200 μg from each individual sample in each category.
2.5 Urinary proteome sample preparation
Samples were desalted using Micron Ultracel YM-3 centrifugal filters MWCO 3000 (Millipore, Billerica, MA, USA) prior to the tryptic digestion following the manufacturer's protocol. Protein concentration was verified after buffer exchange using a BCA Protein Assay. A mixture of three standard proteins, purchased individually from Sigma-Aldrich (horse apomyoglobin, rabbit glyceraldehyde-3-phosphate dehydrogenase, and bovine ovalbumin), was added for quality control purposes. Proteins were denatured in 50 mM ammonium bicarbonate, pH 7.8, 8 M Urea for 1 h at 37°C and then reduced with 10 mM DTT at 37°C for 1 h. After this they were alkylated with 40 mM iodoacetamide at room temperature for 1 h in the absence of light. Samples were diluted tenfold with 50 mM ammonium bicarbonate, pH 7.8, and sufficient amount of 1 M calcium chloride was added to the samples to obtain a concentration of 1 mM in the sample. Sequencing grade-modified trypsin was prepared by adding 20 μL of 50 mM ammonium bicarbonate, pH 7.8, to a vial containing 20 μg trypsin and after 10 min incubation at 37°C was used for digestion of the samples. Tryptic digestion was performed for 3 h at 37°C with 1:50 w/w trypsin-to-protein ratio. Rapid freezing of the samples in liquid nitrogen quenched the enzymatic digestion.
Digested samples were desalted by using a SPE C18 column (Discovery DSC-18, SUPELCO, Bellefonte, PA, USA) conditioned with MeOH and rinsed with 0.1% TFA, 1 mL, and washed with 4 mL of 0.1% TFA/5% ACN. Peptides were eluted from the SPE column with 1 mL of 0.1% TFA/80% ACN and concentrated in a Speed-Vac SC 250 Express (Thermo Savant, Holbrook, NY, USA) to a volume of ∼50–100 μL. The peptide concentration was measured using the BCA Protein Assay. Digested samples were stored at −80°C until needed for analysis or further processing.
2.6 Strong cation exchange fractionation
Digested samples (200.0–350.0 μg) were reconstituted with 900 μL of 10 mM ammonium formate, pH 3.0/25% ACN and fractionated by strong cation exchange chromatography on a Polysulfoethyl A 2.1 mm×200 mm, 5 μM, 300 Å column with 2.1 mm×10 mm guard column (PolyLC, Columbia, MD, USA) using an Agilent 1100 series HPLC system (Agilent technologies, Palo Alto, CA, USA) The flow rate was 200 μL/min, and mobile phases were 10 mM ammonium formate, pH 3.0/25% ACN (A), and 500 mM ammonium formate, pH 6.8/25% ACN (B). After loading 900 μL of sample onto the column, the mobile phase was maintained at 100% A for 10 min. Peptides were then separated using a gradient from 0 to 50% B over 40 min, followed by a gradient of 50–100% B the following 10 min. The mobile phase was held at 100% B for 10 min, followed by H2O rinsing for the next 20 min and final re-conditioning with A for 10 min. A total of 60 fractions over 90 min separation were collected for each depleted sample, and each fraction was dried under vacuum in a Speed-Vac. The fractions were dissolved in 25 μL of 25 mM ammonium bicarbonate, pH 7.8, and combined into 32 fractions for LC-MS/MS analysis. The first 20 fractions were combined into one and were desalted by C18 SPE column (Discovery DSC-18, SUPELCO, Bellefonte), the next 30 fractions were not pooled and each was analyzed separately, and 5.0 μL of each of the last ten fractions were combined together into fraction number 32. A 5.0 μL aliquot of each fraction was analyzed by capillary LC-MS/MS.
2.7 Capillary LC-MS/MS analysis
The HPLC system consisted of a custom configuration of 100-mL Isco Model 100DM syringe pumps (Isco, Lincoln, NE, USA), two-position Valco valves (Valco Instruments, Houston, TX, USA), and a PAL autosampler (Leap Technologies, Carrboro, NC, USA), allowing for fully automated sample analysis across four separate HPLC columns 18. Reversed phase capillary HPLC columns were manufactured in-house by slurry packing 3-μm Jupiter C18 stationary phase (Phenomenex, Torrence, CA, USA) into a 60-cm length of 360 μm od×75 μm id fused-silica capillary tubing (Polymicro Technologies, Phoenix, AZ, USA) that incorporated a 2.0-μm retaining screen in a 1/16′′ 75 μm id union (Valco Instruments). Mobile phase consisted of 0.2% acetic acid and 0.05% TFA in water (A) and 0.1% TFA in 90% ACN/10% water (B). The mobile phase was degassed by using an in-line Degassex Model DG4400 vacuum degasser (Phenomenex). The HPLC system was equilibrated at 10 k psi with 100% mobile phase A, and then a mobile phase selection valve was switched 20 min after injection, which created a near-exponential gradient as mobile phase B displaced A in a 2.5 mL active mixer. A 30-cm length of 360 μm od×15 μm id fused-silica tubing was used to split ∼20 μL/min of flow before it reached the injection valve (5 μL sample loop). The split flow controlled the gradient speed under conditions of constant pressure operation (10 k psi). Flow rate through the capillary HPLC column was ∼900 nL/min. A ThermoScientific LTQ linear ion trap mass spectrometer (ThermoScientific, San Jose, CA, USA) was coupled with the LC-system using an in-house ESI interface for all sample analysis. Home-made 150 μm od×20 μm id chemically etched electrospray emitters were used 19. The heated capillary temperature and spray voltage were 200°C and 2.2 kV, respectively. Data was acquired for 90 min, beginning 30 min after sample injection (10 min into gradient). Full spectra (AGC setting: 3×104) were collected from 400–2000 m/z followed by data-dependent ion trap MS/MS spectra (AGC setting: 1×104) of the ten most abundant ions applying collision energy of 35%. A dynamic exclusion time of 60 s was applied.
2.8 Peptide and protein identification using MS/MS spectra
Peptides were identified from MS/MS spectra by matching them with predicted peptides from the protein FASTA file from the human International Protein Index (IPI – European Bioinformatics Institute) database (version 3.20, released at August 22, 2006) containing 61 225 protein entries using the SEQUEST™ algorithm 20. A standard parameter file allowing for a dynamic addition of oxidation to the methionine residue and a static (non-variable) carboxamidomethylation modification to the cysteine residue, with a mass error window of 3 Da units for precursor mass and 1 Da unit for fragmentation mass was used. The searches were allowed for all possible peptide termini, i.e. not limited by tryptic-only termini. Not limiting by tryptic termini means that we did not require the peptide to end in a K, R, or be the termini of the protein. Peptide identifications were considered acceptable if they passed the thresholds determined acceptable for human plasma by Qian et al. 21 and passed an additional filter of a PeptideProphet score of at least 0.7 22. The PeptideProphet score is representative of the quality of the SEQUEST™ identification and is based on a combination of XCorr, delCn, Sp, and a parameter that measures the probability that the identification occurred by random chance. PeptideProphet scores are normalized to a 0 to 1 scale, with 1 being the highest confidence value.
2.9 Protein grouping
Due to the high redundancy of peptide-to-protein relationships inherent in the IPI database, two protein grouping programs were used to consolidate sequence identifications. ProteinProphet 23 uses the identified peptide sequences to weight the probability that the peptide originated from a particular protein. When parent protein distinctions cannot be determined, those proteins are grouped together and assigned an index value. The second method of grouping proteins involved aligning whole-protein sequences and assigning similarity scores based on the amino acid composition. This method utilized the BLAST algorithm to determine similarity of each protein to all other proteins in the input file. Then these similarities are clustered into groups of like similarities using a Perl script.
2.10 Differentially expressed proteins
Protein-level spectral counts were obtained by summing peptide-level spectral counts. To quantitatively compare relative protein abundances between different pools of samples, we considered either presence or absence of a particular protein in different phenotypes. For the proteins that were identified in multiple categories we used a cutoff criteria of ≥2 of fold change in log base(2) of spectral count with at least five spectral counts in one of the phenotypes being compared.
2.11 ELISA assays for Tamm–Horsfall protein (uromodulin)
A total of 60 urine samples (20 AR, 20 STA, and 20 HC) were included. Urine samples were diluted 200-fold in PBS buffer. Aliquots of diluted 100 μL of urine were incubated in Reacti-Bind 96-Well Plates overnight at 4°C. The plate was washed five times with 1×PBS buffer containing 0.05% Tween 20. The wells were then blocked by 100 μL of 25% FCS in PBS to prevent non-specific binding of the antibody. The wells were then incubated with 1:3000-fold diluted anti-Tamm–Horsfall Glycoprotein PAB at room temperature for 1 h. The color was developed by using turbo-tetramethylbenzidine (TMB) (Pierce) and stopped by the addition of 100 μL of 2 M H2SO4 and the plate was read by a SPECTRAMax 190 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
2.12 ELISA for Pigment epithelium-derived factor-PEDF (SERPINF1), and CD44
Sandwich ELISA assays were performed to validate the observed elevated levels of PEDF (SERPINF1) and CD44 in urine collected from an independent set of patients and controls, which included AR (n=20), STA (n=20), NS [n=8 for PEDF (SERPINF1) and 6 for CD44], HC (n=6).
2.13 PEDF (SERPINF1) ELISA
An ELISA kit for PEDF or SERPINF1 (BioProducts, MD) was used for the purpose and the reagents were prepared following the manufacturer's manual. Briefly, after an initial optimizing step for optimal dilution of urine, the urine samples were diluted (1:40) in Assay Diluent. The ELISA plate with 100 μL of standards and the diluted urine specimens was incubated at 37°C for 1 h. After the incubation the plates were washed five times with Plate Wash Buffer. The wells were incubated with 100 μL PEDF (SERPINF1) detector antibody at 37°C for 1 h and washed five times with the wash buffer. This step was followed by incubation of the wells with 100 μL Streptavidin Peroxidase Working solution.
2.14 CD44 ELISA
An ELISA kit for CD44 (ABCam, Cambridge, MA, USA) was used for the purpose and the reagents were prepared following the manufacturer's manual. Briefly, after an initial optimizing step for the optimal dilution of urine, the urine samples were diluted (1:1) in Standard Diluent Buffer. The ELISA plate with 100 μL of standards and the diluted urine specimens was incubated at room temperature for 1 h. After the incubation the plates were washed five times with washing solution. The plate was incubated for 30 min with 50 μL of diluted biotinylated anti-CD44 in all wells. The plate was washed five times with the wash solution and was incubated with 100 μL HRP solution in all the wells for 30 min. This step was followed by a wash step.
All the assays were developed by ready-to-use TMB substrate followed by addition of Stop Solution. All the plates were read by a SPECTRAMax 190 microplate reader (Molecular Devices). Protein concentrations were determined from a standard curve generated from the standards obtained with the kit.
2.15 Correlation analysis between the spectral counts and the quantity observed from ELISA assay
We obtained quantitative data for uromodulin (UMOD), PEDF (SERPINF1), and CD44 using ELISA assays on an independent set of patients. The quantitative data obtained from ELISA was compared with the spectral count data for each protein observed in the discovery phase using the LC-MS/MS platform. p-Values and Pearson correlation coefficients were calculated using SAS® program (SAS Corporate Statistics, Cary, NC, USA). The receiver operating characteristic (ROC) was calculated by logistic regression model for three proteins based on the values of AR versus no-AR that included STA, HC, and NS.
2.16 Enrichment analysis and pathway impact analysis
The enrichment analysis for identified proteins was performed using Ingenuity Pathway Analysis (http://www.ingenuity.com). A list of all human genes was used as reference for computing significance, which was obtained from the Onto-Tools database 24. The pathway analysis was also performed using Pathway-Express 25, 26. Pathway-Express performs a novel impact analysis on signaling pathways, which in addition to the number of proteins in Ingenuity Pathway Analysis considers important biological factors such as the topology of the pathway, position of the protein on the pathway, amount of change in protein expression, and the type of interaction between the protein in each pathway.
3 Results
3.1 Detection of novel UP expands the urinary proteome database
We identified 1446 UP by using LC-MS/MS-based shotgun proteomics on urine from renal patients and healthy individuals. A minimum of two unique, non-redundant peptides per protein to be identified was the criteria for positive protein identification. The false discovery rate (FDR) for protein identifications is ∼0.1% based on target-decoy analysis using Sequest, while the FDR at the unique peptide level is ∼3.0%. The number of total proteins identified in this study with one peptide identified per protein criteria was 3296. The complete list of proteins is provided as Supporting Information data (Supporting Information Table 1).We identified 1001, 1159, 1325, and 1340 proteins respectively in AR, NS, STA, and HC urine, respectively (Fig. 1). We used Ingenuity Pathway Analysis -IPA (Ingenuity® Systems, Redwood City, CA, USA; www.ingenuity.com) on predicted proteins based on the human genome database 27 and mapped the proteins identified with previously annotated UP and proteins of renal origin. A total of 756 UP from our 1446 protein list have been listed as UP. This leaves 690 proteins in our list of UP as novel and were labeled as novel urinary proteins (NUP) (Supporting Information Table 2). We compared the list of UP identified from healthy individuals in this study with 1543 identified by Adachi et al. 12 and 1160 by Gonzales et al. (Fig. 2A) 13. This study has added 560 new proteins to the existing urinary proteome observed in healthy subjects.
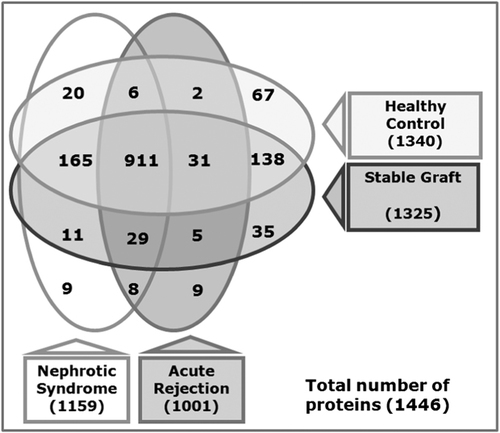
UP were identified from urine collected from healthy as well as renal patients with or without kidney transplant. Number of proteins identified in urine collected from renal transplant patients with biopsy proven AR, renal transplant patients with STA, HC, and renal patients with NS.
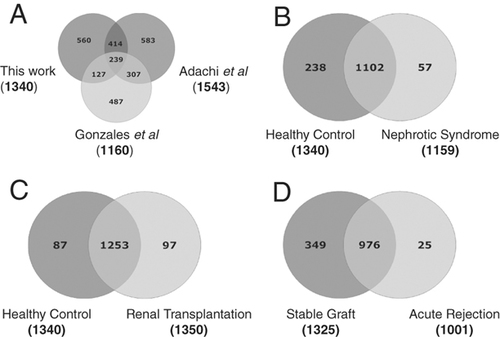
UP identified from different patients groups including the HC were compared. (A) A Venn diagram to compare UP from healthy normal individuals identified in this study with the proteins identified by Adachi et al. 12 and UP identified by 38. (B) A comparison of proteins identified in healthy urine (HC) and urine of NS. (C) A comparison of proteins identified in healthy urine (HC) and urine of renal transplant patients both STA and AR combined. (D) A comparison of proteins identified in urine from STA to urine of AR.
UP are enriched with extracellular proteins, complement, and coagulation, glycan structures–degradation, cell adhesion, and extracellular matrix (ECM)–receptor interaction. Gene ontological classification 28 sub-grouped the 1446 identified proteins into five major groups; 279 were cytoplasmic proteins, 325 were extracellular proteins, 28 were nuclear proteins, 304 were plasma membrane, and 108 had as yet unknown sub-cellular localization. We found that extracellular and plasma membrane proteins were enriched, and nuclear proteins were relatively underrepresented in the urine proteome when compared with the predicted human proteome from the human genome database. This is in agreement with previously published data 27. Hypergeometric analysis revealed that the enrichment of proteins of extracellular origin (p<1.00E-6) and plasma membrane in urine (p<3.00E-6) is highly significant compared with the human proteome. The major representing pathways were complement and coagulation cascades (p=1.95E-12), glycan structures–degradation (p=1.31E-11), cell adhesion molecules (CAMs) (p=1.77E-11), ECM–receptor interaction (p=1.87E-11), cell communication (p=2.04E-11), focal adhesion (p=2.62E-11), axon guidance (p=2.86E-11), regulation of actin cytoskeleton (p=4.97E-09), cytokine–cytokine receptor interaction (p=3.26E-09), and hematopoietic cell lineage (p=4.89E-08).
There was no specific bias towards plasma and renal proteins in the urine of renal patients and depletion of ECM-receptors and integrins in renal patients: We identified the 1420 proteins that were detected in the urine of patients with normal renal function (HC and STA), and only 1206 proteins were found in patients with active renal dysfunction (AR and NS). There was no bias of the health status of the kidney in terms of known urinary, blood, and renal proteins when we used Ingenuity Pathway Analysis® based annotation. Among the total 1420 proteins identified in HC and STA; 578, 463, and 434 proteins were previously known urinary, blood, and renal proteins, while in the total 1206 proteins identified in AR and NS, 504, 405, and 353 proteins were previously known urinary, blood, and renal proteins (Table 2).
| Proteins identified in AR and NS combined | Proteins identified in HC and STA combined | |
|---|---|---|
| Proteins identified in this study | 1206 | 1420 |
| Previously known UP | 504 | 578 |
| Previously known plasma/serum proteins | 405 | 463 |
| Previously known kidney proteins | 353 | 434 |
In total, 67 proteins were uniquely identified only in healthy urine (HC) (Supporting Information Table 3). EH-domain-containing protein 1 (EDH1) and creatinine kinase B-type were the two most abundant proteins identified in this group. Among these proteins a significant number of proteins are known to be involved in cell morphology (CEACAM6, CR1, CRYAB, ERK, GNA12, GNA13, GNAQ, KDR, NOS3, PAFAH1B1, PP1CB, PTPRF, RAB4A, RYR2), metabolic disease and lipid metabolism (ACO1, CD7, DDC, EHD1, EXTL2, FAM125A, FLRT3, LPHN3, MAN2A2, PPIC, RAB4B, RAB5B, SORD, VPS28, and VPS37D).
3.2 Correlation between data obtained based on spectral count and ELISA assays
The relative abundance of identified UP was measured by protein-level spectral counts adopting a weighted fold-change statistic, and assigning increased weight for the more frequently observed proteins. Observed abundances of highly abundant proteins are less likely to be biased in a particular direction due to random variations in the peptide detection from sample to sample. The higher the abundance of the proteins involved, the more confident we are that the observed fold-change difference is close to the actual fold-change difference. A complete data set of identified proteins and observed spectral counts for each protein for all the phenotypes tested is provided in (Supporting Information Table 4). UMOD, SERPINF1 (PEDF), and CD44 proteins were analyzed by ELISA assay to obtain concentrations for the proteins. The spectral counts for the proteins measured by LC-MS were compared and correlated to the concentration calculated from ELISA assays on an independent set of the urine samples from similar phenotypes that were used in the discovery phase (Table 3). We observed a good correlation in between the spectral counts and quantitative data measured from ELISA assays. An ELISA assay was run for urinary Tamm–Horsfall protein (THP). It was performed on an independent validation set of samples with AR (n=20), STA (n=20), and HC (n=20). The mean UMOD concentration in AR urine (5.50±0.85 μg/mL) STA urine (13.95±2.94 μg/mL), and healthy normal control urine (19.80±2.71 μg/mL) has been compared with the spectral counts for AR (122), STA (363), and HC (567) with a correlation coefficient of 0.99 and p-value 0.02. The mean PEDF (SERPINF1) concentration in AR urine (0.370±0.350 ng/mL), STA urine (0.006±0.009 ng/mL, and HC urine (0.01±0.009 ng/mL) was compared with the spectral counts for AR (75), STA (54), and HC (15) with a correlation coefficient of 0.78. When we assayed CD44 in an independent sample set of urine samples, we measured a mean CD44 concentration in AR urine (1.67±1.17 ng/mL), STA urine (2.81±1.10 ng/mL, and HC urine (2.54±1.41 ng/mL). This was in good correlation with spectral counts observed from LC-MS experiments for AR urine (15), STA (42), and HC (126) with a correlation coefficient of 0.59. When we combined total concentration measured from ELISA assay and compared with the spectral counts for corresponding samples, there was an excellent correlation (R2=0.84) with a p-value <0.0012 (Table 3).
| Protein name | Samples | Concentration measured by ELISA assays (ng/μL) | Spectral count | |||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Median | Mean | |||
| THPa) | AR (n=20) | 216.00 | 13 000.00 | 4150 | 5504.50 | 122 |
| THP | STA (n=20) | 374.00 | 56 828.00 | 10 248 | 13 951.90 | 363 |
| THP | HC (n=20) | 7424.00 | 66 622.00 | 17 865 | 19 798.10 | 567 |
| PEDFb) | AR (n=20) | 10.00 | 1357.00 | 327 | 395.95 | 75 |
| PEDF | STA (n=20) | 0.00 | 40.00 | 0 | 6.00 | 54 |
| PEDF | HC (n=8) | 0.00 | 30.00 | 10 | 10.00 | 15 |
| PEDF | NS (n=6) | 0.00 | 96.00 | 5 | 19.33 | 124 |
| CD44 | AR (n=20) | 0.34 | 3.96 | 1.27 | 1.67 | 15 |
| CD44 | STA (n=20) | 3.42 | 19.87 | 13.2 | 12.57 | 42 |
| CD44 | HC (n=6) | 4.06 | 19.87 | 11.1 | 11.76 | 126 |
| CD44 | NS (n=6) | 1.99 | 17.97 | 6.51 | 8.54 | 20 |
| Correlation | 0.84 | |||||
| Cumulative correlation among all the concentration and spectral counts for three proteins | p Value | <0.0012 | ||||
- a Protein concentrations for these proteins were measured by ELISA and correlated with the concentration obtained with the spectral count data observed from label-free LC MS.
- a) a) THP: UMOD.
- b) b) PEDF:SERPINF1.
3.3 UP specific to nephrotic syndrome
We analyzed the urine from patients with NS. Nine proteins were uniquely identified in the nephritic syndrome (NS) urine, which included proteins like keratin 5b (KRT78), Isoform DPI of desmoplakin (DESP), Protein-glutamine γ-glutamyltransferase 4 (TGM4), secretogranin-3 (SCG3), Periplakin (PPL), collagen type V alpha 1 (COL5A1), and cell growth regulator with EF hand domain 1 (CGREF1) (Supporting Information Table 5). We also identified 20 proteins present in both HC and NS that were altogether absent from the renal transplant patients (Supporting Information Table 6). The proteins that were either only present in NS urine or up-regulated in NS urine were involved in the acute phase response signaling (p=4.53E-21), the coagulation system (p=2.17E-11), and the complement system (p=1.63E-02).
3.4 Differential expression of proteins in AR
We analyzed the relative abundance of proteins that were identified in both renal transplant patients with AR episode and those with STA. There were nine proteins that were identified only in AR urine, but not in urine of HC, STA, and NS phenotypes. These included HLA class II histocompatibility antigen, DP(W4) β chain (HLA-DBP), HLA class II histocompatibility antigen, DRB1-8 β chain (IgHM), C4b-binding protein α chain (C4BPA), MHC class II antigen (HLA-DR), Myosin light chain 1 (MYL6B), HLA class II histocompatibility antigen DQ(3) β chain (HLA-DQB1) (Table 4A). In addition a total of 68 proteins that were absent in AR, but present in HC, STA, and NS categories, included Isoform 1 of Melanotransferrin (MFI2), Isoform 1 of FRAS1-related extracellular matrix protein 2 (FREM2), Isoform 2 of FRAS1-related extracellular matrix protein 2 (ROR1), Isoform 2 of Neural CAM L1-like protein (PLD3), Golgi apparatus protein 1 (CRYL1), and Thyrotropin-releasing hormone-degrading ectoenzyme (Table 4B). For a more rigorous quantitative analysis of proteins that were identified in AR and STA, we used spectral counts observed for each protein. Protein-level spectral counts were obtained by summing up peptide-level spectral counts. A total of 284 proteins were observed to be either up-regulated or down-regulated in the AR urine when compared with the patients with STA with a cut-off criteria of ≥2 of fold change in terms of log base(2) of spectral counts (Supporting Information Table 7). The list of 23 up-regulated proteins included ceruloplasmin (CP), complement C5 (C5), hemoglobin subunit α (HBA1), prostate-specific antigen, complement C2 (C2), etc. and are listed in Table 5A. The proteins in this group were associated mainly with acute phase response signaling (p=3.77E-08) and complement system (p=5.46E-07). The top 23 down-regulated proteins out of 261 included α-1-microglobulin (AMBP), cubulin (CUBN), THP (UMOD), amylase, α (AMY2A), fibrilin1 (FBN1), and Collagen type XII, alpha1 (COL12A1) proteins and are listed in Table 5B. From their spectral count evaluations all nine identified collagens, COL5A3, COL4A2, COL1A2, COL27, COL1A1, COL15A1, COL6A1, COL12A1 were decreased in AR urine including type IV collagenase (matrix metalloproteinase 9) and its inhibitor TIMP-1. A number of SERPIN family members SERPING, SERPINB12, SERPINB3, and SERPINB4 were decreased in AR urine; whereas two members SERPINC1 and SERPINF1 (PEDF) were increased. The down-regulated proteins were found to be involved in ECM–receptor interaction, cell communication, and Glycan structure degradation (all with p≤0.0005). We used up-regulated proteins in AR to generate a heat map (Fig. 3), which demonstrate some of the injury mechanisms in AR.
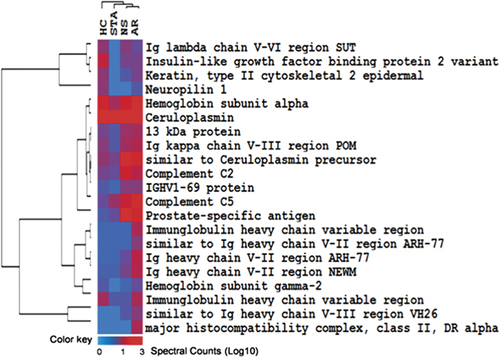
A heat map demonstrating level of elevated proteins in AR compared with STA when compared with healthy urine and NS.
| S. no. | IPI ID | Gene symbol | Protein name |
|---|---|---|---|
| (A) | |||
| 1 | IPI00103082.7 | HLA-DBP | HLA class II histocompatibility antigen, DP(W4) β chain |
| 2 | IPI00005180.2 | IgHM | HLA class II histocompatibility antigen, DRB1-8 β chain |
| 3 | IPI00021727.1 | C4BPA | C4b-binding protein α chain |
| 4 | IPI00641889.1 | KIAA1522 | 25 kDa protein |
| 5 | IPI00746396.1 | 302 kDa protein | |
| 6 | IPI00760688.2 | HLA-DR | MHC class II antigen (fragment) |
| 7 | IPI00027255.1 | MYL6B | Myosin light chain 1, slow-twitch muscle A isoform |
| 8 | IPI00783351.1 | SUMF2 | Sulfatase modifying factor 2 isoform d |
| 9 | IPI00743218.1 | HLA-DQB1 | HLA class II histocompatibility antigen, DQ(3) β chain |
| (B) | |||
| 1 | IPI00029275.1 | MFI2 | Isoform 1 of melanotransferrin |
| 2 | IPI00180707.8 | FREM2 | Isoform 1 of FRAS1-related extracellular matrix protein 2 |
| 3 | IPI00644416.1 | ROR1 | Isoform 2 of FRAS1-related extracellular matrix protein 2 |
| 4 | IPI00328243.1 | PLD3 | Isoform 2 of neural cell adhesion molecule L1-like protein |
| 5 | IPI00645031.1 | CRYL1 | Golgi apparatus protein 1 |
| 6 | IPI00007798.1 | TRHDE | Thyrotropin-releasing hormone-degrading ectoenzyme |
| 7 | IPI00556267.1 | GPC1 | FAT tumor suppressor homolog 4 |
| 8 | IPI00218795.1 | SELL | Isoform 2 of melanotransferrin |
| 9 | IPI00156171.2 | ENPP2 | Isoform 1 of ectonucleotide pyrophosphatase/phosphodiesterase 2 |
| 10 | IPI00641153.2 | GLG1 | Glypican 1 variant (fragment) |
| 11 | IPI00007244.1 | MPO | Isoform H17 of myeloperoxidase |
| 12 | IPI00604773.1 | PODXL | Target of Nesh-SH3 (Tarsh) (Nesh binding protein) |
| 13 | IPI00010790.1 | BGN | Biglycan |
| 14 | IPI00064262.1 | DCHS1 | Protocadherin-16 |
| 15 | IPI00641672.1 | CD320 | Isoform 3 of Crumbs homolog 2 |
| 16 | IPI00019907.1 | GPC3 | Glypican-3 |
| 17 | IPI00025240.1 | CDH16 | Isoform 1 of Cadherin-16 |
| 18 | IPI00182728.2 | VPS4B | Vacuolar sorting protein 4b |
| 19 | IPI00413016.3 | CADM2 | CDNA FLJ35635 fis, clone SPLEN2011805 |
| 20 | IPI00413781.3 | CXCL12 | Vacuolar sorting protein 4a |
| 21 | IPI00419215.3 | A2ML1 | Hypothetical protein DKFZp761G128 |
| 22 | IPI00385751.3 | FUCA1 | δ-Notch-like EGF repeat-containing transmembrane |
| 23 | IPI00025512.2 | HSPB1 | Heat-shock protein β-1 |
| 24 | IPI00165360.4 | MPST | 3-Mercaptopyruvate sulfurtransferase |
| 25 | IPI00017672.4 | NP | Hypothetical protein FLJ25678 |
| 26 | IPI00103175.1 | CANT1 | Isoform 1 of soluble calcium-activated nucleotidase 1 |
| 27 | IPI00217253.2 | GCHFR | GTP cyclohydrolase 1 feedback regulatory protein |
| 28 | IPI00645085.2 | LOC653163 | Intrinsic factor-vitamin B12 receptor |
| 29 | IPI00646907.1 | SLC12A3 | ROR1 protein |
| 30 | IPI00011564.1 | SDC4 | Syndecan-4 |
| 31 | IPI00023014.1 | VWF | von Willebrand factor |
| 32 | IPI00024012.4 | FZD7 | Frizzled-7 |
| 33 | IPI00027769.1 | ELA2 | Leukocyte elastase |
| 34 | IPI00333140.7 | DNER | Phospholipase D3, isoform 1 |
| 35 | IPI00009294.1 | CRIM1 | Cysteine-rich motor neuron 1 protein |
| 36 | IPI00017160.3 | VTA1 | Protein C6orf55 |
| 37 | IPI00027510.1 | IL2RB | Interleukin-2 receptor β chain |
| 38 | IPI00719786.1 | CD248 | λ-Crystallin |
| 39 | IPI00759832.1 | YWHAB | PREDICTED: similar to Von Ebners gland protein |
| 40 | IPI00157414.3 | ENPP6 | Ectonucleotide pyrophosphatase/phosphodiesterase 6 |
| 41 | IPI00220253.1 | FGFR3 | Erythrocyte band 7 integral membrane protein |
| 42 | IPI00291737.1 | ITLN1 | Xaa-pro dipeptidase |
| 43 | IPI00296922.3 | LAMB2 | Intelectin-1 |
| 44 | IPI00004946.8 | CXCL16 | chemokine (C-X-C motif) ligand 16 |
| 45 | IPI00024896.2 | PBLD | Probable isomerase MAWBP |
| 46 | IPI00030887.1 | TYRO3 | Tyrosine-protein kinase receptor TYRO3 |
| 47 | IPI00299059.5 | CHL1 | Tumor-associated calcium signal transducer 2 |
| 48 | IPI00607804.1 | CRB2 | Thioredoxin |
| 49 | IPI00002441.1 | SDC1 | Syndecan-1 |
| 50 | IPI00005517.1 | EFNA5 | Ephrin-A5 |
| 51 | IPI00008554.1 | ANG | Angiogenin |
| 52 | IPI00016027.3 | FZD2 | Frizzled-2 |
| 53 | IPI00021812.1 | AHNAK | Neuroblast differentiation-associated protein AHNAK (fragment) |
| 54 | IPI00025252.1 | PDIA3 | Protein disulfide-isomerase A3 |
| 55 | IPI00158145.4 | LILRA5 | Leukocyte immunoglobulin-like receptor subfamily A member 5 isoform 1 |
| 56 | IPI00297910.1 | TACSTD2 | Laminin β-2 chain |
| 57 | IPI00411356.5 | VPS4A | Stomatin isoform b |
| 58 | IPI00440824.2 | ABI3BP | Chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) isoform γ |
| 59 | IPI00552768.1 | TXN | CDNA FLJ41598 fis, clone CTONG2025496 |
| 60 | IPI00646217.2 | IL10RB | 24 kDa protein |
| 61 | IPI00000861.1 | LASP1 | Isoform 1 of LIM and SH3 domain protein 1 |
| 62 | IPI00006601.5 | CHGB | Secretogranin-1 |
| 63 | IPI00021034.1 | COL4A1 | Collagen α-1(IV) chain |
| 64 | IPI00219682.5 | STOM | l-selectin |
| 65 | IPI00413641.6 | AKR1B1 | CDNA FLJ35595 fis, clone SPLEN2008007 |
| 66 | IPI00257882.6 | PEPD | Isoform 2 of fibroblast growth factor receptor 3 |
| 67 | IPI00419327.2 | FAT4 | Aldose reductase |
| 68 | IPI00641203.1 | CUBN | Podocalyxin-like isoform 1 |
- a (A) List of proteins identified only in AR urine. (B) List of proteins identified in all other phenotypes (STA, HS, and NS) but not identified in AR urine.
| S. no. | IPI ID | Gene symbol | Protein name | AR spectral counts | STA spectral count | Fold change (log 2) |
|---|---|---|---|---|---|---|
| (A) | ||||||
| 1 | IPI00017601.1 | CP | Ceruloplasmin | 439 | 141 | 2 |
| 2 | IPI00032291.1 | C5 | Complement C5 | 26 | 8 | 2 |
| 3 | IPI00410714.4 | HBA1 | Hemoglobin subunit α | 30 | 9 | 2 |
| 4 | IPI00010858.1 | KLK3 | Prostate-specific antigen | 21 | 4 | 2 |
| 5 | IPI00303963.1 | C2 | Complement C2 | 12 | 4 | 2 |
| 6 | IPI00747314.1 | 13 kDa protein | 15 | 4 | 2 | |
| 7 | IPI00477804.2 | Immunglobulin heavy chain variable region | 10 | 3 | 2 | |
| 8 | IPI00464948.3 | HLA-DRA | Major histocompatibility complex, class II, DR α | 10 | 1 | 3 |
| 9 | IPI00021304.1 | KRT2 | Keratin, type II cytoskeletal 2 epidermal | 5 | 1 | 2 |
| 10 | IPI00741163.1 | LOC65265 | PREDICTED: similar to Ig heavy chain V-II region ARH-77 | 6 | 2 | 2 |
| 11 | IPI00783393.1 | Immunglobulin heavy chain variable region | 10 | 2 | 2 | |
| 12 | IPI00745363.1 | LOC652113 | PREDICTED: similar to Ig heavy chain V-III region VH26 | 6 | 2 | 2 |
| 13 | IPI00386142.1 | Ig heavy chain V-II region ARH-77 | 12 | 2 | 3 | |
| 14 | IPI00737304.1 | LOC652141 | PREDICTED: similar to Ig heavy chain V-III region VH26 | 6 | 1 | 3 |
| 15 | IPI00556442.1 | IGFBP2 | Insulin-like growth factor binding protein 2 variant | 5 | 1 | 2 |
| 16 | IPI00736985.1 | LOC441368 | PREDICTED: similar to Ceruloplasmin | 21 | 5 | 2 |
| 17 | IPI00477540.2 | 13 kDa protein | 9 | 3 | 2 | |
| 18 | IPI00382540.1 | Ig heavy chain V-II region NEWM | 11 | 2 | 2 | |
| 19 | IPI00386135.1 | Ig λ chain V-VI region SUT | 4 | 1 | 2 | |
| 20 | IPI00554676.1 | HBE1 | Hemoglobin subunit γ-2 | 4 | 1 | 2 |
| 21 | IPI00387119.1 | Ig κ chain V-III region POM | 11 | 3 | 2 | |
| 22 | IPI00419517.1 | IGHV1-69 | IGHV1-69 protein | 6 | 2 | 2 |
| (B) | ||||||
| 1 | IPI00022426.1 | AMBP | AMBP protein | 724 | 2201 | 2 |
| 2 | IPI00160130.3 | CUBN | Cubilin | 59 | 209 | 2 |
| 3 | IPI00012503.1 | PSAP | Isoform Sapmu0 of Proactivator polypeptide | 93 | 427 | 2 |
| 4 | IPI00640271.1 | UMOD | Tamm–Horsefall protein | 122 | 363 | 2 |
| 5 | IPI00745705.1 | AMY2A | Amylase, α 2A; pancreatic variant | 89 | 264 | 2 |
| 6 | IPI00744362.1 | FN1 | Hypothetical protein DKFZp686K08164 | 36 | 126 | 2 |
| 7 | IPI00021885.1 | FGA | Isoform 1 of fibrinogen α chain | 60 | 176 | 2 |
| 8 | IPI00784458.1 | FBN1 | 312 kDa protein | 30 | 112 | 2 |
| 9 | IPI00000073.1 | EGF | Proepidermal growth factor | 37 | 140 | 2 |
| 10 | IPI00328113.2 | FBN1 | Fibrillin1 | 20 | 76 | 2 |
| 11 | IPI00744835.1 | PSAP | Isoform Sapmu9 of proactivator polypeptide | 71 | 312 | 2 |
| 12 | IPI00641961.1 | COL12A1 | Collagen, type XII, α 1 | 39 | 128 | 2 |
| 13 | IPI00783446.1 | GAA | Lysosomal αglucosidase | 29 | 120 | 2 |
| 14 | IPI00329573.8 | COL12A1 | Isoform long of collagen α1(XII) chain | 32 | 117 | 2 |
| 15 | IPI00023673.1 | LGALS3BP | Galectin3-binding protein | 46 | 134 | 2 |
| 16 | IPI00385896.1 | SPP1 | Isoform D of osteopontin | 27 | 109 | 2 |
| 17 | IPI00293088.4 | GAA | 106 kDa protein | 28 | 114 | 2 |
| 18 | IPI00008787.3 | NAGLU | α-Nacetylglucosaminidase | 27 | 96 | 2 |
| 19 | IPI00741768.1 | LOC64213 | PREDICTED: similar to maltaseglucoamylase, intestinal | 25 | 114 | 2 |
| 20 | IPI00003919.1 | QPCT | Glutaminylpeptide cyclotransferase | 30 | 87 | 2 |
| 21 | IPI00783792.1 | MGAM | 192 kDa protein | 10 | 43 | 2 |
| 22 | IPI00220143.2 | MGAM | Maltaseglucoamylase, intestinal | 22 | 97 | 2 |
| 23 | IPI00240345.3 | CLEC14A | Ctype lectin domain family 14 member A | 5 | 29 | 3 |
3.5 Verification of AR-associated proteins THP (UMOD), PEDF (SERPINF1), and CD44
We performed an ELISA assay on UMOD, PEDF (SERPINF1), and CD44 as AR specific NUP for verification. We verified the decreased UMOD in AR patients. An ELISA assay was run for urinary UMOD, and was performed on an independent validation set of samples with AR (n=20), STA (n=20), and HC (n=20). The mean UMOD concentration in AR urine (5.50±0.85 μg/mL) was significantly lower than the STA urine (13.95±2.94 μg/mL (p<0.01) and the healthy normal control urine (19.80±2.71 μg/mL) (p<0.001) (Fig. 4A). In another experiment we observed an elevated concentration of PEDF (SERPINF1) in the AR urine compared with the urine collected from the urine from patients with STA function and other controls that included healthy normal control and non-specific proteinuric patients. The mean PEDF (SERPINF1) concentration in AR urine (0.0.396±0.0.344 μg/mL) was significantly higher than STA urine (0.006±0.009 μg/mL (p=0.0001), NS urine (0.019±0.038 μg/mL) (p=0.005), and HC urine (0.01±0.009 μg/mL) (p=0.005) (Fig. 4B). When we assayed CD44 in an independent sample set of individual urine samples, we observed a decreased concentration of CD44 in the AR urine compared with the urine collected from STA function and other controls that included the healthy normals and non-specific proteinuric patients. The mean CD44 concentration in AR urine (1.67±1.17 ng/mL) was significantly lower than STA urine (2.81±1.10 ng/mL (p=0.0001), NS urine (1.83±1.63 ng/mL) (p=0.005), and the HC urine (2.54±1.41 ng/mL) (p=0.005) (Fig. 4C). The ROC analysis was performed by the logistic regression model for the three proteins based on the values of AR versus no-AR including STA, HC, and NS. The area under the curve for AR classification of CD44 is 97.3% with p=0.0058, PEDF is 93.2% with p=0.0205 and UMOD is 84.6% with p=0.0005 (Fig. 5).
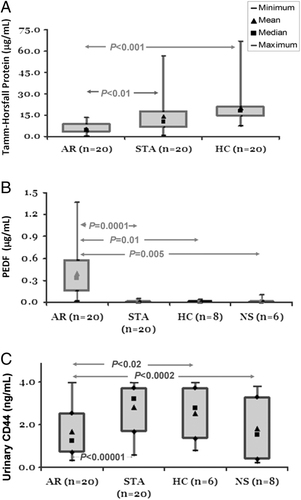
Verification of discovery of potential biomarker candidates by ELISA assay. UP level of three candidate proteins, THP, PEDF (SERPINF1), and CD44 were measured by ELISA using an independent set of samples from different phenotypes. (A) A decreased level of THP was observed in AR urine (n=20, mean concentration 5.50 μg/mL) when compared with STA urine (n=20, mean concentration 13.95 μg/mL) with p<0.01 and HC urine (n=20, mean concentration 19.80 μg/mL) with p<0.001. (B) An increased level of PEDF protein was observed in AR urine (n=20, mean concentration 0.40 μg/mL) when compared with STA urine (n=20, mean concentration 0.01 μg/mL) with p=0.0001, with HC urine (n=8, mean concentration 0.01 μg/mL) with p=0.02, and with NS urine (n=6, mean concentration 0.02 μg/mL) with p=0.005. (C) A decreased level of CD44 protein was observed in AR urine (n=20, mean concentration 1.67 ng/mL) when compared with STA urine (n=20, mean concentration 12.57 ng/mL) with p<0.00001, with HC urine (n=6, mean concentration 11.76 ng/mL) with p<0.02, and with NS urine (n=6, mean concentration 8.54 ng/mL) with p<0.0002. The boxes in the box plots are bounded by 75th and 25th percentiles of the data and the whiskers extend to the minimum and maximum values. As there are no statistically significant differences in urine osmolality between the samples groups (data not shown), the differences in concentration between groups are unlikely to be in part due to differences in water excretion, variably diluting, or concentrating the markers.
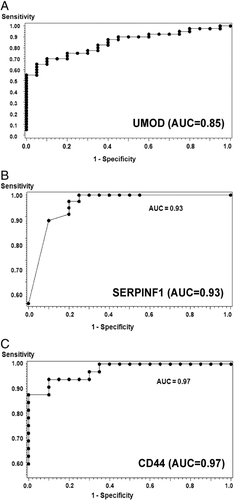
Verification of discovery of potential biomarker candidates by ELISA assay. UP level of three candidate proteins, THP (A), PEDF (SERPINF1) (B), and CD44 (C), were measured by ELISA using an independent set of samples from different phenotypes. The ROC curves used to distinguish AR from non-AR, the areas under the curves were 0.802, 0.844, and 0.738 for PECAM1, CXCL9, and CD44, respectively.
4 Discussion
This study describes the application of shotgun proteomics to expand the existing healthy normal urinary proteome database and its use in the identification and the verification of three potential biomarkers specific for AR in renal transplantation. Urine is the most relevant biofluid in biomarker discovery efforts for kidney diseases. Thus, the analysis of its proteome is very relevant 29, 30. MS-based proteomics provides a fast and accurate means of obtaining protein identification from complex samples and allows for rapid screening for disease markers 31. Renal transplantation has remained the optimal treatment for patients with end-stage kidney disease 14. Even though improvement in the short-term survival of grafts has been reported, AR of renal transplant still remains the primary risk factor for graft functional decline, chronic rejection, and graft loss 32-35. Therefore, AR-specific biomarkers are important for patient and allograft surveillance. Herein, we used LC-MS-based proteomics to investigate urine from kidney transplant patients. Using this approach, we have discovered the potential protein biomarker candidates that could provide a way to diagnose AR of renal transplant effectively and non-invasively. For this discovery step, using pediatric and young adult patient samples, we applied a pooling approach to minimize individual and disease heterogeneity.
Different proteomics approaches have been applied to analyze the urinary proteome in the past, which has helped to build up the list of UP identified to date 7-10, 12, 13. Early studies used gel-based techniques to identify a relatively smaller number of proteins, whereas use of gel-free LC-MS has proven to be an efficient way to identify a greater number of proteins. Adachi et al. identified 1492 proteins using the urine collected from the healthy individuals 12. In a recent report Gonzales et al. have identified 1160 from human urinary exosomes 13 (summarized in Fig. 2). In this report we have identified a new set of UP with a stringent criteria of a minimum of two unique, non-redundant peptides per protein with ∼0.1% FDR for protein identification. As summarized in Fig. 2 there is a significant overlap among the list of proteins identified by Adachi et al. 36 and Gonzales et al. 13. Yet, there are new proteins identified in each study, which will eventually help to build a comprehensive human urinary proteome database. Apart from contributing to the existing UP database, we have analyzed UP identified from the healthy normal controls to those with nephrotic syndrome and renal transplantation, which yielded specific proteins related to renal injury associated with NS as well as renal transplantation that included AR and STA function.
One of the challenges of translational research is that there is such a wide range (approximately as high as 10 orders of magnitude) of protein concentrations present in the bio-specimen, especially in blood and urine. The experimental design applied in this study has provided us with protein identifications for high abundance proteins such as UMOD with a concentration measured at five orders of magnitude (∼0.07 mg/mL). This is more than the concentration measured for protein S100 calcium binding A4 protein (∼2 ng/mL) in urine. In this study we calculated spectral counts as a semi-quantitative means for comparison and a weighted fold-change was used to derive a list of potential biomarker proteins. We tested three proteins whose concentrations differed by four orders of magnitude, whereas there was a nearly perfect correlation to a good correlation for the proteins ranging from mean spectral counts 9–360 (r2=0.59–0.99). The data suggest that the label-free LC-MS/MS spectral count data provides a relatively good quantification for high abundance to moderate abundance proteins. If the spectral count is low, it has a poor correlation with the real concentration in the sample and may require a more stringent labeling methods such as either iTRAQ 37 or the 18O/16O labeling method 38 to achieve more accurate quantification. In this study, we used spectral counts as our measure of relative abundance to list potential AR-specific proteins.
Given the scope of the study, we took three relevant protein candidates to verify their validity as being AR-specific as discovered by the label-free approach using LC-MS/MS. Since the ELISA assay is known to be robust, sensitive for performing quantitative measurements of proteins in a simple setting unlike multiple reaction monitoring, we performed the ELISA assay on THP (UMOD), PEDF (SERPINF1), and CD44 as AR-specific NUP. We have demonstrated that the reduced levels of THP (UMOD) and CD44 and the elevated level of PEDF (SERPINF1) in AR urine could be verified as both a highly specific and sensitive method to detect AR within the transplanted kidney, regardless of the confounding effect of proteinuria, immunosuppression, age, or gender.
The Tamm–Horsfall protein (UMOD) is localized in the epithelial cells of the thick ascending limbs of Henle's loop and the most proximal part of the distal convoluted tubule 39. This protein is suggested to be involved in the constitutive inhibition of calcium crystallization 40. Mutation of the UMOD gene has been linked to familial juvenile hyperuricemic nephropathy as well as autosomal-dominant medullary cystic kidney disease (MCKD2) in children 41. It has also been reported to be involved in prevention of urinary tract infection 42. This protein has intrigued nephrologists for a long time because of its high abundance in healthy urine with no obvious role 39. Kaden et al. observed reduced urinary UMOD delayed onset of transplanted function and increased urinary UMOD with the recovery of kidney health 43. However, the use of UMOD as a diagnostic parameter was not recommended. Sejdiu et al. have recently related decreased UMOD in urine to the development of renal failure and cardiovascular death within 20 years in type 1 but not in type 2 diabetes 44. Our observation of the reduced level of THP in AR does agree with the pattern of low urinary UMOD in naturally occurring peptides that is seen in AR and chronic allograft rejection and may need to be further validated with a larger cohort of patient samples 30, 45.
PEDF precursor is also known as serpin peptidase inhibitor. Clade F (SERPINF1) is a member of serine protease inhibitors and is known to be a potent inhibitor of angiogenesis in the eye 46. PEDF (SERPINF1) was detected as one of the proteins whose level was elevated in the AR urine. PEDF (SERPINF1) is one of the major inhibitors of angiogenesis and is involved in physiological activities including wound healing, ischemia reperfusion injury, and cancer metastasis to name a few examples. No direct correlation has been established for PEDF (SERPINF1) in renal injury. In a recent report Matsuyama et al. observed an increased PEDF (SERPINF1) level in the serum of diabetic patients with both diabetic retinopathy and nephropathy and suggested this could be a reflection of microvascular damage 47. Our observation of the increased level of PEDF (SERPINF1) in AR urine could provide a new way to monitor the health status of renal transplant. It would take further investigation to understand the underlying mechanism related to its involvement in AR.
CD44 is a cell-surface glycoprotein known to be involved in cell–cell interactions and cell adhesion and migration 48. It acts as a receptor for hyaluronic acid, osteopontin, collagens, and matrix metalloproteinases 49. A number of activities for this protein have been reported. These include lymphocyte activation, recirculation and homing, hematopoiesis, and tumor metastasis. Transcripts for this gene undergo a complex alternative splicing that results in many functionally distinct isoforms; however, the full length nature of some of these variants has not yet been determined.
High-throughput genomics or proteomics studies generate not only a list of disease-specific genes/proteins, but also help in understanding the underlying molecular pathways and events. Their biological activity and their association to different pathways provides a better understanding of the AR event, which is generally known to be mediated by T-cell responses to antigens from the donor organs that are different than the ones in the recipient. This study has provided a broad view of underlying events in the kidney at the time of AR. We observed up-regulation of MHC proteins that are involved in the presentation of foreign antigens to T cells. This observation parallels an observation of increased expression of MHC class II genes in AR 50, increased recruitment of MHC class II-positive leukocytes into the kidney due to injuries in kidney after renal transplantation 51, and an increase of MHC class II antibodies with acute and chronic dysfunction 52, 53. By impact analysis on signaling pathways, we identified a number of AR-specific UP that are part of the acute phase response, complement and coagulation cascades. On the other hand, there is a significant down-regulation of proteins involved with ECM, cytoarchitecture in AR urine when compared with STA and HC. This suggests a significant turnover of ECM during an AR episode.
In summary, for the first time, we have demonstrated that shotgun proteomics is a viable way to discover potential biomarkers in transplantation. The outcome of this study demonstrates that comparative analysis strategy using pooled samples is a simple and effective way to achieve a list of potential biomarkers that can track with normal and disease states. Cross-validation of selected results from these studies, by an economically viable and convenient ELISA assay with an independent set of urine samples, demonstrates the feasibility of the translation of this approach to clinical practice. In conclusion, this label-free, semi-quantitative approach to analyze the urinary proteome in normal and disease states provides a robust and sensitive method for detection of UP for serial, non-invasive clinical monitoring for graft rejection after kidney transplantation. Since this study included pediatric and young adult patients as study subjects, a similar study on adult renal transplant patients will contribute in finding biomarkers that are more effective. In order to further validate the effectiveness of this approach in identifying AR-specific marker proteins, a longitudinal response analysis on prospectively collected urine samples from a larger independent cohort of patients is warranted.
Clinical Relevance
Kidney transplantation is the treatment of choice for patients with end stage kidney disease. At present, renal transplant physicians do not have a non-invasive means to monitor acute rejection that contributes to dysfunction of transplanted kidney. Monitoring serum creatinine is not specific and renal biopsy is an invasive process with many complications. Here in this study, we applied shotgun proteomics to discover potential urine protein biomarkers in acute rejection of renal transplantation. Because of its close association with kidney and its being non-invasive, urine is an important source of biomarkers to diagnose and monitor kidney transplant dysfunction. The data presented here demonstrate that a biomarker discovery strategy using pooled samples is a simple and effective way to achieve a list of potential biomarkers. We have cross-validated the discovered selected marker proteins by ELISA assays with an independent set of urine samples which demonstrates the feasibility of the translation of this approach to clinical practice. Discovery of urine protein biomarkers for assessing the rejection status of patients following kidney transplant could significantly improve patient outcomes and decrease the cost of care. This strategy of discovery of non-invasive biomarkers can potentially be applied to other solid organ transplantations in the future.
Acknowledgements
The authors thank the NIH (grant RR018522 to R.D.S.) for support of portions of this research and the Environmental Molecular Sciences Laboratory (EMSL) for use of the instrumentation applied in this research. EMSL is a U.S. Department of Energy (DOE) national scientific user facility located at PNNL in Richland, Washington. PNNL is a multi-program national laboratory operated by Battelle Memorial Institute for the DOE under Contract DE-AC05-76RL01830. The authors thank Dr. Atul Butte, Dr. Li Li, Dr. Rong Chen, Dr. Purvesh Khatri, Szu-chuan Hsieh, Amery Chen, Mary Hansen, and the other members of the Sarwal Lab for their help in the manuscript preparation and Dr. Frank Golich for critically reading the manuscript.
The authors have declared no conflict of interest.




