Lower respiratory tract infections in children with congenital lung abnormalities
Abstract
Objective
We aimed to determine if the incidence of lower respiratory tract infections (LRTI) among children with asymptomatic, observationally managed congenital lung abnormalities (CLA) differed from that of symptomatic patients who underwent surgery. Second, we sought to compare the pre- and post-resection incidence of LRTI in patients who underwent surgery.
Methods
This retrospective cohort study included patients born between 1999 and 2021 with CLA confirmed by CT scan who were enrolled in a prospective longitudinal follow-up program. The LRTI incidence rates at 1, 2, 5, 8, and 12 years were compared between surgically and observationally managed patients using incidence rate ratios (IRR). Differences in pre- and post-resection LRTI incidence rates among patients who underwent CLA-related surgery were assessed through IRR.
Results
Among 217 included patients, 81 (37%) had undergone surgery and 136 (63%) had been observationally managed. The LRTI incidence rates did not significantly differ at any follow-up moment between the surgical and observational groups. Among the children who underwent CLA-related surgery, the pre-resection LRTI incidence rates were significantly higher than the post-resection LRTI incidence rates (IRR of 3.57, 95% confidence interval: [2.00; 6.33], p < .001).
Conclusion
We could not demonstrate differences in LRTI incidence throughout childhood between patients with surgically and observationally managed CLA. We recommend discussing cases of LRTI in patients with CLA in a multidisciplinary setting, using additional diagnostics such as chest X-ray to screen for CLA involvement, enabling a well-considered decision on surgical resection of the lesion.
1 INTRODUCTION
Congenital lung abnormalities (CLA) comprise a variety of respiratory tract anomalies including congenital pulmonary airway malformation (CPAM), bronchopulmonary sequestration (BPS), bronchogenic cyst (BC), congenital lobar overinflation (CLO), bronchial atresia, and hybrid lesions.1 CLA occurs in approximately 1 in 2500–3000 live births, with an increase in prenatally identified lesions over the last decades, partially attributed to routine prenatal ultrasound screening—initiated in 2007 in the Netherlands—and advancements in ultrasound imaging resolution.2, 3 Postnatal symptoms may manifest as respiratory insufficiency, cardiac overload, and (recurrent) lower respiratory tract infections (LRTI).4-6 Currently, children with symptomatic CLA undergo surgery based on international consensus. However, there is ongoing disagreement regarding the optimal management of children without symptoms, in part due to lack of evidence.7, 8 Some medical centers adopt a conservative approach through routine follow-up, as only 3%–24% of these children develop symptoms throughout childhood.9-15 Conversely, others opt for resection of all CLA in the first year of life.16 Recently, a core outcome set for patients with CPAM was developed to facilitate prospective research in this population.17 One of the parameters incorporated in this core outcome set is the occurrence of respiratory symptoms, such as doctor-diagnosed LRTI.17
Despite a global decrease in mortality and morbidity over the past 30 years, LRTI remain the leading cause of infection-related deaths in children under 5 years.18, 19 The estimated incidence of LRTI in Western European children aged 0–5 years is currently 1940 per 100,000 person-years.19 The occurrence of LRTI in childhood is clinically relevant, given their association with unfavorable lung function trajectories and chronic respiratory morbidity.20, 21 Children with CLA could be at higher risk for LRTI, especially in the first years of life.22 Advocates of surgery in asymptomatic children with CLA cite prevention of LRTI as one of their arguments.14, 16 However, a recent study has shown higher rates of LRTI in patients with CLA, before and after surgery, compared to healthy controls.13 Furthermore, there appears to be a paradoxical increase in LRTI occurrences in the months following CLA-related surgery.23 To the best of our knowledge, to date no studies have investigated the incidence of LRTI throughout childhood in patients initially managed observationally for asymptomatic CLA. Insight into these incidence rates could provide valuable guidance for counseling parents of children with asymptomatic CLA.
This study primarily aimed to determine whether LRTI incidence rates in children with observationally managed CLA differed from those in patients who underwent surgery. Second, in patients who underwent surgery for CLA due to symptom development, we wanted to compare the pre- and post-resection LRTI incidence rates. We hypothesized that incidence of LRTI among patients who underwent resection of CLA would be lower than that among observationally managed patients in whom the CLA was still present.
2 MATERIALS AND METHODS
2.1 Population and data collection
This retrospective cohort study included all children born between January 1999 and July 2021, diagnosed with CLA confirmed by chest CT-scan, and treated in our hospital. At our tertiary university hospital, all children with CLA are prospectively enrolled in a structured longitudinal follow-up program offering regular outpatient visits between the ages of 6 months and 17 years, along with several assessments of lung function and exercise capacity, as described earlier by Hijkoop et al.24, 25 The Medical Ethical Review Board at the Erasmus University Medical Center Rotterdam approved this retrospective study design and waived the need for informed consent (MEC-2022-0498). All parents and children were informed that data gathered from participation in the follow-up program could be used for research purposes.
2.2 Collected characteristics
We analyzed data from outpatient visits at the ages of 1, 2, 5, 8, and 12 years. During these visits, parents are queried how many doctor-diagnosed LRTI—defined as bronchitis, bronchiolitis or pneumonia—their child has suffered during the 12 months before the outpatient visit. Additionally, information regarding the administration of antibiotics and the use of diagnostic procedures, such as radiological assessments, is collected.
The following perinatal, clinical, and CLA-specific characteristics were extracted from the electronic patient files: sex, gestational age at birth, birthweight, Apgar scores at 1, 5, and 10 min, type of CLA, location of CLA (lobar localization), associated morbidities, admission to the intensive care unit (yes/no/only perinatally or perioperatively), and need for extracorporeal membrane oxygenation (yes/no).26
Additionally, we recorded surgery-related characteristics of patients who underwent CLA resection, including age at surgery, surgical setting (emergency/elective), indications for surgery (respiratory insufficiency, recurrent infection, cardiac overload, lesion size), the surgical approach (thoracoscopic, thoracotomy, endovascular), and type of intervention (lobectomy, segmentectomy, pneumonectomy, coiling/embolization, intrathoracic prosthesis, cystectomy, trachea reconstruction).
2.3 Primary outcomes
- 1.
The LRTI incidence rates at each follow-up moment, calculated by dividing the number of LRTI cases by the person-years of follow-up.
- 2.
The LRTI incidence rates before and after surgery for CLA, calculated by dividing the number of LRTI by the number of person-years patients spent in follow-up.
2.4 Secondary outcomes
The secondary outcomes included the proportion of LRTI treated with antibiotics, the proportion of LRTI that were investigated with chest X-ray (CXR), LRTI requiring hospitalization, and the proportion of patients on daily low-dose antibiotic maintenance for prevention of bacterial superinfections at each follow- up moment.
2.5 Data analysis
Data were presented as mean ± standard deviation for parametric data and median (interquartile range) for non-parametric data. General patient characteristics were compared between patients who underwent surgery at any point during follow-up and those who were observationally managed throughout the follow-up period using the t-test for numeric normally distributed variables, the Mann–Whitney U-test for numeric non-normally distributed variables, and the Chi-square test for categorical variables. Normality was assessed using the Kolmogorov–Smirnov test.
LcolorAt each follow-up point, the LRTI incidence rates were compared between patients who had undergone surgery before that follow-up point and patients who had been managed observationally up to that moment. Univariable negative binomial regression analysis was employed for this comparison, yielding incidence rate ratios (IRR). Last, pre- and post-resection LRTI incidence rates were calculated and compared for all surgically managed patients using univariable negative binomial regression analysis. For data analysis we used SPSS Statistics (version 28, IBM SPSS). All p-values <.05 were considered significant.
3 RESULTS
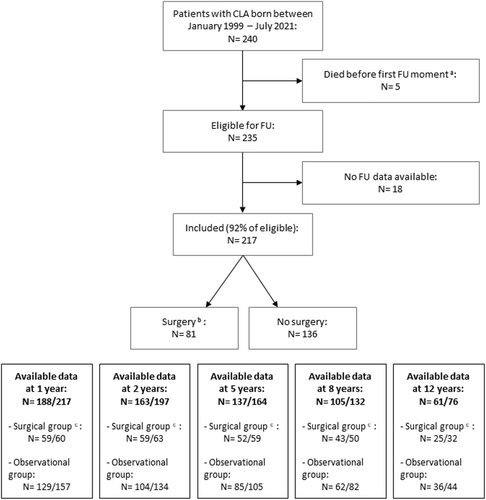
Two hundred and forty patients with radiologically confirmed CLA, born between January 1999 and July 2021, were identified in our database at the time of this study. Five of them had died before the first follow-up moment due to a complicated clinical course of CLA (N = 3) or associated morbidities (N = 2). Consequently, 235 patients were eligible for follow-up. For 18 of them, no follow-up data were available. Ultimately, we included 217 patients, among whom 81 (37%) underwent surgery at any point during follow-up, and 136 (63%) remained observationally managed throughout follow-up (Figure 1).
3.1 Characteristics
LcolorThe general demographic and clinical patient characteristics are presented in Table 1. Patients who underwent surgery at any point throughout follow-up had a lower gestational age at birth (median: 38.6 vs. 39.1 weeks, p = .006), lower birthweight (mean: 3140 vs. 3340 g, p = .02), and significantly more often associated morbidities (31% vs. 12%, p < .001) than patients who were observationally managed. Across all patients, CPAM was the most frequent type of CLA (N = 107; 49%), followed by BPS (N = 41; 19%). We assessed outcomes at various time points, reclassifying patients from the observational to the surgical group if they had undergone surgery between assessment intervals. A table detailing patient characteristics for the observational and surgical groups at each time point is provided in Supporting Information File S1. In Table 2, characteristics of patients who underwent surgery (N = 81) are provided. The median age at surgery was 62 days (interquartile range: 15–381). Three-quarters of these patients underwent surgery in an elective setting. Respiratory insufficiency was the most prevalent indication for surgical intervention (N = 45; 56%), followed by respiratory infections (N = 15; 19%). Overall, thoracotomy was the most frequently performed surgical approach (N = 58; 72%) and lobectomy was the most frequently conducted type of resection (N = 50; 62%).
| Surgery at any time during FU | No surgery throughout FU | ||||
|---|---|---|---|---|---|
| N total = 81 | Na | N total = 136 | Na | p-value | |
| Male sex | 45 (56%) | 81 | 74 (54%) | 136 | .89 |
| Gestational age (weeks) | 38.6 [37.2; 40.0] | 73 | 39.1 [38.5; 40.6] | 126 | .006 |
| Birthweight (grams) | 3140 ± 600 | 69 | 3340 ± 530 | 121 | .02 |
| Apgar score | |||||
| At 1 min | 8 [8; 9] | 52 | 9 [8; 9] | 109 | .14 |
| At 5 min | 9 [8; 10] | 52 | 9 [9; 10] | 109 | .20 |
| At 10 min | 10 [8; 10] | 41 | 10 [9; 10] | 90 | .08 |
| ECMO treatment | 5 (7%) | 74 | 0 | 134 | .005 |
| Associated morbiditiesb | 24 (31%) | 78 | 15 (12%) | 130 | <.001 |
| Cardiovascular | 8 | 8 | |||
| Airway | 5 | 1 | |||
| CDH | 4 | 0 | |||
| Gastro-intestinal | 0 | 1 | |||
| Neurological | 1 | 0 | |||
| Urogenital | 2 | 4 | |||
| Chromosomal | 2 | 1 | |||
| Other | 2 | 0 | |||
| ICU stay | 72 | 129 | <.001 | ||
| No | 8 (11%) | 64 (49%) | |||
| Only perinatally or perioperatively | 31 (43%) | 55 (43%) | |||
| Yes—for clinical care | 33 (46%) | 10 (8%) | |||
| Type of CLA | 81 | 136 | <.001 | ||
| CPAM | 31 (38%) | 76 (56%) | |||
| BPS | 19 (23%) | 22 (16%) | |||
| BC | 10 (12%) | 1 (1%) | |||
| CLO | 12 (15%) | 13 (9%) | |||
| Lung agenesis | 1 (1%) | 0 | |||
| Hybrid | 6 (7%) | 16 (12%) | |||
| Bronchial atresia | 1 (1%) | 7 (5%) | |||
| Otherc | 1 (1%) | 1 (1%) | |||
| Location of CLA | 67 | 121 | <.001 | ||
| Left upper lobe | 8 (12%) | 13 (11%) | |||
| Left lower lobe | 17 (25%) | 43 (36%) | |||
| Right upper lobe | 5 (7%) | 10 (8%) | |||
| Right middle lobe | 11 (16%) | 1 (1%) | |||
| Right lower lobe | 13 (19%) | 44 (36%) | |||
| Other/hybrid | 13 (19%) | 10 (8%) |
- Note: Data presented as N (%), mean (±SD) or median [interquartile range]. p < .05 is considered statistically significant.
- Abbreviations: BC, bronchogenic cyst; BPS, bronchopulmonary sequestration; CDH, congenital diaphragmatic hernia; CLA, congenital lung abnormality; CLO, congenital lobar overinflation; CPAM, congenital pulmonary airway malformation; ECMO, extracorporeal membrane oxygenation; FU, follow-up; ICU, intensive care unit.
- a N = number of patients for whom data is available.
- b Associated morbidities: cardiovascular (ASD, VSD, Ebstein malformation, Tetralogy of Fallot, left heart hypoplasia…), airway (stenosis trachea/bronchus, lung hypoplasia, esophageal atresia…), gastro-intestinal (anorectal malformation), neurological (cerebral bleeding), urogenital (duplex kidney, ureterocele, exstrophy bladder…), chromosomal (Triplication chromosome 7, Klinefelter, 47 XXX), other (chronic hemolytic anemia, diaphragmatic eventration)
- c Other type of CLA: bullae (N = 1, observational group), persisting pneumatocele (N = 1, surgical group).
| All operated patients (N = 81) | |
|---|---|
| Median age at surgery (days) | 62 [15; 381] |
| Timing of surgery | |
| Neonatal period | 31 (38%) |
| 1–6 months | 19 (23%) |
| 6–12 months | 10 (12%) |
| 12–24 months | 8 (10%) |
| 2–5 years | 9 (11%) |
| 5–8 years | 3 (4%) |
| After 8 years | 1 (1%) |
| Acute/elective setting of surgery | |
| Emergency | 20 (25%) |
| Elective | 61 (75%) |
| Surgical indications | |
| Respiratory insufficiency | 45 (56%) |
| (Recurrent) infections | 15 (19%) |
| Cardiac overload | 5 (6%) |
| Size/increase lesion | 6 (7%) |
| Othera | 6 (7%) |
| Unclear/missing | 4 (5%) |
| Surgical approach | |
| Thoraco- (± laparo)tomy | 58 (72%) |
| Thoracoscopic | 17 (21%) |
| Vascular access | 4 (5%) |
| Unclear/missing | 2 (2%) |
| Type of intervention | |
| Lobectomy | 50 (62%) |
| Segmentectomy | 4 (5%) |
| Pneumonectomy | 3 (4%) |
| Coiling/embolization | 3 (4%) |
| Intrathoracic prosthesis | 1 (1%) |
| Resection of BC/EL BPS | 18 (22%) |
| Trachea reconstruction | 1 (1%) |
| Unclear | 1 (1%) |
- Note: Data presented as N (%), median [interquartile range]. p < .05 is considered statistically significant.
- Abbreviations: BC, bronchogenic cyst; EL BPS, extralobar brochopulmonary sequestration.
- a Other surgical indications: leakage sternal dimple (N = 1), failure to thrive (N = 1), mediastinal shift (N = 1), asymptomatic patient with secundary esophageal atresia (N = 1) or congenital diaphragmatic hernia (N = 1), unfavorable location of intralobar bronchopulmonary sequestration (N = 1).
3.2 Respiratory outcomes
There were no significant differences in incidence rates of LRTI between the surgical and observational group at any of the follow-up moments (Table 3). When comparing pre- and post-resection LRTI incidence rates for all patients who underwent surgery (N = 81), we found an IRR of 3.57 (95% confidence interval [CI]: [2.00; 6.33], p < .001) indicating a higher incidence of LRTI before surgery than after surgery.
| Total number of LRTIa in last 12 months | Person-years of follow-up | IRRb surgery vs. observational group with 95% CI | p-value | |
|---|---|---|---|---|
| At 1 year | ||||
| Surgical group | 6 | 59 | ||
| Observational group | 18 | 129 | ||
| Total | 24 | 188 | 0.73 [0.27; 1.94] | .53 |
| At 2 years | ||||
| Surgical group | 13 | 59 | ||
| Observational group | 22 | 104 | ||
| Total | 35 | 163 | 0.97 [0.44; 2.48] | .93 |
| At 5 years | ||||
| Surgical group | 13 | 52 | ||
| Observational group | 16 | 85 | ||
| Total | 29 | 137 | 1.33 [0.55; 3.21] | .53 |
| At 8 years | ||||
| Surgical group | 4 | 43 | ||
| Observational group | 2 | 62 | ||
| Total | 6 | 105 | 2.88 [0.53; 15.74] | .22 |
| At 12 years | ||||
| Surgical group | 1 | 25 | ||
| Observational group | 2 | 36 | ||
| Total | 3 | 61 | 0.72 [0.07; 7.94] | .79 |
| Number of LRTIa | Person-years of follow-up | IRRb pre- vs. post-resection with 95% CI | p-value | |
|---|---|---|---|---|
| Pre-resection | 22 | 41 | ||
| Post-resection | 34 | 226 | ||
| 3.57 [2.00; 6.33] | <.001 |
- Note: p < .05 is considered statistically significant.
- Abbreviation: CI, confidence interval.
- a LRTI = lower respiratory tract infection.
- b IRR = incidence rate ratio.
Antibiotic treatment was administered in 95% of all LRTI cases. Forty-one percent of all LRTI were investigated with CXR (Table 4). Percentages of LRTI requiring hospitalization were comparable between the observational and surgical groups across ages (Table 4). The use of daily low-dose maintenance antibiotics for prevention of bacterial superinfections decreased with age and was more prevalent in surgically managed patients (Table 4).
| Surgical group | Observational group | Total group | |
|---|---|---|---|
| At 1 year | |||
| LRTIa treated with antibiotics | 5/6 (83%) | 17/18 (94%) | 22/24 (92%) |
| LRTIa investigated with CXRb | 1/6 (17%) | 12/18 (67%) | 13/24 (54%) |
| LRTIa requiring hospitalization | 4/6 (67%) | 10/18 (56%) | 14/24 (58%) |
| Patients on daily low-dose antibioticsc | 10/59 (17%) | 6/129 (5%) | 16/188 (9%) |
| At 2 years | |||
| LRTIa treated with antibiotics | 13/13 (100%) | 21/22 (95%) | 34/35 (97%) |
| LRTIa investigated with CXRb | 3/13 (23%) | 11/22 (50%) | 14/35 (40%) |
| LRTIa requiring hospitalization | 4/13 (31%) | 7/22 (32%) | 11/35 (31%) |
| Patients on daily low-dose antibioticsc | 9/59 (15%) | 4/104 (4%) | 13/163 (8%) |
| At 5 years | |||
| LRTIa treated with antibiotics | 12/13 (92%) | 16/16 (100%) | 28/29 (97%) |
| LRTIa investigated with CXRb | 3/13 (23%) | 8/16 (50%) | 11/29 (38%) |
| LRTIa requiring hospitalization | 1/13 (8%) | 3/16 (19%) | 4/29 (14%) |
| Patients on daily low-dose antibioticsc | 3/49 (3%) | 1/84 (1%) | 4/137 (3%) |
| At 8 years | |||
| LRTIa treated with antibiotics | 4/4 (100%) | 2/2 (100%) | 6/6 (100%) |
| LRTIa investigated with CXRb | 1/4 (25%) | 0/2 | 1/6 (17%) |
| LRTIa requiring hospitalization | 0/4 | 0/2 | 0/6 |
| Patients on daily low-dose antibioticsc | 2/43 (5%) | 0/62 | 2/105 (2%) |
| At 12 years | |||
| LRTIa treated with antibiotics | 0/1 | 2/2 (100%) | 2/3 (67%) |
| LRTIa investigated with CXRb | 0/1 | 1/2 (50%) | 1/3 (33%) |
| LRTIa requiring hospitalization | 0/1 | 0/2 | 0/3 |
| Patients on daily low-dose antibioticsc | 1/25 (4%) | 0/36 | 1/61 (2%) |
- a LRTI = lower respiratory tract infection.
- b CXR = chest X-ray.
- c Patients on daily low-dose maintenance antibiotics, prescribed in children who are deemed vulnerable, to prevent bacterial superinfections of LRTI.
4 DISCUSSION
Our evaluation of LRTI incidences in surgically and observationally managed patients with CLA aged between 1 and 12 years yielded no significant differences between these groups. Among the patients who underwent surgery, the post-resection LRTI incidence rates were significantly lower than the pre-resection rates, with an IRR of 3.57 (95% CI: [2.00; 6.33], p < .001).
Several studies have reported respiratory outcomes in patients with CLA. Previously, no significant differences in LRTI incidence were found between children with surgically and observationally managed CLA at the ages of 6 and 12 months.27 LRTI rates in patients who underwent surgery remained elevated to 80 months postoperatively compared to healthy controls.28 Analysis of data from a Canadian data repository demonstrated higher LRTI rates in patients who underwent surgery for CLA both pre- and post-resection compared to age-matched controls.13 A recent paper reported that 27% of patients undergoing surgery for CLA, at a median age of 3 months, experienced at least one LRTI before the age of 4 years.29 Although our observations on the incidence rates of LRTI are in line with those of previous studies, we additionally learned that the incidence of LRTI decreases after symptomatic patients with CLA undergo surgery. A possible explanation for this observation is that the occurrence of LRTI in the pediatric population tends to decrease with ageing after the age of 2–3 years.30 Therefore, we cannot attribute the lower post-resection LRTI rates solely to the effect of the surgical intervention, as LRTI were reported less frequently after the age of 5 years in both the surgical and observational groups. Nevertheless, previously asymptomatic children who develop a LRTI with CLA involvement should be deemed symptomatic, and surgery should be considered. To identify possible CLA involvement, we advocate for additional imaging (CXR) and multidisciplinary consultation upon suspicion of a LRTI in children with CLA. We also recommend centralizing the care of CLA-afflicted children as much as possible and setting up international registries whose data would allow to evaluate management strategies.
There are some important drawbacks associated with our study. First, we based our LRTI-data on parent-reported events concerning the 12 months before each outpatient visit, as privacy laws prohibit using health registries to track medication prescriptions and ICD-10 diagnoses. Even though experienced pediatricians thoroughly and systematically conduct the patients natural history and parents are specifically asked about doctor-diagnosed events, these parent-reported LRTI-numbers might be skewed, and recall bias might have influenced the completeness of parent-reported LRTI numbers. To minimize misdiagnosis during follow-up visits, pediatricians confirm LRTI history by collecting details about where and by whom patients were diagnosed. Second, knowledge of a CLA diagnosis could lead to increased vigilance in parents and healthcare professionals. This implies that parents might seek medical help if their child displays mild symptoms, in contrast to parents of children without CLA. Doctors may also be more inclined to prescribe antibiotics and diagnose respiratory symptoms as LRTI sooner in children with CLA. These phenomena could have led to overestimation of LRTI occurrences in our cohort. A third limitation is the lack of a control group representing Dutch peers. Markel and colleagues utilized an administrative population-based data repository to generate a 10:1 age-matched control cohort.13 A repository of this kind is not available in the Netherlands. A comparison of our data to general data representing the Dutch general population revealed that LRTI occurred more often in our cohort.30, 31 A similar comparison with pooled data from 53 European pregnancy and birth cohorts revealed similar prevalences.32
Before 2007—the year when prenatal ultrasound screening was introduced in the Netherlands—it was more challenging to detect asymptomatic children with CLA. Consequently, in our cohort, asymptomatic, observationally managed children may have been underrepresented among the participants born before 2007. Nevertheless, given the numbers of 22 observationally managed children versus 24 surgically treated children born before 2007, this effect seems limited.
The retrospective design of this study is also a limitation, as it does not allow for definitive conclusions on the relation between surgery for CLA and LRTI incidence. The ongoing CONNECT Trial, which prospectively randomizes patients with asymptomatic CPAM in a surgically and observationally managed group, will provide conclusive data on LRTI incidence.33 Moreover, future research could focus on examining further potential correlations of LRTI with other clinical outcomes in patients with CLA—such as lung function or exercise capacity.
In conclusion, we could not demonstrate differences in LRTI incidence throughout childhood between patients with surgically and observationally managed CLA. After CLA resection in symptomatic children the LRTI rates dropped. For children with CLA who develop a LRTI, we recommend conducting a CXR and arranging a multidisciplinary consultation to assess potential infectious involvement of the CLA, as surgical resection may be justified in these children.
AUTHOR CONTRIBUTIONS
Louis Dossche, Casper Kersten, Hanneke IJsselstijn, and Johannes Schnater contributed to conceptualization and design of the study, acquired data, carried out analyzes and interpreted the data. Joost van Rosmalen provided statistical expertize and helped interpret the conducted analyzes. Rene Wijnen contributed to conception and design of the study and revised the draft versions for important intellectual content. All authors have read and approved the final version of the manuscript for publication. Moreover, they agree to be accountable for all aspects of the work and will ensure that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENTS
The authors thank Ko Hagoort for critically reviewing the manuscript and providing editorial advice.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The Medical Ethical Review Board of Erasmus University Medical Center approved this retrospective study design and waived the need for informed consent (MEC-2022-0498). All parents and children were informed that outcome data were used for research purposes.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.