Intra-tracheal surfactant/budesonide versus surfactant alone: Comparison of two consecutive cohorts of extremely preterm infants
Laura Moschino and Daniel Nardo contributed equally to this manuscript.
Some of the results of this study have been previously reported in the form of abstract at the Joint European Neonatal Societies Congress 2019, Maastricht.
Abstract
Objectives
To compare the efficacy of intra-tracheal (IT) surfactant/budesonide (SB) with that of surfactant alone (S) in reducing the rate of bronchopulmonary dysplasia (BPD) at 36 weeks post-menstrual age (PMA), we included extremely preterm very low birth weight (VLBW) infants with severe respiratory distress syndrome (RDS) in our tertiary neonatal level of care unit (Padua, Italy).
Study Design
A retrospective chart review of two cohorts of extremely preterm VLBW neonates (<28+0 gestation weeks, birth weight [BW] < 1500 g) born in two consequent epochs (2017–2018/2018–2019) were compared. The SB group received surfactant (200 mg/kg 1st dose) and budesonide (0.25 mg/kg), while the S group received surfactant alone.
Results
Among 68 neonates with RDS Grades III–IV, FiO2 ≥ 0.3 within 12 h of life, 18 were included in each group after matching for perinatal, clinical, and laboratory characteristics. IT SB did not affect the rate of BPD (Vermont Oxford Network, Jensen's, and National Institute of Child Health and Human Development BPD Workshop 2018 definitions), death, BPD, or death at 36 weeks PMA. Hypotension requiring inotropic support within the first 5 days was lower in those receiving the combined treatment (p = .03). The SB group had fewer admissions to pediatric ward due to respiratory causes up to 12 months of corrected age (p = .03).
Conclusion
The preliminary results of this retrospective study suggest that in extremely preterm VLBW infants, IT SB for severe RDS did not affect the incidence of BPD, death, and BPD or death at 36 weeks PMA, compared to surfactant alone. The combined therapy proved to be safe in this population. Further studies are warranted to explore the role of early IT steroids on respiratory morbidity in preterm infants.
Abbreviations
-
- BPD
-
- bronchopulmonary dysplasia
-
- BW
-
- birth weight
-
- CPAP
-
- continuous positive airway pressure
-
- CRP
-
- C-reactive protein
-
- DOL
-
- day of life
-
- EOS
-
- early-onset sepsis
-
- GA
-
- gestational age
-
- GW
-
- gestational weeks
-
- HSPDA
-
- hemodynamically significant patent ductus arteriosus
-
- IT
-
- intra-tracheal
-
- IVH
-
- intra-ventricular hemorrhage
-
- LOS
-
- late-onset sepsis
-
- MV
-
- mechanical ventilation
-
- NEC
-
- necrotizing enterocolitis
-
- NICHD
-
- National Institute of Child Health and Human Development
-
- NICU
-
- neonatal intensive care unit
-
- NIPPV
-
- non-invasive positive pressure ventilation
-
- PCA
-
- principal component analysis
-
- PDA
-
- patent ductus arteriosus
-
- PICU
-
- Paediatric Intensive Care Unit
-
- PMA
-
- post-menstrual age
-
- PPV
-
- positive pressure ventilation
-
- PVL
-
- periventricular leukomalacia
-
- RDS
-
- respiratory distress syndrome
-
- ROP
-
- retinopathy of prematurity
-
- S
-
- surfactant
-
- SB
-
- surfactant/budesonide
-
- SGA
-
- small for gestational age
-
- SVIA
-
- spontaneous ventilation in room air
-
- TPN
-
- total parenteral nutrition
-
- VLBW
-
- very low birth weight
-
- VON
-
- Vermont Oxford Network
-
- WBC
-
- white blood cell
1 INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most important cause of mortality and long-term morbidity in preterm infants.1 Despite the wide implementation of gentle mechanical ventilation (MV) and noninvasive respiratory support by neonatologists in recent years, BPD still affects about 10%–89% of preterm infants regardless of gestational age (GA) and definition used.2
The morbidity burden of BPD starts from the first months of life,3 to span to adult age with potential impaired lung function.4-6
BPD survivors are therefore likely to be frequent users of healthcare systems, and research acting toward the reduction of BPD is strongly encouraged.
The pathophysiology of BPD is highly complex, but one of the key roles is played by inflammation. This is demonstrated by the presence of elevated levels of pro-inflammatory chemokines and cytokines in the amniotic fluid and tracheal aspirates of infants who subsequently developed BPD compared to those who did not.7 Therefore, attention has turned to the use of corticosteroids as potential therapy for the prevention and management of BPD.8, 9 These drugs are potent anti-inflammatory agents which act by decreasing both pulmonary recruitment of macrophages and leukocytes and lung edema.9 For these reasons, post-natal corticosteroids have been extensively trialed to prevent or treat BPD in preterm infants. However, there have been concerns regarding systemically administered corticosteroids due to their potential side effects on death and neurodevelopment.10
Studies and meta-analyses have demonstrated promising effects of early inhaled steroids11-14 in reducing the incidence of death or chronic lung disease (CLD) at 36 weeks post-menstrual age (PMA) in preterm infants.11, 12, 14 Airway administration of corticosteroids is assumed to cause less side effects than systemic treatment.15 Particularly, instillation of budesonide using surfactant as a vehicle has proven to be an attractive and effective approach.16-19 Yeh et al.16, 18 compared the administration of surfactant and budesonide with that of surfactant only in very low birth weight (VLBW) infants with severe respiratory distress syndrome (RDS). They demonstrated a significantly lower incidence of the combined outcome of death or BPD at 36 weeks PMA in the intervention group without adverse effects at 2 years of age.17 The systematic review and meta-analysis of these studies confirmed a significant 40% reduction in the composite outcome, with 43% reduction in the risk of BPD alone.20
In light of these promising findings, we aimed to retrospectively analyze the effect of intra-tracheal (IT) administration of surfactant/budesonide (SB) compared to that of surfactant alone (S) on the incidence of BPD in two cohorts of extremely preterm VLBW infants with severe RDS, respectively, before and after the introduction of this combined strategy in our NICU.
Some of the results of this study have been previously reported in the form of abstract at the Joint European Neonatal Societies Congress 2019, Maastricht.
2 MATERIALS AND METHODS
2.1 Study design and population
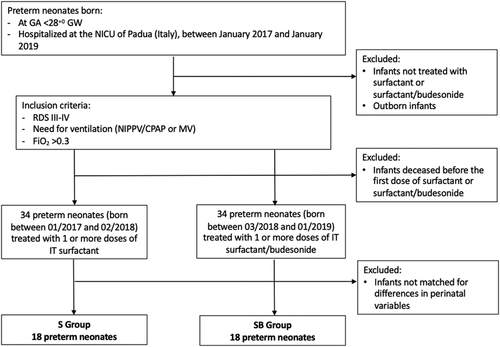
This retrospective analysis included two cohorts of neonates born in two consecutive epochs (January 2017–February 2018 and March 2018–January 2019), at our tertiary level of care Neonatal Intensive Care Unit (NICU) of Padova University Hospital (Italy).
Inborn patients were included if born <28+0 gestational weeks (GW) with a birth weight (BW) < 1500 g and demonstrated radiographic evidence of severe RDS (Grades III–IV) requiring respiratory support with FiO2 ≥ 0.3 within 12 h from birth. These infants were considered to be at high risk of developing BPD. Infants born <23 GW, with a birth weight <400 g, or with congenital abnormalities or lethal disorders compromising the respiratory system were excluded.
The recruited neonates were partitioned into two subsets, one receiving only surfactant and the other treated with SB, without significant differences in the perinatal characteristics using a one-to-one matching procedure.
In our Unit, the instillation of budesonide using surfactant as a vehicle for this high-risk population was introduced in March 2018 in light of the results of the studies by Yeh and co-workers.16-18 After these studies, our department protocol for the prevention and management of BPD was updated. Medical team and management protocol of VLBW infants did not change between the two epochs. Revision and publication of these data have been approved by the Ethics Committee of the Padova University Hospital (EC number 148, Protocol 18909).
2.2 Intra-tracheal SB versus surfactant alone (S)
The cohort born in the first epoch of the study period, defined as S group, received intra-tracheal surfactant first dose of 200 mg/kg (2.5 ml/kg), followed, if needed, by subsequent doses of 100 mg/kg (1.25 ml/kg) (Curosurf® intratracheal suspension 80 mg/ml; Chiesi Farmaceutici S.p.A.).
The cohort born in the second epoch of the study period, defined as SB group, received intra-tracheal surfactant first dose of 200 mg/kg, followed, if needed, by subsequent doses of 100 mg/kg (Curosurf® intratracheal suspension 80 mg/ml; Chiesi Farmaceutici S.p.A.), each dose combined with budesonide 0.25 mg/kg (0.5 ml/kg) (Aircort® suspension for nebulization 0.5 mg/ml; Italchimici S.p.A.). Therefore, a first-dose solution with a concentration ratio of 1:0.2 was obtained, with final surfactant and budesonide concentrations of 66.67 and 0.083 mg/ml, respectively. Under sterile conditions, surfactant and budesonide were mixed in the same syringe that was gently vortexed to obtain a clear and uniform solution before instillation. SB mixture was administered with the same technique to that of routine surfactant therapy. As in the study by Yeh et al.,16 treatment with surfactant or with SB could be repeated every 8 h until the infant required a FiO2 < 0.3, extubation, or had reached the maximum of six doses.
2.3 Respiratory care and fluid management
For infants who had respiratory distress shortly after birth, a trial of nasal continuous positive airway pressure (nCPAP) was initiated in the delivery room. According to our policy of early rescue surfactant, we considered candidates for the first surfactant administration those infants with clinical signs of RDS on noninvasive ventilation (nCPAP or non-invasive positive pressure ventilation NIPPV) with a FiO2 requirement >0.3 to maintain pre-ductal O2 saturation (SpO2) of 90%–95%. In the case of persistent work of breathing with still high oxygen requirement (FiO2 > 0.4) and significant X-ray changes within 8 h, a second dose of surfactant could be administered. Following additional doses could be given according to the clinician's decision.
Infants met criteria for intubation, according to our local protocol, if they showed inadequate or absent respiratory drive (more than 6 episodes of apnea in an hour or severe apnea requiring PPV),21 or excessive work of breathing, or RDS with FiO2 > 0.6 after surfactant with 6 cmH2O of positive end-expiratory pressure (PEEP), or pCO2 > 65 mmHg and pH < 7.20 in the first 3 days of life. Infants were considered ready for extubation when fulfilling SpO2 criteria with FiO2 ≤ 0.3, mean airway pressure between 8 and 10 cmH2O, and when having a good respiratory drive. After extubation, infants were non-invasively ventilated using nCPAP or NIPPV, and progressively weaned to high flow nasal cannulae or hood O2. Postnatal systemic dexamethasone for extubation was reserved for infants who had severe underlying lung disease receiving intermittent mandatory ventilation with a FiO2 > 0.6 or high-frequency oscillatory ventilation (HFOV) after 14 days of life (DOL). In such cases, a short course of dexamethasone at low doses was given after collegial agreement and if the infant was free from infections.
Infants were screened for patent ductus arteriosus (PDA) within the first 48 h of life. A PDA was considered of hemodynamic significance if at least three parameters of shunt volume were present (PDA diameter >2 mm or >1.4 mm/kg, pulsatile PDA flow pattern, bidirectional shunt, or pure right-to-left shunt, left ventricular output >300 ml/kg/min, left atrium over aortic ratio > 1.5, mitral E/A > 1, large left ventricle end-diastolic diameter). Infants with hemodynamically significant PDA (HSPDA) received either ibuprofen or paracetamol treatment, according to local protocol.
Within the first 2 h of life, infants were inserted a central umbilical vein catheter for parenteral nutrition (personalized total parenteral nutrition [TPN] or Numeta G13%E Preterm; Baxter) and therapies. An umbilical artery catheter was placed for the routine monitor of blood gases, laboratory tests, and arterial blood pressure.
Hypotension was defined as a mean arterial blood pressure (MABP) in mmHg equal to or below the estimated gestational age (EGA) in weeks of the infant.22 In this case, a normal saline fluid bolus 10 ml/kg was administered or inotropic therapy with dopamine or dobutamine was started.
2.4 Outcome measurements
Primary outcomes were death or BPD at 36 weeks PMA, death, and BPD at 36 weeks PMA. For the analysis, we considered three definitions of BPD, in particular the one adopted by the Vermont Oxford Network (VON),23, 24 the definition by Jensen and co-workers published in 201925 and the one proposed by the Executive Summary of a Workshop in 2018.26 We investigated whether there was a difference in severity of BPD according to NICHD BPD Workshop Grading 2018 and Jensen's definition.
As secondary respiratory outcomes, we considered extubation failure within 24 h, need for reintubation for any respiratory cause up to discharge, duration of mechanical ventilation, post-natal treatment with dexamethasone for extubation, or BPD prevention.
Among the relevant non-respiratory secondary outcomes, we considered: hypotension with the need for inotropic support within the first 5 DOL; the presence of HSPDA requiring medical and/or surgical treatment; documented (positive blood culture) or suspected (increased C-reactive protein CRP and suggestive signs and symptoms) early-onset sepsis (EOS) within 72 h from life)23; documented or suspected late-onset sepsis (LOS) after 72 h from life; severe intra-ventricular hemorrhage (IVH grade ≥ III); cerebellar hemorrhage; periventricular leukomalacia at any cerebral ultrasound scan or magnetic resonance imaging; necrotizing enterocolitis (NEC) Bell's stage ≥ II27; retinopathy of prematurity (ROP) grade ≥ II, metabolic bone disease. Regarding long-term respiratory outcomes, we studied the type of respiratory support at 12 months of corrected age, the number of hospitalizations for respiratory causes, and that of admissions to the Paediatric Intensive Care Unit (PICU) for respiratory insufficiency in the first year of life among infants survived at that age.
We considered as potential adverse effects related to the instillation of budesonide: hyperglycemia (two consecutive blood values ≥200 mg/dl); need for insulin therapy within 72 h (after a reduction of glucidic supplementation to 4.5 g/kg/die as per local protocol); leucocytosis (white blood cell count WBC > 30,000/mm3 in the first 5 DOL); Candida infections (positive blood culture in the first 14 days).
2.5 Statistical analysis
Medical records of the preterm infants who received either surfactant or SB during the study period were reviewed to collect demographic, clinical, and laboratory data.
A one-to-one matching procedure was applied to extract a subset of infants who received surfactant alone, and a subset of infants treated with SB without significant differences in the perinatal characteristics. The matching procedure is described in the Supporting Information.
Qualitative variables were expressed by counts and analyzed by Fisher's exact tests. Numerical variables normally distributed were expressed by mean (standard deviation [SD]) and analyzed by Student's t test, whereas variables non-normally distributed were expressed by median [IQR] and analyzed by Mann–Whitney test. Normality test with Shapiro–Wilk was conducted. A significant level of α = .05 was assumed and post hoc power analysis was performed.
The dichotomous outcomes were investigated by multivariate logistic regression. Since perinatal data resulted in being strongly correlated and logistic regression is not robust when factors are correlated, perinatal characteristics with the exclusion of the type of treatment were compressed by principal component analysis (PCA) to obtain independent score variables. Thus, the design matrix was obtained joining the scores and the factor “treatment” (with the two levels surfactant and budesonide/surfactant). Post hoc power analysis was performed by simulation following a similar procedure to that described by Gail et al.28 and is detailly described in the Supporting Information. A significant level α = .05 was considered.
In the case of numerical outcomes, data were investigated by ordinary least squares (OLS) regression. As for logistic regression, perinatal data were compressed by PCA and joined with the factor “treatment” to obtain the design matrix. Post hoc power was estimated by a procedure based on a simulation similar to that implemented for logistic regression. A level α = .05 was considered significant. In the text, the value of p and the post hoc power of the most relevant factors are reported for the model discussed.
Data analysis was performed by R 3.6.3 platform (R Foundation for Statistical Computing) using in-house developed R-functions.
3 RESULTS
Sixty-eight neonates fulfilling the inclusion criteria were enrolled, of which 34 received surfactant alone and 34 the combination of SB. The perinatal characteristics of the two groups are reported in Table 1. Since the group of neonates receiving surfactant alone and that treated with budesonide differed for BW (p = .01), total parenteral nutrition at Day 1 (p < .01) and glucidic supplementation at Day 2 (p < .01), we applied a one-to-one matching procedure to obtain two groups of subjects with no significant differences in the perinatal variables. In fact, the emerged perinatal differences could generate false discoveries because perinatal variables could behave as confounding factors with respect to the treatment, preventing the investigation of the relationships between treatment and BPD or adverse outcomes.
A group composed of 18 infants treated with SB (SB group) and a group of 18 infants treated with surfactant alone (S group) were extracted from the original cohorts and considered for the analysis (Figure 1). Table 2 summarizes the distributions of perinatal variables in the two matched groups.
| Group treated with surfactant (n = 34) | Group treated with SB (n = 34) | p | |
|---|---|---|---|
| GA (weeks) | 26 [24–27] | 25 [25–27] | .59 |
| BW (g) | 787 (187) | 681 (140) | .01 |
| SGA | 2 (5.9%) | 8 (23.5%) | .08 |
| Gender (male) | 20 (58.8%) | 18 (52.9%) | .80 |
| Caucasian | 25 (73.5%) | 26 (76.5%) | 1.00 |
| Singleton birth | 28 (82.4%) | 26 (76.5%) | .76 |
| ACS | .28 | ||
| Not performed | 11 | 7 | |
| Incomplete | 3 | 7 | |
| Complete | 20 | 20 | |
| Maternal clinical chorioamnionitis | 14 (41.2%) | 19 (55.9%) | .33 |
| Apgar 5’ | 7.0 [1.0] | 7.0 [1.0] | .54 |
| First pH | 7.33 [0.09] | 7.28 [0.13] | .22 |
| First pCO2 | 42.6 (9.9) | 45.0 (10.8) | .34 |
First 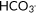
|
21.7 [4.0] | 21.9 [5.3] | .31 |
| Doses of S or SB | |||
| 1 | 21 | 20 | 1.00 |
| 2 | 11 | 11 | 1.00 |
| 3 | 2 | 3 | 1.00 |
| Total parenteral nutrition at D1 | 8 (23.5%) | 20 (58.8) | <.01 |
| Glucidic supplementation at D1 (g/kg/day) | 6.55 [0.73] | 6.70 [0.75] | .21 |
| Glucidic supplementation at D2 (g/kg/day) | 7.5 [1.8] | 6.5 [2.4] | <.01 |
- Note: Data are expressed by mean (SD) for normally distributed data, median [IQR] for non-normally distributed data, and count (%) for qualitative data.
- Abbreviations: ACS, antenatal corticosteroids; BW, birth weight; GA, gestational age; S, surfactant; SB, surfactant/budesonide; SGA, small for gestational age.
| S group (n = 18) | SB group (n = 18) | p | |
|---|---|---|---|
| GA (weeks) | 25.5 [2.1] | 25.5 [2.2] | .96 |
| BW (g) | 793 (139) | 728 (136) | .17 |
| SGA | 0 (0) | 3 (16.7%) | .23 |
| Gender (male) | 11 (61.1%) | 11 (61.1%) | 1.00 |
| Ethnicity (Caucasian) | 14 (77.8%) | 15 (83.3%) | 1.00 |
| Singleton birth | 18 (100%) | 18 (100%) | 1.00 |
| Antenatal corticosteroids | 1.00 | ||
| Not performed | 4 | 5 | |
| Incomplete | 2 | 2 | |
| Complete | 12 | 11 | |
| Maternal clinical chorioamnionitis | 10 (55.5%) | 10 (55.5%) | 1.00 |
| Apgar 5' | 7.00 [0] | 7.00 [0.75] | .76 |
| First pH | 7.30 [0.10]) | 7.33 [0.10] | .49 |
| First pCO2 | 42 (11) | 45 (11) | .43 |
First 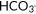
|
21.8 [2.7] | 22.0 [4.2] | .68 |
| Doses of surfactant or SB | .73 | ||
| 1 | 8 | 10 | |
| 2 | 8 | 7 | |
| 3 | 2 | 1 | |
| Total parenteral nutrition at D1 | 5 (27.8%) | 11 (61.1%) | .09 |
| Glucidic supplementation at D1 (g/kg/day) | 6.45 [0.65] | 6.50 [0.83] | .27 |
| Glucidic supplementation at D2 (g/kg/day) | 7.25 [3.2] | 7.50 [1.3] | .42 |
- Note: Data are expressed by mean (SD) for normally distributed data, median [IQR] for non-normally distributed data, and count (%) for qualitative data.
- Abbreviations: BW, birth weight; GA, gestational age; S, surfactant; SB, surfactant/budesonide; SGA, small for gestational age.
The perinatal data with the exclusion of the type of treatment were compressed by PCA obtaining four independent score variables that explained more than 70% of the total variance of the data. The score variables and the factor “treatment” were included in the design matrix that showed a conditional number equal to 4.9. Since the factor “treatment” was uncorrelated to the other four factors, we were able to assess if the treatment with surfactant alone and with SB affected the outcomes independently of the effects of the other perinatal variables.
3.1 Primary outcomes (BPD or death)
In the univariate analysis, there were no significant differences in the rates of BPD, death, and BPD or death at 36 weeks PMA between the S and the SB group (Table 3) considering all three definitions of BPD (VON definition, Jensen's definition, NICHD BPD Workshop Grading 2018) (p > .70 and power < .15).
| S group (n = 18) | SB group (n = 18) | p | Power | |
|---|---|---|---|---|
| Primary outcomes | ||||
| BPD VON definition | 9 (50.0%) | 9 (50.0%) | 1.00 | 0.05 |
| BPD Jensen's definition | 9 (50.0%) | 9 (50.0%) | 1.00 | 0.05 |
| Severity | ||||
| Grade 1 | 4 | 2 | .66 | 0.11 |
| Grade 2 | 2 | 1 | 1.00 | 0.02 |
| Grade 3 | 3 | 6 | .44 | 0.02 |
| BPD NICHD BPD Workshop Grading 2018 | 9 (50.0%) | 11 (61.1%) | .74 | 0.12 |
| Severity | ||||
| Grade I | 1 | 2 | 1.00 | 0.02 |
| Grade II | 5 | 2 | .40 | 0.21 |
| Grade III | 3 | 6 | .44 | 0.02 |
| Grade IIIA | 0 | 0 | – | |
| Death at 36 weeks PMA | 1 (5.5%) | 0(0) | 1.00 | <0.01 |
| BPD or death at 36 weeks PMA (VON definition) | 10 (55.5%) | 9 (50.0%) | 1.00 | 0.07 |
| BPD or death at 36 weeks PMA (Jensen's definition) | 10 (55.5%) | 9 (50.0%) | 1.00 | 0.07 |
| BPD or death at 36 weeks PMA (NICHD BPD Workshop Grading 2018) | 10 (55.5%) | 11 (61.1%) | 1.00 | 0.07 |
| Secondary respiratory outcomes | ||||
| Extubation failure within 24 h | 4 (22.2%) | 1 (5.5%) | .33 | 0.21 |
| No. of reintubations for respiratory causes | 9 (50.0%) | 6 (33.3%) | .50 | 0.20 |
| Duration of MV (days) | 17 [15] | 25 [23] | .25 | 0.35 |
| Post-natal dexamethasone treatment | 5 (27.8%) | 7 (38.9%) | .72 | 0.11 |
| Ventilation at 36 weeks PMA | 17 evaluated | 18 evaluated | ||
| SVIA | 8 | 7 | .74 | 0.07 |
| Hood oxygen | 4 | 2 | .40 | 0.15 |
| High flows | 2 | 3 | 1.00 | 0.03 |
| CPAP/NIPPV | 3 | 5 | .69 | 0.09 |
| MV | 0 | 1 | 1.00 | <0.01 |
| FiO2 at 36 weeks PMA | 23 [13] | 28 [19] | .55 | 0.29 |
| Secondary non-respiratory outcomes | ||||
| Hypotension (inotropic support within first 5 days of life) | 9 (50.0%) | 2 (11.1%) | .03 | 0.76 |
| PDA | 11 (61.1%) | 10 (55.5%) | 1.00 | 0.07 |
| Requiring medical treatment | 10 | 10 | 1.00 | 0.05 |
| Requiring surgical ligation | 2 | 4 | .66 | 0.11 |
| Certain EOS (positive blood culture within the first 72 h of life) | 2 (11.1%) | 0(0) | .49 | 0.04 |
| Suspected EOS (clinical symptoms and increased CRP within the first 72 h of life) | 6 (33.3%) | 8 (44.4%) | .73 | 0.12 |
| Certain LOS (clinical symptoms and increased CRP after 72 h of life) | 7 (38.9%) | 6 (33.3%) | 1.00 | 0.07 |
| Suspected LOS (positive blood culture after 72 h of life) | 4 (22.2%) | 3 (16.7%) | 1.00 | 0.05 |
| IVH ≥ III | 5 (27.8%) | 4 (22.2%) | 1.00 | 0.06 |
| Cerebellar hemorrhage | 1 (5.5%) | 1 (5.5%) | 1.00 | <0.01 |
| PVL | 2 (11.1%) | 3 (16.7%) | .66 | 0.04 |
| NEC Bell's stage ≥ II | 3 (16.7%) | 1 (5.5%) | .60 | 0.09 |
| ROP ≥ II | 11 (61.1%) | 10 (55.5%) | 1.00 | 0.07 |
| Metabolic Bone Disease | 2 (11.1%) | 3 (16.7%) | 1.00 | 0.04 |
| Long-term respiratory outcomes | ||||
| Respiratory support at 12 months of corrected age | 16 evaluated | 17 evaluated | 1.00 | <0.01 |
| 100% SVIA | 100% SVIA | |||
| Hospitalizations for respiratory causes within the first year of life | 14 evaluated over 17 | 17 evaluated over 17 | ||
| 0 | 8 | 16 | .03 | 0.65 |
| 1 | 5 | 0 | .01 | 0.80 |
| 2 | 1 | 1 | 1.00 | <0.01 |
| Admissions to PICU for respiratory insufficiency within the first year of life | 14 evaluated over 16 | 17 evaluated over 17 | ||
| 1 | 2 | 1.00 | 0.02 | |
- Note: Data are expressed by mean (SD) for normally distributed data, median [IQR] for non-normally distributed data, and by count (%) for qualitative data.
- Abbreviations: BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure; CRP, C-reactive protein; EOS, early-onset sepsis; IVH, intra-ventricular hemorrhage; LOS, late-onset sepsis; MV, mechanical ventilation; NEC, necrotizing enterocolitis; NICHD, National Institute of Child Health and Human Development; NIPPV, non-invasive positive pressure ventilation; PDA, patent ductus arteriosus; PICU, Paediatric Intensive Care Unit; PMA, post-menstrual age; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; S, surfactant; SB, surfactant/budesonide; SVIA, spontaneous ventilation in room air; VON, Vermont Oxford Network.
The logistic regression-based analysis led to models where only the score part of the design matrix was significantly relevant. Specifically, scores mainly based on GA and BW were related to BPD at 36 weeks PMA and BPD or death at 36 weeks PMA (p and power between 0.01 and 0.02 and between 0.40 and 0.64, respectively). Then, the most relevant variables associated to these two primary outcomes resulted in gestational age and birth weight, whereas the combined therapy of SB did not affect their incidence, independently of the definition used for BPD (p and power between 0.30 and 0.82 and between 0.10 and 0.23, respectively).
Both univariate and multivariate data analysis led to the conclusion that the incidence of BPD was independent of the treatment with budesonide.
3.2 Secondary respiratory and non-respiratory outcomes
Receiving SB versus surfactant only did not change the rate of secondary respiratory outcomes, nor the type of ventilation and the FiO2 at 36 weeks PMA.
The SB group was characterized by a lower rate of hypotension requiring inotropic support within the first 5 DOL (p = .03, power = .76) (Table 3).
Significant models by logistic regression emerged only for two secondary outcomes. Specifically, need of reintubations for respiratory causes (p < .01, power = 0.89) and hypotension requiring inotropic support within the first 5 DOL (power = 0.75). Confirming the finding from the univariate analysis, only the last outcome was affected by the type of treatment, with the SB group characterized by a lower rate of hypotension (p = .02, power = 0.69).
3.3 Long-term respiratory outcomes
Considering infants who survived at 12 months of corrected age for whom data on the respiratory follow-up were available, from the univariate analysis those receiving SB had fewer admissions to a pediatric ward due to respiratory causes (p = .03, power = 0.65). None of the included infants required any respiratory support at 1 year of corrected age (Table 3).
3.4 Adverse effects
The incidence of potential adverse effects related to the intra-tracheal administration of budesonide did not differ between the two groups (p > .05, power < 0.35) (Table 4).
| S group (n = 18) | SB group (n = 18) | p | Power | |
|---|---|---|---|---|
| Hyperglycemia (2 consecutive values ≥ 200 mg/dl in the first 3 DOL) | 6 (33.3%) | 8 (44.4%) | .73 | 0.12 |
| Insulin therapy (within first 72 h of life, despite reduction of glucidic supplementation to 4.5 g/kg/day) | 1 (5.5%) | 4 (22.2%) | .34 | 0.22 |
| Leucocytosis (WBC > 30,000/mm3 in the first 5 DOL) | 2 (11.1%) | 6 (33.3%) | .29 | 0.35 |
| Candida infection (within first 14 DOL) | 1 (5.5%) | 1 (5.5%) | 1.00 | <0.01 |
- Note: Data are expressed by count (%).
- Abbreviations: DOL, day of life; S, surfactant; SB, surfactant/budesonide; WBC, white blood cell.
4 DISCUSSION
In 2018, we began administering a combination of budesonide and surfactant to all infants <28+0 GW and severe RDS to decrease the burden of BPD, in light of the recent evidence from the studies by Yeh et al.16, 18 This new strategy has been touted as a unique opportunity (a “magic bullet”) for the prevention of BPD, specifically targeting the lung and minimizing the risk of adverse effects.29
Airway administration (inhalation or instillation) of corticosteroids theoretically increases pulmonary deposition, reduces systemic bioavailability, and therefore potential adverse effects.19, 30 Available data suggest that the mixture of SB maintains an equivalent surface tension reduction of surfactant and at the same time the anti-inflammatory properties of budesonide. To verify the effectiveness of this strategy and to evaluate the side effects potentially related to the use of the combined therapy, we retrospectively analyzed respiratory and neonatal outcomes in the period before and immediately after the introduction of SB administration in our Unit.
Curosurf® (poractant alfa) intra-tracheal suspension, a natural surfactant prepared from porcine lungs, has been demonstrated to have good performance when supplemented with budesonide in preliminary studies in vitro31 and in animal studies.32 Histological examinations of the lung parenchyma of surfactant-depleted adult rabbits show significantly lower inflammation and edema scores in those treated with the combination of poractant alfa and budesonide in comparison to the animal group treated with poractant alfa only.32 Similar results of decreased lung and systemic inflammation, associated with improved pulmonary physiology, have been found in preterm sheep treated with surfactant plus budesonide.33, 34
In 2008 Yeh et al.16 suggested that intra-tracheally administered surfactant maximizes distribution and efficacy of budesonide when acting as a vehicle to the latter. The effects of budesonide in humans consist in a potent anti-inflammatory action by reducing inflammatory cells' recruitment, and an improvement in pulmonary edema possibly via a diuretic effect, which causes a fluid offload from the lungs when administered locally. This contributes to better gas exchange and lung mechanics.9 Budesonide undergoes extensive biotransformation in the liver with low systemic potency of its metabolites.35
In this retrospective chart review, the overall rates of BPD were comparable with those reported in the literature for extremely preterm VLBW infants.2 We considered neonates born <28+0 GW and a BW < 1500 g similar to the studies conducted by Yeh et al.,18 where the mean GA of VLBWI was 26.5 GW in the intervention group. Unlike their findings, however, our results show that the combination of surfactant and budesonide did not affect the incidence of BPD, death, and BPD or death at 36 weeks PMA considering the more frequent definitions of BPD currently used (VON's, Jensen's, and 2018 NICHD BPD Workshop's).25, 36 The logistic regression analysis confirmed the findings from the univariate analysis, showing no reliable models for BPD depending on budesonide, but a reliable relationship between death or BPD at 36 weeks PMA and gestational age and SGA condition. This is in accordance with evidence from several studies demonstrating a higher rate of morbidities with lower gestational age, and in particular in the small for gestational age infants.37
In contrast with the results from the studies by Yeh et al.16, 18 and Kothe et al.38 we found no differences in the duration of mechanical ventilation between the two groups, nor in other secondary short-term respiratory outcomes. Nevertheless, infants treated with the combination of SB showed a lower rate of admissions to a pediatric ward due to respiratory infections within the first year of corrected age. This result is similar to the one from the follow-up of infants included in the clinical trial by Yeh et al in 2016,18 where upper respiratory infections occurred less frequently in the intervention group (16.9% vs. 27.6%). However, this is difficult to interpret as several factors may have influenced this outcome (presence of siblings, nutrition, social aspects, etc.).
As regards the secondary non-respiratory outcomes, interestingly hypotension with the need for inotropic support within the first 5 days of life was decreased by the use of the combined therapy. This effect may be related to the action of budesonide in the regulation of vascular tone through adrenergic receptors, and to the modulation of adrenal insufficiency in these extremely preterm infants.9 This finding highlights that, despite acting locally at the bronchial tree and the lung parenchyma, budesonide may be systemically absorbed and determine other unexpected effects. Systemic absorption of budesonide from the lungs has been reported in both preterm sheep33, 34 and infants,39 with high initial plasma levels of the drug (170 ng/ml) sharply decreasing at 24 h, and a rapid clearance from both lungs and plasma. In addition, budesonide-exposed lambs show more stable blood pressures.33 Further studies of pharmacokinetics and pharmacodynamics using dried blood spot or urine assays,39 are warranted to explore systemic absorption and effects of budesonide in preterm infants.
In our cohort, no clinically significant adverse effects were observed that may potentially be related to the combined mixture.16
We used different inclusion criteria compared to the ones by Yeh et al, as we chose to select only extremely preterm VLBW infants who failed CPAP according to the oxygen requirement of >0.3 as proposed by Dargaville et al.,40 while the threshold for oxygen supplementation in previous studies was 0.5–0.6. Additionally, the 95th percentile of BW in our selected neonates was <1000 g and there were approximately 50% of cases of maternal chorioamnionitis in both groups. The cohort investigated by Yeh et al.18 covered a greater range of BW and showed a less incidence of cases of chorioamnionitis.
There are several limitations inherent to our study. Firstly, the retrospective nature of our analysis and the small sample size compared to previous studies dampen firm conclusions from our results, which should therefore be taken with caution. For these same reasons, the study is underpowered for most of the primary and secondary outcomes, with the exception of hypotension and need of reintubations for respiratory causes.
The major strength of this study is the accurate matching of the study groups to improve causal inferences and reduce confounding biases given by the demographic and clinical data, in particular the ones related to BW and SGA infants. Our study adds further data to the paucity of studies comparing instilled steroids to gold-standard therapies for RDS treatment and BPD prevention. Larger randomized controlled trials, like the Australasian PLUSS trial led by the Royal Women's Hospital in Melbourne,41 are needed to shed light on this topic. The analysis of the metabolic profile of instilled steroids may also help in the understanding of their potential beneficial and side effects in preterm infants.
5 CONCLUSIONS
The preliminary results of this retrospective study provides further data on the use of intra-tracheal budesonide with surfactant in extremely preterm VLBW infants with severe RDS in the first hours of life. In our tertiary level of care NICU we did not find an efficacy of the combined therapy on the incidence of BPD, death, and BPD or death at 36 weeks PMA. Further randomized controlled trials are needed to explore the benefits of early administration of IT steroids on short- and long-term outcomes in the preterm population.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Laura Moschino: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); resources (equal); visualization (equal); writing—original draft (equal). Daniel Nardo: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); resources (equal); supervision (equal); writing—original draft (equal). Luca Bonadies: Data curation (equal); resources (equal); visualization (equal); writing—review and editing (equal). Matteo Stocchero: Data curation (equal); formal analysis (lead); software (lead); writing—original draft (equal). Giulia Res: Data curation (equal); writing—review and editing (equal). Elena Priante: Writing—review and editing (equal). Sabrina Salvadori: Writing—review and editing (equal). Eugenio Baraldi: Conceptualization (lead); project administration (lead); supervision (lead); visualization (equal); writing—review and editing (equal).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




