Proportional assist and neurally adjusted ventilation: Clinical knowledge and future trials in newborn infants
Abstract
Different types of patient triggered ventilator modes have become the mainstay of ventilation in term and preterm newborn infants. Maintaining spontaneous breathing has allowed for earlier weaning and the additive effects of respiratory efforts combined with pre-set mechanical inflations have reduced mean airway pressures, both of which are important components in trying to avoid lung injury and promote normal lung development. New sophisticated modes of assisted ventilation have been developed during the last decades where the control of ventilator support is turned over to the patient. The ventilator detects the respiratory effort and adjusts ventilatory assistance proportionally to each phase of the respiratory cycle, thus enabling the patient to have full control of the start, the duration and the amount of ventilatory assistance. In this paper we will review the literature on the ventilatory modes of proportional assist ventilation and neurally adjusted ventilatory assistance, examine the different ways the signals are analyzed, propose future studies, and suggest ways to apply these modes in the clinical environment.
1 INTRODUCTION
Promoting spontaneous breathing during invasive ventilation in term and especially in preterm infants has been a prerequisite for early weaning to noninvasive ventilatory assistance. But spontaneous breathing during ventilation necessitates synchrony with the mechanical inflations, as asynchrony can cause volutrauma, that is, pulmonary air leaks,1, 2 fluctuations in cerebral blood flow with risk of developing periventricular hemorrhage,3, 4 and suboptimal ventilation and oxygenation.5 Consequently, comparisons of patient-triggered ventilation with intermittent mandatory ventilation showed improved oxygenation,6 increased and more consistent tidal volumes,7 decreased fluctuations in arterial and cerebral blood flow,8, 9 and decreased work of breathing.10 Encountered problems with triggering devices are delayed trigger time, missed spontaneous breaths, triggering on expiration, or inappropriate triggering, that is, auto-cycling due to artefactual signals.11 But there might also be interactions between the onset of ventilatory inflations and the pulmonary reflexes, such as the Hering Breuer inspiratory inhibitory reflex and Head's paradoxical reflex,12-14 where too rapidly inflated or/and too large volumes might inhibit inspiration, and where superimposed sighs might be non-synchronously triggered, problems that might be mitigated with more proportionally adjusted modes.
The newest addition to patient-triggered ventilation is the development of modes that proportionally adjust the ventilator inflation pressure in response to the breathing effort of the patient, that is, patient control of the applied ventilator pressures. To provide this ventilatory assistance, the ventilator needs a reliable signal from the patient representing the breathing effort. The three most common ways that provide such a signal are: (1) airflow changes detected by highly sensitive pneumotachographs (PNT), such as proportional assist ventilation (PAV); (2) diaphragmatic electrical activity (EAdi), such as neurally adjusted ventilatory assistance (NAVA); and (3) external devices, such as computerized Graseby capsules or plethysmographs detecting the diaphragmatic contractions as abdominal movements and/or thoracic movements. Although the external devices generate comparable breathing curves as airflow integrated tidal volumes and transcutaneous/esophageal electromyography of the diaphragm, they have mainly been studied in noninvasive modes and as initiators of pre-set mechanical breaths, that is, synchronized noninvasive intermittent positive pressure ventilation, and as such will not be dealt with in this review.
2 PROPORTIONAL ASSIST VENTILATION
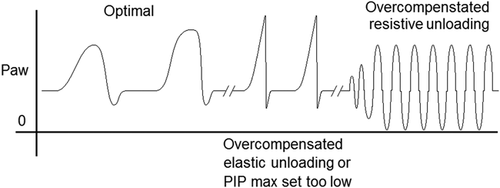
During PAV pressure and/or flow are applied proportionally to the spontaneous breathing effort as detected by inspired airflow throughout the entire respiratory cycle,15, 16 allowing the patient full control of the amplitude and timing of each breath. The servo control of ventilatory pressure or flow is accomplished by a rapid computing (<10 ms) of the airflow signal from a sensitive laminar PNT,17 to keep the ∆P/∆V constant to the preset unloading of the total system elastance, which is the combined elastance of the ventilator in series with the elastance of the lung (Figure 1). The resistance can be unloaded in a similar way by keeping ∆P/airflow constant, where the approximated endotracheal tube (ETT) resistance can be included in the calculation. Performance may be affected by many possible sources of error, including leakages around un-cuffed ETT, secretions or water in the PNT, ETT occlusions or malpositions. The lung elastance (EL) can be calculated by inverting the effective lung compliance (CL), as measured by the ventilator, giving EL = 1/CL; (to unload 75% of EL, set ventilator elastance [EV] at 0.75 × EL or start with 80%–90% of EL and lower this unloading until good breathing movements and corresponding pressure curves are detected). Excessive elastic unloading might lead to overcompensation of inspiratory pressures to the patient that may inhibit the breathing efforts and consequently cause the ventilator to revert to the backup mode of flow triggered ventilation (Figure 2). The unloading of airway resistance during PAV is less evident as the cross-sectional area of the lungs related to lung volume, that is, specific airway resistance, is low in neonates, whereby only compensation for the resistance of the smallest ETTs is usually needed (Table 1), both during inspiration and expiration.18-20 It should be noted that an excessive resistive unloading can potentially induce oscillations corresponding to the inert elastic elements of the lung18, 19 (Figure 2), and completely inhibit phrenic nerve activity.21 Clinical settings and adjustments of PAV are presented in Table 1.
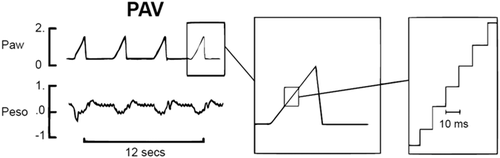
| Before start of PAV: |
| ▪ Set the backup ventilation as during the previous ventilation for PIP, PEEP, I:E, and trigger level. |
| ▪ Use sinusoidal pressure curve to promote spontaneous breathing |
| ▪ Apneic time: depending on GA and lung function (usually 2–4 s) |
| ▪ Backup time: minimal time that backup will be maintained before PAV starts again (usually 10 s) |
| Monitoring during PAV: |
| ▪ tcpCO2, tcpO2, SaO2, ECG |
| ▪ PIP, MAP, PEEP, RR |
| Initial setting PAV: |
| 1. Set VTlimit: upper allowed limit depending on GA/PMA and lung function (6 ml/kg if applied early; 10–15 ml/kg if applied later) |
| 2. Set RV–during inspiration and expiration (R = 25 cmH2O/L/s for 2.0 mm ET; R = 12.5 cmH2O/L/s for 2.5 mm ET; R = 0 cmH2O/L/s for 3.0 mm ET; for severe BPD individualized R) |
| 3. Set EV–unload 75% of lung elastance, that is, 0.75 × 1/CL (e.g., CL = 0.5 ml/cmH2O; lung elastance = 1/CL = 1/0.5 = 2; 75% unloading of lung elastance, i.e., 0.75 × EL = 0.75 × 1/CL = 0.75 × 2 = 1.5 cmH2O/ml → set EV = elastic unloading = 1.5 cmH2O/ml; the resulting total C will be 1/[EL–EV] = 1/[2–1.5] = 1/0.5 = 2 ml/cmH2O) |
| 4. Set PEEP +1 cmH2O as during the previous ventilation; to compensate for leakage during PAV and lower FRC |
| 5. PAV is started by changing to “CPAP mode” and pushing the green button for “Unloading” |
| 6. Alarm limits set for PIP |
| Further adjustments of EV should be based on observations of the pressure curves where a soft and harmonious curve during the entire breath indicates optimal unloading. If the resulting pressure curve is short and sharp, this could indicate: (a) overcompensation, that is, too high EV → lower EV; (b) too stiff lungs which necessitates higher PIP limits → increase PIP limit (see Figure 2) |
| Documentation and controls: |
| ▪ MAP gives information on how much unloading is necessary for a certain E (monitor trend curves) |
| ▪ tcpCO2 just before and during PAV (observe that increased breathing effort produces CO2) |
| ▪ Respiratory rate; usually increased due to elastic unloading and/or too high set resistive unloading |
| ▪ How often back-up sets in, that is, apneic episodes |
| ▪ Check PAV settings 3–6 times a day |
| Suggested treatment strategy: |
| Start with 1 h PAV + 2 h previous ventilation; increase time on PAV to 2 + 2 h previous ventilation, then 2 + 1 and 3 + 1 h, and then 24 h. |
| Adjustments: |
| ▪ If decreased MAP → lower EV |
| ▪ If increased pCO2 → increase EV and PEEP (or shorten time on PAV; check and increase PIP limits) |
| ▪ If increased FiO2 →increase EV and/or increase PIP limits |
| Weaning and extubation to nCPAP: |
| ▪ Continually on PAV for 12-24 h |
| ▪ Decreased pCO2 and/or FiO2 |
| ▪ Decreased EV, usually to ≤1.0 |
- Abbreviations: CL, lung compliance; CT, total compliance; EV, elastic unloading; FiO2, fraction of inspired oxygen; FRC, forced respiratory capacity; I:E, inspiratory to expiratory ratio; MAP, mean airway pressure; PAV, proportional assist ventilation; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; RR, respiratory rate; RV, resistive unloading; SaO2, oxygen saturation; tcpCO2, transcutaneous carbon dioxide pressure; tcpO2, transcutaneous oxygen pressure; VT, tidal volume.
Even though extensively studied in animal models of different lung injuries,22, 23 the clinical application of PAV has only been evaluated in small cohorts of neonates during short periods focusing mainly on feasibility,24, 25 and less in prospective randomized controlled trials (RCTs). Clinical studies have shown short-term benefits in neonates with acute respiratory distress syndrome (RDS) and evolving bronchopulmonary dysplasia (BPD) in terms of maintaining stable gas exchange and lower oxygen index at lower mean airway pressures (MAPs), compared to other modes of patient triggered ventilations24-28 (Table 2), but no long term RCTs have been performed to evaluate outcomes such as the development of BPD and/or duration of mechanical ventilation. Both the facilitated spontaneous breathing and the lower MAP without compromised oxygenation or ventilation, suggest the possibility of using PAV to minimize ventilator induced lung injury in the early phase of life in extremely preterm infants, and later during the weaning of long term mechanically ventilated infants, applications that need future large controlled studies. However, the use of PAV during the transition from invasive to noninvasive ventilation is partly limited by the fact that it is difficult to apply PAV non-invasively as the PNT will not be able to measure correctly the volumes and pressures to unload adequately the elastance. Despite being clinically available for over 20 years, PAV has not gained widespread acceptance. Still, PAV is an attractive concept, but there remains an absence of convincing evidence surrounding clinically important outcomes.
| N | GA (weeks ± days/range) | PNA (days) | Duration | Indication | Comparison | Outcome | |
|---|---|---|---|---|---|---|---|
| Schulze et al.24 | 36 | 26.9 ± 2.3 | 2.7 ± 1.7 | 45 min | RDS | A/C and IMV | Lower MAP, PIP, and OI |
| Schulze et al.25 | 22 | 25.6 ± 2.0 | 22.9 ± 15.6 | 4 × 4 h | BPDE | A/C and SIMV | Lower MAP and PIP |
| Bhat et al.26 | 12 | 25 (24–26) | 43 (8–86) | 1 h | BPDE | A/C | Lower MAP, PIP, OI, and Ptp |
| Shetty et al.27 | 8 | 25 (24–33) | 19 (10–105) | 4 h | BPDE | A/C | Lower MAP and OI |
| Hunt et al.28 | 18 | 25.3 (23–30) | 20.5 (8–58) | 2 h | BPDE | NAVA | Lower MAP; higher A-a; same OIa |
- Abbreviations: A-a, alveolar-arterial oxygen gradient; A/C, assist control ventilation; BPDE, evolving BPD; GA, gestational age; IMV, intermittent mechanical ventilation; MAP, mean airway pressure; N, number of infants; NAVA, neurally adjusted ventilatory assist; OI, oxygen index; PAV, proportional assist ventilation; PIP, peak inspiratory pressure; PNA, postnatal age; Ptp, transpulmonary pressure; RDS, respiratory distress syndrome.
- a Both PAV and NAVA had lower MAP and OI compared to baseline settings.
3 NEURALLY ADJUSTED VENTILATORY ASSIST
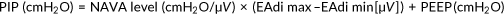
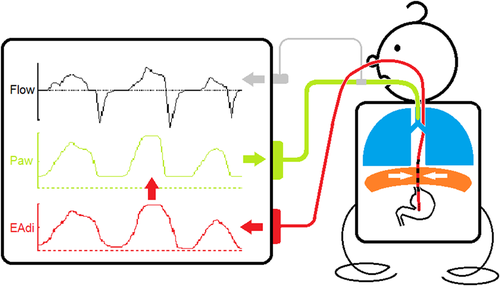
Clinical settings and adjustments of NAVA level are presented in Table 3. As in all applications of patient triggered ventilatory assistance, NAVA is dependent on the spontaneous respiratory drive and what might influence this drive. It would be easy to adjust the proportional assist ventilation during NAVA and PAV, if the breathing effort was solely dependent on chemoreceptor activation, that is, changes in CO2 and O2. But given that the respiratory drive of premature infants is not fully developed and there is an immature response to changes in CO2 and O2, a reduced capacity to compensate for changes in gas exchange, a risk of developing respiratory acidosis and a heterogenic mechanoreceptor activity, it is difficult to base changes in the proportional assist ventilation during NAVA and PAV on a single set of variables. In this special patient population it is important to adjust the ventilation settings, and also the alarm settings, in concordance with the observed ventilatory and maturational status of each individual infant. It is also important to consider the immaturity of other organs, such as the kidneys that commonly lead to transient renal tubular acidosis in the first few days of life. Because the respiratory control is driven by pH, not simply PCO2, clinicians must not focus solely on PCO2 targets but rather evaluate the patient's respiratory drive in the context of pH. Further it is possible to over assist the patient. For example, increasing the NAVA level can unload the respiratory muscles to a point where the CO2 induced breathing activity is diminished, and thereby reduce the EAdi and the effectuated tidal volumes, that is, reaching the breakpoint for NAVA setting.34, 35 Interestingly the breakpoint might sometimes be difficult to estimate in preterm infants, especially with NAVA gains above 2 cmH2O/µV where reduced EAdi and shallow breathing (hypoventilation) might be observed in combination with periods of excessively high EAdi and high tidal volumes,36 possibly because the Hering Breuer reflex is overridden by pronounced respiratory acidosis and gasping. The manufacturer of NAVA provides a simultaneous flow or pressure triggered pressure support ventilation mode in addition to the backup ventilation in case of inadequate EAdi signal. Compared to other triggering devices, EAdi is a relatively stable signal that has minimal trigger delay and is unaffected by movement or airflow artefacts.37 Besides selecting the right size of the EAdi catheter, it is important to ensure the correct position of the catheter at the level of the diaphragm as dislodgement poses the greatest problem to synchronizing the ventilator with the patient's breathing activity38 (Figure 3). Because it does not depend on a pneumatic trigger and is unaffected by leakage in an open system NAVA is ideally suited for noninvasive ventilation with a high level of synchrony.30, 39 Studies of spontaneously breathing term and mildly preterm infants without ventilatory assistance have given us some directions as to the normal levels of maximal and minimal EAdi.31
| Generally: |
| • Respiratory drive controls the EAdi signal together with the set NAVA level (pressure/EAdi; cmH2O/µV), resulting in a certain inspiratory pressure |
| • Respiratory drive is a prerequisite for NAVA ventilation. Respiratory drive is affected by CO2 variations and infant maturity, wherefore increasing the NAVA level does not necessarily lead to an improved ventilation. The lung mechanics of the patient may also require other types of ventilation |
| Note: If repeated alarms for apnea, despite correct electrode placement → put patient on other ventilatory assistance during continued EAdi monitoring, and try to normoventilate the patient. If reduced VT in combination with reduced EAdi max and increased RR → time to put the patient on more assisted ventilation |
| Monitoring during NAVA: |
| ▪ tcpCO2, tcpO2, SaO2 |
| ▪ EAdi signal trends (see "Trends") |
| ▪ Trends for VT, PIP, MAP, RR, FiO2 (note all before starting NAVA and then during NAVA) |
| Initial setting invasive NAVA: |
| 1. Set the same PEEP as during the previous ventilation. Consider increasing PEEP if EAdi min is constantly >2 µV, that is,elevated baseline activity, suggesting that the diaphragm is activated all the time during expiration to maintain FRC |
| 2. Set the initial NAVA level so as the test curve achieves the same PIP level as during the previous ventilation (usually 1–2 cmH2O/µV) |
| 3. Adjust NAVA level according to the EAdi maximum; should be 5–10 (−15) µV |
| (a) Lower NAVA level if EAdi max <5 µV |
| (b) Increase NAVA level if EAdi max >15 µV |
| (c) Lower and increase NAVA level by 0.1–0.2 cmH2O/µV at a time |
| 4. Set the trigger EAdi = 0.5 µV |
| 5. Set the Backup according to earlier ventilator settings |
| 6. Alarm settings: max PIP (+2–4 cmH2O above previous setting) and apneic time (2–4 s) according to maturity and postnatal age |
| Further adjustments should be based on trend curves and specifically the pendants, as the snapshot varies considerably during NAVA |
| Setting noninvasive NAVA (NIV-NAVA): |
| 1. Consider NIV-NAVA if NAVA levels <0.5–1.0 cmH2O/µV. Not a definitive requirement for extubating the patient to NIV-NAVA. Try lowering the NAVA level and see if EAdi activity and VT are maintained |
| 2. NIV-NAVA level is usually lower (0.5–1.0 cmH2O/µV) compared to invasive NAVA |
| (a) Lower NAVA level if EAdi max <5 µV |
| (b) Increase NAVA level if EAdi max >20 µV |
| (c) Lower and increase NAVA level by 0.1–0.2 cmH2O/µV at a time |
| 3. At EAdi <10 µV with NIV-NAVA <0.5 cmH2O/µV, consider nCPAP without NAVA |
| Note: If NIV-NAVA level >2.0 cmH2O/µV → consider other ventilatory options |
| Documentation and controls: |
| • NAVA level, PEEP, FiO2 and regular PDMS; check position EAdi catheter every 8th hour |
| • Back-up: pressure over PEEP; RR = 50–60/min (slightly lower than RR during NAVA); Tinsp or I:E |
| • Check NAVA settings 3–6 times a day |
- Abbreviations: EAdi, electric activity of diaphragm; FiO2, fraction of inspired oxygen; FRC, forced respiratory capacity; MAP, mean airway pressure; NAVA, neurally adjusted ventilatory assistance; nCPAP, nasal continuous positive airway pressure; NIV-NAVA, noninvasive NAVA; PDMS, patient data monitoring system; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; RR, respiratory rate; SaO2, oxygen saturation; tcpCO2, transcutaneous carbon dioxide pressure; tcpO2, transcutaneous oxygen pressure; Tinsp, inspiratory time; VT, tidal volume.
The clinical use of EAdi can be divided into either the direct ventilatory application, that is, NAVA, or as a sensitive detector of breathing activity40 in diseases, such as congenital central hypoventilation syndrome,41, 42 postoperatively after diaphragmatic hernia,43 phrenic nerve injury after cardiac surgery44 etc.
The clinical application of NAVA has been studied both in preterm and term newborn infants, and compared to different patient triggered modalities, but few long term RCTs have been performed. In a Cochrane Review from 2017, where the inclusion criteria of randomized controlled trials, and with the predefined objectives of primary or rescue treatment with NAVA in reducing BPD, mortality, or other morbidities (intraventricular hemorrhage [IVH], periventricular leukomalacia or pneumothorax), were only met by one study out of 17 selected studies, and thus not considered a fully validated mode of ventilation.45, 46 In the single study included in this Cochrane Review, the authors observed a lower PIP during NAVA compared to conventional ventilation in infants born between 28 + 0 to 36 + 6 weeks GA with RDS, but with no other difference in outcomes, such as time on mechanical ventilation, rate of BPD, pneumothorax or IVH.46 The same study revealed some of the difficulties in performing RCTs with early applications of proportionally adjusted ventilatory modes, where due time was taken to stabilize the patients before inserting the EAdi catheter, so that some patients were included after 24 h, which made the reviewers consider this study more of a rescue mode evaluation of NAVA than a primary mode evaluation.45, 46 Additionally, periods of apnea and low EAdi activity were reported which necessitated back up ventilation and/or switching to other modes of ventilation in some infants.46 In multiple small prospective cross-over studies in infants with RDS or evolving BPD, NAVA has been shown to reduce the PIP, the fraction of inspired oxygen, and the work of breathing compared with other patient-triggered ventilatory modes28, 46-55 (Table 4). In a multi-center review of current clinical practice, NAVA was successful as a weaning mode of ventilation in infants with severe BPD 67% of the time.56
| N | GA (weeks ± days/range) | PNA (days) | Duration (hours) | Indication | Comparison | Outcome | |
|---|---|---|---|---|---|---|---|
| aBreatnach et al.47 | 16 | Term | 2–22 | 4 | VOC, CDH | PSV (RCO) | Lower PIP and MAP |
| aLee et al.48 | 19 | 29 ± 3 | 7 (2–70) | 4 + 4 | Premat. | SIMV-PS (RCO) | Lower PIP and WoB |
| aStein and Howard49 | 52 | 26 ± 3 | 15 (0–56) | 24 | RDS, BPDE | SIMV-PS (retrosp.) | Lower PIP, FiO2, CO2 |
| aStein et al.50 | 5 | 26 ± 1 | 24 (6–34) | 4 + 4 | RDS, BPDE | SIMV-PS (RCO) | Lower PIP, FiO2, CO2, WoB |
| aChen et al.51 | 10 | Preterm | ? | 1 | RDS | SIMV (RCO) | Lower PIP and WoB |
| aLonghini et al.52 | 14 | 32 ± 3 | 3 (1–7) | 1–6–12 | RDS, sepsis | PSV-VG (prosp. NR) | Lower PIP, VT; Higher RR |
| aKallio et al.46 | 60 | 32 (28–36) | 9 (2–49) h | 35 (23–68) | RDS | PCV, HFOV (RCT) | Lower PIP |
| aJung et al.53 | 29 | 25 (23–30) | 32 (26-43) | 1–4–12–24 | BPD, BPDE | SIMV (retrosp.) | Lower PIP, MAP, WoB, CO2, FiO2 |
| aShetty et al.54 | 9 | 25 (22–27) | 20 (8–84) | 1 | BPD, BPDE | PCV (RCO) | Lower PIP, MAP, FiO2, OI |
| aRosterman et al.55 | 22 | 26 (23–39) | 40 (3–135) | 12 | RDS, BPDE | SIMV-PS (RCO) | Lower PIP, WoB, VT; Higher RR |
| Kallio et al.57 | 40 | 33 (28–36) | 0–2 | 12 (–LOS) | RDS | nCPAP (RCT) | No diff. FiO2, RR, intub., CO2, surf., duration NIV; Longer LOM |
| Yagui et al.58 | 123 | 29 ± 2 | 0–2 | 12–72 (−LOS) | RDS | nCPAP (RCT) | No diff. intub., surf., duration NIV; Shorter LOM |
| Yagui et al.59 | 49 | <28 | ? | 0–72 | RDS, BPDE | nCPAP (retrosp.) | Less extub. failure |
| Lee et al.60 | 32 | 25–27 | 4–34 | 0–72 | RDS, BPDE | nCPAP (retrosp.) | Less extub. failure |
| aHunt et al.28 | 18 | 25.3 (23–30) | 20.5 (8–58) | 2 | BPDE | PAV | Higher MAP; Lower A-a; Same OIb |
- Abbreviations: A-a, alveolar-arterial oxygen gradient; BPDE, evolving bronchopulmonary dysplasia; CDH, congenital diaphragmatic hernia; CO2, arterial carbon dioxide pressure; diff., difference; extub., extubation; FiO2, fraction of inspired oxygen; HFOV, high frequency oscillatory ventilation; intub., intubation; LOM, length of mechanical ventilation; LOS, length of stay; MAP, mean airway pressure; N, number of infants; NAVA, neurally adjusted ventilatory assist; nCPAP, nasal continuous positive airway pressure; NIV, duration of noninvasive ventilation; OI, oxygen index; PAV, proportional assist ventilation; PIP, peak inspiratory pressure; Premat., prematurity; prosp.NR, prospective non-randomized study; PSV, pressure support ventilation; PS, pressure support; PSV, pressure support ventilation; RCO, randomized cross-over study; retrosp., retrospective study; RCT, randomized controlled trial; RDS, respiratory distress syndrome; RR, respiratory rate; SIMV, synchronized intermittent mechanical ventilation; VOC, vitium of cordis; VG, volume guarantee; VT, tidal volume; WoB, work of breathing.
- a Invasive NAVA.
- b Both PAV and NAVA had lower MAP and OI compared to baseline settings.
Similarly, no long-term studies have been performed with the noninvasive mode of NAVA. Two recent randomized controlled studies comparing NIV-NAVA with nasal CPAP during the early stages of RDS in moderately preterm infants, did not show any benefit in avoiding and/or time to intubation57, 58 (Table 4). Even though no difference was observed between NIV-NAVA and nCPAP with respect to need of intubation in both these studies (35 vs. 50%, Kallio et al.; 20.3 vs. 15.6%, Yagui et al.), a longer duration of mechanical ventilation in the NIV-NAVA group was reported in the study by Kallio et al., whereas a shorter duration could be observed by Yagui et al., possibly explained by surfactant being administered non-invasively and earlier in the latter study but contradicted by the fact that NIV-NAVA combined with early surfactant did not result in later intubation compared to nasal CPAP with early surfactant in that study57, 58 (Table 4). Using NIV-NAVA after extubation seems more promising where two small retrospective studies observed more successful weaning with NIV-NAVA than nCPAP, measured as failure within 72 h of extubation.59, 60 A Cochrane review from 2020 evaluating the safety and effectiveness of NIV-NAVA versus NIPPV included only two studies and found that there was not enough evidence to draw conclusions on their primary outcomes of safety and efficacy.61 Clearly, there is an urgent need to do prospective large controlled studies that involves both early and late use of NAVA, and during the transition from invasive to noninvasive ventilation, that is, to explore the adequate application of this sophisticated mode of ventilation.
4 DISCUSSION
The concept of allowing the patient to drive the magnitude of support received from the ventilator is very attractive and is shared by both PAV and NAVA. There are obvious similarities between these two proportionally adjusted modes, but the delay between the diaphragmatic electric activity and the concomitant start of inspiratory airflow presents the user with differences in trigger delay already at the start of the inspiration as NAVA is using the former and PAV the latter as trigger signals.37, 62 Another difference is how the pressure support is generated where a proportional but constant gain is set during NAVA independent of ongoing changes in compliance whereas the gain during PAV is proportional not only to the generated volume, as integrated from the airflow signal, but also to the pressure required to accomplish a constant unloading of this volume to obtain the set total compliance. Even though the proportional pressure support during NAVA do produce an airflow that might reduce the resistance during inspiration, the additional possibility during PAV of setting resistive unloading during both inspiration and expiration, implies that this method adjusts not only the driving pressures but also the airflows.
NAVA has the additional advantage of a diaphragmatic trigger that is not affected by ETT leakage and has a near instantaneous response time.37 However, both modalities are positive feedback loops that assume that the patient's respiratory effort is appropriate, but that weak muscles or poor lung compliance limit the patient's ability to generate an adequate tidal volume. In other words, these modes assume a mature respiratory control center. Therefore, there is a potential for unintended consequences when such techniques are applied to extremely low birth weight infants with a great deal of periodic breathing, frequent apneas and intermittent excessive inspiratory effort when disturbed—as all of these phenomena would be amplified by the positive feedback mechanism thereby giving rise to both lung collapse (atelectrauma) and/or excessive inflations (volutrauma). It should be noted that whereas PAV has a possibility to set an alarm limit for maximal volume, NAVA has only an alarm limit for maximal pressure, which should be set with care as strong breathing efforts might generate inadvertently high pressures and consequently high tidal volumes that might cause air leaks, especially when applied early during evolving lung diseases.63 The reduced MAP reported in many studies of PAV and NAVA, should also be taken with caution as to the possibility of reducing over inflation and BPD, as the same transpulmonary pressures are probably effectuated by the combination of gain or unloading and spontaneous breathing similar to other assisted ventilatory modes.64 More vigorous breathing activity might also generate larger fluctuations in end-expiratory pressures, usually necessitating optimization of PEEP during PAV and NAVA.
There is still a need for thorough evaluation of the stability of delivered tidal volume and optimal PEEP with regard to different lung conditions encountered in preterm infants, such as RDS and evolving or established BPD, but also in view of differences in the control of breathing in preterm infants at different gestational and postmenstrual ages. With due respect to efforts to evaluate proportionally adjusted modes in infants during the early acute phase of RDS, this necessitates knowledge and experience in applying these sophisticated methods, knowledge that might be more easily acquired in more stable infants and in infants being weaned off invasive ventilation and/or being rescued from invasive ventilation when deteriorating on other noninvasive ventilatory modes. Another field of interest in applying a more adapted ventilation is to investigate the possibility to reduce the use of sedatives without causing increased stress, aspects that have been observed in some clinical investigations. It's evident that there is an urgent need for large well-designed randomized trials to evaluate important long-term outcomes, such as intraventricular hemorrhage, time on mechanical ventilation, bronchopulmonary dysplasia and other complications of extreme prematurity.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Richard Sindelar: conceptualization (lead); data curation (lead); formal analysis (lead); writing original draft (lead); writing review and editing (lead). Robin L McKinney: conceptualization (supporting); data curation (supporting); formal analysis (supporting); writing original draft (supporting); writing review and editing (supporting). Linda Wallström: conceptualization (supporting); data curation (supporting); formal analysis (supporting); writing original draft (supporting); writing review and editing (supporting). Martin Keszler: conceptualization (supporting); data curation (supporting); formal analysis (supporting); writing original draft (supporting); writing review and editing (supporting).




