Advances and approaches for chemical recycling of plastic waste
Funding information: Directorate for Mathematical and Physical Sciences, Grant/Award Number: CHE-1708844
Abstract
The global production and consumption of plastics has increased at an alarming rate over the last few decades. The accumulation of pervasive and persistent waste plastic has concomitantly increased in landfills and the environment. The societal, ecological, and economic problems of plastic waste/pollution demand immediate and decisive action. In 2015, only 9% of plastic waste was successfully recycled in the United States. The major current recycling processes focus on the mechanical recycling of plastic waste; however, even this process is limited by the sorting/pretreatment of plastic waste and degradation of plastics during the process. An alternative to mechanical processes is chemical recycling of plastic waste. Efficient chemical recycling would allow for the production of feedstocks for various uses including fuels and chemical feedstocks to replace petrochemicals. This review focuses on the most recent advances for the chemical recycling of three major polymers found in plastic waste: PET, PE, and PP. Commercial processes for recycling hydrolysable polymers like polyesters or polyamides, polyolefins, or mixed waste streams are also discussed.
Graphical Abstract
The global production and consumption of plastics have increased significantly over the years. The major current recycling processes are limited by the sorting and pretreatment of plastic waster and degradation. This review covers the most recent academic and industrial efforts to recycle conventional plastics and mixed waste.
1 INTRODUCTION
The term “plastics,” in common verbiage, refers to synthetic polymers that are ubiquitous in modern society, to the extent that each person consumes 50 kg per year in the European Union and 68 kg per year in the United States.[1 ] Plastics pervade daily life as packaging,[2 ] clothing and sports equipment,[3 ] biomedical devices,[4 ] electronic components,[5 ] and in a panoply of other applications. Unfortunately, the majority of high market-share plastics are obtained from the use of nonrenewable and ecologically devastating petroleum/natural gas feedstocks and processing techniques. The imperative to access new technologies for recycling and repurposing plastics is clear given their unsustainable origins and dogged environmental persistence in the oceans[6-8 ] and on land.[9 ] The current review aims to highlight contemporary academic efforts to develop new methods for recycling some of the most abundantly produced plastics. Specifically, efforts to chemically recycle poly(ethylene terephthalate) (PET), polyethylene (PE), and polypropylene (PP), in their various forms, will be discussed. These approaches demonstrate some of the chemical methods that are effective in recycling hydrolyzable and nonhydrolyzable plastics. Many of these approaches are leveraged by industry to address recycling of mixed plastic waste streams, as will be discussed in the second half of this review.
The Society of the Plastics Industry (SPI) has assigned different plastics with Codes 1–6 in order to more easily identify the polymer used in the production of the material as well as to expedite the recycling process.[10 ] Table 1 summarizes common polymers classified in this way, with some of their most familiar uses highlighted. Organized disposal of vast quantities of plastic waste consists primarily of landfill techniques, while practices of dumping into waterways still persist, particularly in less environmentally regulated areas of the world. Beyond ecological effects of these behaviors, there are economic and geopolitical concerns of ongoing reliance on the dwindling petroleum/natural gas supply available for a growing population.
| SPI Code | Polymer | Structure | Uses |
|---|---|---|---|
| 1 | Poly(ethylene terephthalate) (PET) | 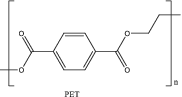
|
Soda bottles, water bottles, medicine jars, and salad dressing bottles |
| 2 | High density polyethylene (HDPE) | 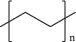
|
Soap bottles, detergent and bleach containers, and trash bags |
| 3 | Polyvinyl chloride (PVC) | 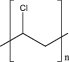
|
Plumbing pipes, cables, and fencing |
| 4 | Low density polyethylene (LDPE) | 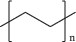
|
Cling wrap, sandwich bags, and grocery bags |
| 5 | Polypropylene (PP) | 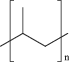
|
Reusable food containers, prescription bottles, and bottle caps |
| 6 | Polystyrene (PS) | 
|
Plastic utensils, packaging peanuts, and styrofoam |
| 7 | Other |
- Abbreviation: SPI, society of plastics industry.
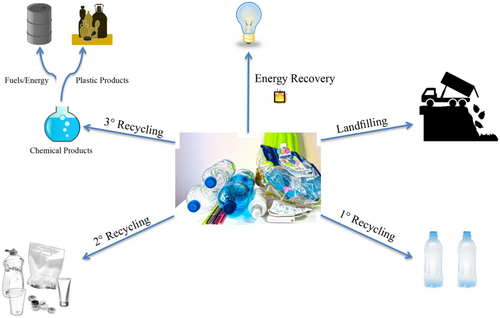
Efforts to recycle plastic waste have grown in meteoric fashion over the past two decades, yet the challenges associated with harnessing postconsumer plastics as feedstock for new products are sufficiently severe that the relative amount of plastics recycled remains embarrassingly low.[11 ] In 2015, approximately 262 Mt of municipal solid waste (MSW) was generated in the United States.[12 ] Of the 262 Mt of MSW, a full 13% (34.5 Mt) was constituted of putatively recyclable plastic waste. Of these 34.5 Mt of plastic waste, however, only 9% was recycled. This compares with 16% that was incinerated and 75% that was landfilled.
Contrast these low recycling numbers with the striking fact that an energy saving to society of approximately seven barrels of oils is accrued for every ton of mixed plastic waste that is recycled,[13 ] and it becomes self-evident that oil and energy conservation could be astronomical if we could implement an effective recycling strategy. Should we reach the apex of accomplishment by recycling all of the plastic waste, consumption of nearly a quarter billion barrels of oil could be saved each year from recycling of U.S. plastic alone.
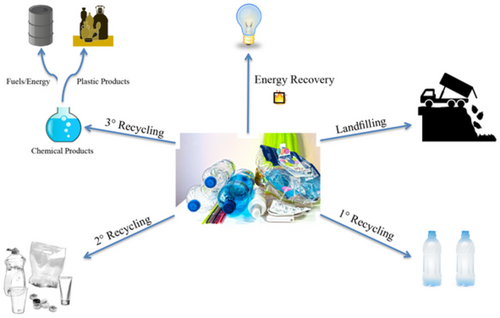
Certainly, not all plastics can be as easily recycled as others. In 2015, about 18% of PET was recycled, while only 10% of high-density polyethylene (HDPE), 6% of low-density polyethylene (LDPE)/linear low-density polyethylene (LLDPE), and < 1% of PP were recycled.[12 ] A plethora of complexities affect recycling rates, from practical considerations of collection, sorting, and pretreatment (e.g., what contaminants, adhesives, colorants, or residues might be present), to more technical considerations such as chemical reactivity.10 To better understand the state-of-the-art in addressing these complexities, it is instructive to introduce the four classifications of recycling: primary recycling, secondary recycling, tertiary recycling, and energy recovery. Primary recycling, also known as closed-loop recycling, is the process of taking uncontaminated discarded plastics and directly turning that material into the same “new” product, ideally without loss of properties.[14 ] A familiar example of this would be to use clean aluminum pipes and use the metal to make new aluminum pipes. Secondary recycling refers to mechanical recycling, wherein the chemical identity of the polymer is unchanged, but the polymer is in some way physically reprocessed, and thus generally used for a different purpose than its original use.[15 ] An example of this is taking waste tires and using the rubber crumb as an additive in rubber flooring or park benches. In the context of polymers, the most prevalent problems with primary and secondary recycling are related to stability. As the polymers are continually reprocessed, the polymer may degrade to varying extents, which will have drastic effects on the mechanical properties of the postrecycled product.[15 ] Additionally, the need for pure/clean plastic waste is a significant barrier when postconsumer, mixed-source plastic is targeted for recycling.
Tertiary recycling, sometimes referred to as chemical recycling, uses chemical processes to break down the polymer into value-added commodities. Typical processes include hydrolysis[14 ] and pyrolysis[16 ] of waste plastics. The product obtained is then used as a feedstock for the production of fuels and polymers.[17, 18 ] The last form of recycling is incineration of the polymer for energy recovery. In this process, the polymer is incinerated, and some amount of energy is recovered in the form of heat. This is generally a “last resort” process when no more value-added application is achievable. Incineration of many plastics also releases hazardous gases and leave behind toxic residues, presenting undesirable hazardous waste remediation and collection costs and downstream ecological consequences.[14 ]
The overall efficiency of recycling plastic waste begins with the sorting and pretreatment process of plastic waste. Different plastics have different properties and thus have different recycling methods. From the typical mixed waste model, each type of plastic must first be sorted. Contaminated plastics in the waste stream can lead to unwanted decomposition reactions that will decrease the efficiency of plastic recycling as well as alter the end product. While there are many different types of sorting processes that have been utilized and are currently being studied for optimization,[19, 20 ] this review will focus on chemical recycling methods of plastic waste that occur once sorting is complete.
2 ACADEMIC ADVANCES IN PLASTIC RECYCLING
2.1 Recycling of poly(ethylene terephthalate) (SPI Code 1)
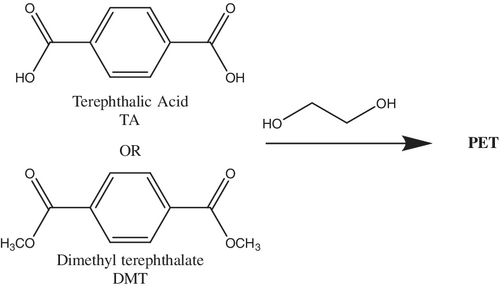
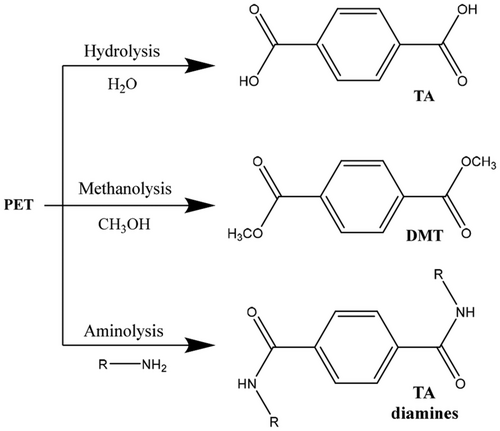
Poly(ethylene terephthalate), often abbreviated PET or PETE, is a semicrystalline, thermoplastic polymer that is known for its high strength. Industrially, PET is synthesized through a polycondensation reaction between ethylene glycol (EG) and terephthalic acid (TA) or through a transesterification reaction between dimethyl terephthalate (DMT) and EG (Scheme 1).[21 ] Efficient recycling of PET has reached the most advanced stage of maturity among the common plastics, and a variety of methods have proven utility on a large scale.[21-23 ] There have been instances where PET undergoes primary and secondary recycling, that is, the recycling of plastic bottles. However, a significant remaining problem with recycling of PET is that the mechanical properties of the nonvirgin material are greatly reduced with each reuse. The strain-at-break (the percent of the length that a sample can be stretched before the sample breaks) for virgin PET, for example, is 42%, whereas after only the fifth cycle of extrusion, the strain-at-break was only 0.7%.[24 ] This downcycling process limits the ability to thermo-mechanically recycle PET. For this reason, tertiary recycling via chemical processes has been the main focus of research in the past few years.[21, 25 ]
PET can undergo pyrolysis to yield its precursor monomers, TA, and EG. Kenny et al.[26 ] have shown that the pyrolysis of PET at 450°C yields TA and oligomers thereof, which can be further hydrolyzed to obtain the TA monomer. Despite such promising advances, the pyrolysis of PET is seldom used as a method to depolymerize PET into its monomeric units on an industrial scale because pyrolysis generally leads to other liquid and gaseous side-products, reducing process efficiency, and necessitating costly separation steps.[21 ]
Du et al.[27 ] used PET from carpet waste as a source and studied the thermal and catalytic decomposition of this waste into oils. The catalytic degradation focused on using an aluminosilicate zeolite, ZSM-5, or CaO as the catalyst. They also looked at how steam would affect the final decomposition products. They found that using a catalyst, namely CaO and steam during the pyrolysis process would yield large percentages of benzene in high purity. These studies foreshadow the promise held by many decades of plastic waste to serve as the next source of what are typically viewed as petrochemicals.
Another chemical process that has shown great promise for the depolymerization of PET into its monomeric units is hydrolysis (reaction with water at elevated temperatures and/or with a catalyst).[28 ] The products yielded from this method are TA (or a terephthalate salt) and EG. There are three different types of hydrolysis that have been studied in greatest detail: acidic, alkaline, or neutral hydrolysis. While acidic hydrolysis can take place using concentrated acids, such as phosphoric or nitric, the most common acid used is sulfuric acid.[29-31 ] Although the yields obtained from this method are generally high, separation of the EG from the highly acidic solution is a major drawback of this method. Additionally, the amount of acid needed to industrialize this process pose economic, process, and environmental problems.
Alkaline hydrolysis generally employs aqueous solutions of 4–20 wt% NaOH.[30 ] This process yields the sodium terephthalate and EG in relatively good yields, up to 100% PET conversion. However, longer reaction times (3–5 hr) and high temperatures (>200°C) than needed for acidic hydrolysis techniques are notable drawbacks of this method. Polk et al.[32 ] have recently improved on the alkaline hydrolysis process, demonstrating that the addition of a phase transfer catalyst (trioctylmethylammonium bromide) facilitated the reaction at lower temperatures (70–95°C) while yielding high purity (99.6%) TA in up to 93% yield.
Neutral hydrolysis employs water or steam in the presence of catalysts.[30 ] This process uses high temperatures (200–300°C) and elevated pressures (1–4 MPa). Neutral hydrolysis, without the need for stoichiometric acid or base, would be ideal, but these processes generally produce low purity monomers and have relatively slow rate of reaction.[30 ] This process does not take into account for any mechanical impurities, such as sand and particulates, which can reduce the purity of the monomer for further use. Campanelli et al.[33 ] found that the use of large ratios of water:PET (5:1) are needed for the complete depolymerization of PET.
Another method to depolymerize PET into its monomers is through methanolysis. In this process, methanol reacts with PET at high temperatures (180–280°C) and pressures (20–40 atm) in the presence of a catalyst, most commonly zinc acetate.[34, 35 ] This reaction leads to the formation of DMT and EG, which can then be used to resynthesize PET through a transesterification reaction. A major drawback of this method, outside of the high temperatures and pressures, is again the purification process. The crude product contains not only DMT and EG but also other alcohols and phthalate derivatives.[21 ] A noteworthy advance was developed by Tang et al.,[36 ] who demonstrated that DMT recovered from methanolysis of PET could be exploited as a starting material for the synthesis of gasoline and jet fuel.
Aminolysis of PET is an area that has not been widely exploited, likely because this process requires an amine (which is often toxic or expensive) to depolymerize PET, yielding diamides of TA. The reaction temperatures generally range from 20 to 100°C.[21 ] In a study done by Teotia et al.,[37 ] four different amines—methylamine, ethylenediamine, ethanolamine, and butylamine—were reacted with PET. Unprecedented conversion of PET into lower molecular weight oligomers was achieved at ambient temperatures and pressures, but this required reaction times ranging from 10 to 85 days. Soni et al.[38 ] also studied aminolysis of PET with a variety of amines and achieved complete degradation of the PET to the diamide after 45 days of reaction. Hoang et al.[39 ] showed that ethelyenediamine was even more effective and was able to depolymerize PET to yield a range of oligomers after only 17 hr at 100°C. Significantly, longer reaction times were needed to achieve degradation to small molecules; however, several catalysts, such as dibutyl tin oxide, sodium acetate, and cetyltrimethylammonium bromide, are under development that show promise for shorter reaction times and improved selectivity, but these have not yet demonstrated large-scale applicability.[40, 41 ]
Glycolysis of PET is an area that has been widely studied. This is a very versatile process due to the various potential applications of the products obtained. In this process, PET is depolymerized by glycols to form monomers, oligomers, and/or polyols, which can then be used for different applications. Some of the glycols that have drawn particular recent interest for this application include ethylene glycol, diethylene glycol (DEG), propylene glycol, butylene glycol, and dipropyleneglycol (DPG).[42, 43 ] If EG is used to depolymerize PET, the major product formed is bis(2-hydroxyethyl) terephthalate (BHET), which can be used to synthesize PET. A challenge in these promising reactions is the immiscibility of PET with the polyols. Liu et al.[44 ] sought to address this problem by conducting a careful study on the role of different solvents in the conversion of PET to BHET. DMSO proved most effective among solvents screened for effective cosolvation of EG and PET and thus showed an increase in BHET yield to 82% (when compared to 20% without the addition of DMSO) with remarkably short reaction times of 1 min at 190°C and atmospheric pressure.
Catalyst development has also been an area of active research. For many years, zinc acetate was the primary catalyst used in the glycolysis of PET. Troev et al.[45 ] have recently developed a titanium (IV) phosphate catalyst for the glycolysis of PET using EG, DEG, or 1,2-propylene glycol. This catalyst achieved shorter reaction times than previous catalysts, with increased yield and selectivity for BHET formation. Titanium is advantageous because it is nontoxic, though the catalyst could not be efficiently recycled. Wang et al.[46 ] have sought to exploit sustainably produced organocatalysts such as urea in place of transition metal catalysts. Urea was quite an effective catalyst, facilitating 100% conversion of PET with 74% selectivity of BHET. Additionally, urea could be recycled five times without loss of activity or selectivity for BHET.
Ionic liquids can also be used as a catalyst for the glycolysis of PET.[47, 48 ] Despite their high initial cost, ionic liquids have become increasingly attractive tools for green chemistry because of their low volatility and recyclability. Consequently, Yue et al.[49 ] have explored the utility of 1-butyl-3-methylimidazolium hydroxide, ([Bmim]OH) to serve as a catalyst. A 100% conversion of the PET with a selectivity of 72% of BHET was achieved, compared to only 11% conversion of PET without the addition of the ionic liquid as a catalyst. In a follow-up study,[50 ] these researchers found that Lewis acidic ([Bmim]ZnCl3) ionic liquid likewise facilitated a 100% PET conversion but with improved selectivity of 84% for BHET and minimal catalyst loading (0.16 wt%). Liu et al.[51 ] reported that deep eutectic solvents could also be used to catalyze the glycolysis of PET with EG. They found that the combination of 1,3-dimethylurea (1-3-DMU) with 5 wt% Zn(OAc)2 was able to convert 100% of the PET with 82% selectivity for BHET at 190°C in just 10 min. They associated the high selectivity with relatively mild reaction conditions due to the acid–base synergistic effects between 1-3-DMU and Zn(OAc)2. The catalyst was also recycled up to five times without any loss in conversion efficiency. However, the zinc content was shown to decrease by 25% after the five cycles, which limits further recycling ability of the catalyst.
Whereas the foregoing discussion highlights efforts to leverage glycolysis of PET with EG to synthesize BHET as a monomer feedstock, an emerging area of interest is the use of the chemically recycled PET products as feedstocks for other polymer formulations. Mecit and Akar[52 ] used different glycols to convert PET into lower-weight oligomers having hydroxyl end groups that were then reacted with toluene diisocyanate to produce urethane oils. The recycled urethane oils showed similar Koenig hardness values and touch/hard to dry times to commercially available oils. Desai et al.[53 ] synthesized polyol blends by reacting PET waste with plant-derived starch. The oligomers so formed were then esterified with fatty acids—themselves primary constituents of low-value, high-volume waste products from other industries.[54-58 ] The esterified materials were then used to synthesize polyurethanes. These authors also demonstrated the facile tunability of adhesion, flexibility, and chemical resistance properties of the polyurethanes as a function of PET products present in the formulation.
Amaro et al.[59 ] used DEG and PET to synthesize oligomers that proved effective as secondary plasticizers in PVC formulations resulting in improved thermal stability and flexibility of the final PVC product. Furthermore, migration of PET-derived plasticizer migration was greatly decreased compared to traditional PVC plasticizers such as, di(2-ethylhexyl)phthalate (DHEP) which leach out over time. Initial PET oligomerization was achieved in this instance by action of a Ca/Zn stearate catalyst at 250°C for only 20 min. Recently, Sirohi et al.[60 ] have described alcoholysis of PET to synthesize oligomers that can be used as a plasticizer in nitrile-PVC rubber blends. They used a ZnCl2 catalyst and 1-decanol as the alcohol with reaction temperatures of 190°C for 4 hr. Once resultant depolymerized products were blended with nitrile-PVC rubber blends, the tensile properties and the aging resistance of the materials was significantly buttressed.
2.2 Polyethylene (SPI Codes 2 and 4)
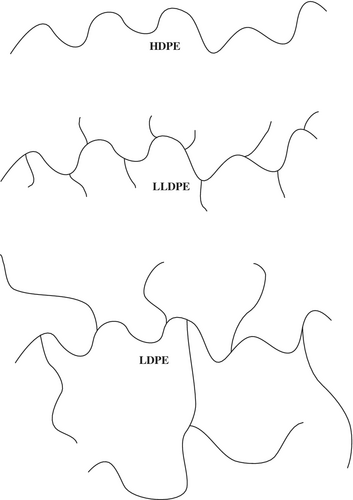
Polyethylene is a lightweight and durable thermoplastic that finds use in films, tubing, packaging, plastic bags and bottles, and even automobile parts. PE is typically made by polymerization of ethylene (C2H4), often facilitated by a Ziegler–Natta or metallocene catalyst.[28 ] The linear alkyl chains comprising the PE backbone lack polar functional groups, and are inert to many chemical reactions, including common acids and bases, under standard conditions. To complicate recycling efforts further, there are multiple types of PE: HDPE, LDPE, LLDPE, ultrahigh molecular weight polyethylene, and many other crosslinked types of PE.[28 ] Each of these different types of PE is used for different purposes depending primarily on the mechanical property profile required. The most-used PEs include HDPE (SPI Code 2) and LDPE/LLDPE (together sharing SPI Code 4). HDPE is flexible, translucent, and shows good toughness at low temperatures. There is little to no branching in HDPE, thus it is said to be a linear polymer. LDPE and LLDPE differ from HDPE in that both are branched polymers. LDPE is semirigid and has branches that are both short and long throughout the polymer backbone. LLDPE differs from LDPE in that this form of PE features only shorter branches (Figure 2).
The structural variability and relative chemical inertness of PE have relegated most studies on PE recycling to variations on pyrolysis. A primary difficulty associated with efforts to recycle PE by pyrolysis is that the thermal degradation of PE usually proceeds via random scissions at CC bonds. This homolytic scission generates two radical chains that can go on to form a complex mixture of olefinic products and highly crosslinked polymeric products.[61 ] Moreover, the melt flow index (a key figure of merit for processability) changes by two orders of magnitude, from 2.25 (reasonable flow) for virgin LDPE to a nearly unprocessable melt flow index of 0.02 g/10 min after 100 extrusion cycles.[61 ] This changing behavior is accompanied by drastic, deleterious drift of mechanical properties of the recycled LDPE, so these approaches do not provide a long term, sustainable path forward for PE recycling. When mechanical processing of PE is no longer feasible, depolymerization of PE through pyrolysis to yield hydrocarbons for fuel/energy applications is often the final stage of use for such materials.
There are two major processes for the pyrolysis of PE: thermal or catalytic pyrolysis. Thermal pyrolysis is simply heating PE at high pressure to break down the polymer backbone to form smaller organic molecules. Catalytic pyrolysis utilizes a catalyst in an effort to reduce the temperature and reaction time and thus improve the economic viability and, in some cases, the selectivity. In a study by Ahmad et al.,[62 ] the thermal pyrolysis of commercially available HDPE pellets at 350°C which yielded a liquid oil product in 81% yield. The oil consisted mainly of paraffinic hydrocarbons, most of which, contained between 6 and 16 carbon atoms. Specificity for a single chemical commodity from PE pyrolysis, however, remains elusive. An intriguing divergent strategy has been devised by Palos et al.,[63 ] who eschewed the selectivity problem and instead sought to take waste HDPE and chemically transform it into a complex mixture akin to “crude” oil. As long as the complex mixture is comprised by molecules similar to those found in petroleum, such a complex mixture could hypothetically be processed by established petroleum refining and cracking techniques. This nascent approach used waste HDPE that had already been sorted, washed, and shredded. The HDPE samples so prepared were then heated in a batch autoclave reactor at 430°C with short reaction times, up to 38 min, to yield what they refer to as “plastic oil,” with impressive yields of up to 85–90 wt% oil recovered. While the oil obtained is not clean enough to be used directly in place of fuel, this plastic oil can be refined by established methodologies to yield target products. The main drawback of this creative approach is the potentially high energy required to produce the plastic oil on large scales.
While many researchers focus on pristine or near-ideal PE sources in their proof-of-principle studies, Das and Tiwari[64 ] examined the important difference between utilizing virgin and waste plastic products. Additionally, a mixed waste feedstock mixture of different polymers was used. The mixture consisted of HDPE, LDPE, and PP. The virgin polymers were obtained commercially, and the waste plastics were obtained in the form primarily of packaging plastics, plastic containers, and bottles. The thermal pyrolysis of this mixed waste was undertaken at 350 or 400°C and the reaction time was 8 hr. Lower temperature reactions yielded lighter hydrocarbons (< C20) while an increase in temperature yielded heavier hydrocarbons (> C20). This offers the potential to tune the oil obtained by varying reaction temperature and holds promise for exploiting mixed waste streams and thereby circumventing some of the challenges associated with waste separation.
Miandad et al.[65 ] likewise recognized the importance of developing strategies to deal with mixed waste. In this study, the plastic waste was collected in the form of disposable plates, grocery bags, and cups and comprised not only linear alkyl polymers (PP and PE) but also aromatic-bearing polystyrene (PS) and PET. The plastic samples were crushed into fine powders and used on their own as well as mixed together in varying ratios (as little as 20 wt% PE) with reaction conditions of 450°C for 75 min. Depending on the ratios of the plastic waste liquid oil yields ranged from 24 to 54%. The initial feedstock used was 1 kg, which shows the potential to scale the system to an industrial level. The oil obtained consisted of large amounts of aromatic compounds and showed higher heating values between 41 and 42 MJ/kg, which is comparable to that of commercially available diesel (43 MJ/kg).
The area of catalytic pyrolysis of PE has also achieved notable advances in the last few years with regard to increasing yields and lowering reaction time/temperature. Santos et al.[66 ] looked at three different zeolites, HZSM-5, USY, and NH4ZSM-5. These catalysts differ based on pore size, acidic sites, and surface area. This study garnered important insight that catalysts with larger pore sizes led to a greater yield of liquid products and that the less acidic catalysts would yield lighter fractions of gas and liquid products, thus affording flexibility in the process based on end use.
An intriguing study by Chattopadhyay et al.[67 ] examined the effects of catalysts on pyrolysis of an exceedingly complex and disparate waste stream of HDPE, PP, PET and paper biomass (consisting primarily of cellulose). Cobalt complexes were used as catalysts with various chelating agent along with Al2O3 and/or CeO2. The catalytic pyrolysis of the paper waste by itself yielded mainly gaseous and solid products, whereas pyrolysis of the paper/PP/HDPE/PET mixture yielded more liquid products that were rich in aromatics and olefins.
The potential for pyrolytic approaches to valorize PE waste is counterbalanced by the high energy cost. A greener avenue that has shown recent promise in the decomposition of plastic waste is the use of supercritical water.[68, 69 ] An added benefit of using supercritical water is that there is no need for a catalyst. Moriya and Enomoto[70 ] undertook one such study on supercritical water as a medium for the thermal cracking of PE for comparison to established pyrolysis techniques. A 5:1 ratio of H2O:HDPE was used, and the mixture was reacted at 425°C for 2 hr, the oil conversion rate was shown to be 90%.
Encouragingly, the yield of organic oil produced was higher for supercritical water cracking, while commensurately less coke/char was formed in the process. The oil product mainly was composed of a majority of alkenes with the presence of some alkanes with very little presence of any aromatic moieties.
While most of the aforementioned approaches to recycling PE have focused on the pyrolysis to form shorter chain molecules that ultimately tend to be used in fuel applications, the pyrolysis products may also be leveraged as feedstocks to produce other polymers. Recently, Bäckström et al.[71 ] depolymerized HDPE waste to synthesize a mixture of succinic, glutaric, and adipic acid. This was accomplished through a microwave-assisted hydrothermal process with the addition of HNO3. Whereas microwave reactions have in the past been dismissed as impractical for large-scale operations, recent advances and implementation on a commercial scale have begun to dispel these preconceptions.[72 ] The products of the microwave reaction were carried forward as plasticizers in a poly(lactic acid) formulation. Incorporating these PE-derivative plasticizers into the PLA formulation increased the strain at break to 144% as compared to 6% for PLA without the use of any plasticizer. This study illuminates a creative path forward to exploit postconsumer petrochemical waste for improved performance of sustainably sourced polymers.
2.3 Polypropylene (SPI Code 5)
Polypropylene (PP) has a similar backbone to PE, but the presence of an additional methyl group as a sidechain on each repeat unit has a significant impact on the properties. PP is a light weight, tough, crystalline thermoplastic polymer that finds use in reusable food containers, the automotive industry, and even the furniture market.[73 ] PP is synthesized from the propylene (C3H6) using either a Ziegler–Natta or metallocene catalyst. PP can have three different types of structures, atactic, isotactic, and syndiotactic,[28 ] depending on the relative disposition of the methyl side chains with respect to one another along the backbone (Figure 3).
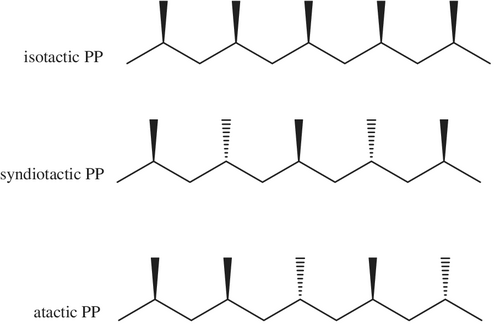
The configuration of the methyl groups has an effect on the crystallinity of the PP and consequently on bulk properties. The tacticity of PP generally has little effect on recycling techniques and consequently will not be discussed further here. Very little of the PP that enters the marketplace—less than 1%—ends up being recycled, largely due to the fact that PP is generally found in mixed waste streams[12 ] such as cable coverings, electronic appliances, and rugs. In each of those materials, PP is not the only polymer that is present, thus, having to wash and separate each component are major barriers to recycling efforts.[73 ]
In cases where separation is effective and practical, there are still drawbacks to melt reprocessing of PP. As with the other polymers discussed herein, recursive heating cycles rapidly degrade the PP backbone. Thermal degradation of PP is more severe than in PE because the tertiary carbon atom present in the PP backbone is susceptible to thermo-oxidative and photo-oxidative degradation.[73 ] While some PP plastics include stabilizer to prevent degradation from occurring, if the PP polymer has been successfully recycled into a new material, this material must then include additional stabilizers to imbue the “newly” made material the same oxidative stability, while the buildup of stabilizer and sacrificial stabilizer degradation products contributes commensurately to the deterioration of PP properties. The elongation-at-break for a virgin, unstabilized sample of PP is 65%; however, after just 10 recycling cycles the elongation-at-break has decreased to 45%.[74 ] When PP can no longer be mechanically recycled through either shredding or melt reprocessing, there are some emerging strategies to convert PP into value-added feedstocks.
Interest in tertiary recycling of PP has been growing rapidly. The depolymerization of PP into propylene has even been accomplished by Guddeti et al.[75 ] In this process, PP was depolymerized in an induction-coupled plasma reactor. Under these conditions, PP was converted to gaseous products (up to 78 wt%) and of the gaseous product formed 94% was identified as propylene. Other studies have used plasma as a media to depolymerize PP into other value added commodities.[76 ] One major advantage of using a plasma reactor is that reaction times are very short; however, the cost to setup and operate plasma reactors on an industrial scale detracts from the practical viability of these approaches. Another drawback of using such high temperatures is that any contaminants present, as would be expected in a waste stream, tend to yield many side reactions and have profound impact on product yields.
As for PE recycling strategies, supercritical water is a privileged media for contemporary PP recycling as well. Chen et al.[77 ] used supercritical water to convert PP into oil. Optimal reaction conditions were found to be at 425°C for 2–4 hr or 450 °C for 0.5–1 hr, in which case up to 91 wt% of the PP was converted into oil. The composition of the oil was found to be olefins, paraffins, cycloalkanes, and aromatics. The analysis of the oil showed that it had similar properties to that of naphtha, which can be further purified to make gasoline. The use of supercritical water to depolymerize PP is a largely sustainable step toward upgrading waste PP into value-added feedstocks.
The decomposition of PP can also yield solid carbonaceous products that can be used for various applications. Liu et al.[78 ] described a two-stage reactor. In the first step of the process, PP is catalytically pyrolyzed to yield gaseous and liquid products. These products are then taken to the second stage of the process in which the products are thermally decomposed to make either carbon nanotubes or gaseous products, which mainly consisted of H2 or CH4. This process allows for the collection of gaseous and solid products that can be used in different applications. In a similar vein, Mishra et al.[79 ] successfully synthesized carbon nanotubes and hydrogen gas from waste PP using a chemical vapor deposition method with a nickel-based catalyst. The carbon nanotubes synthesized by this process showed high transmittance (85%) of light at 550 nm, which can be exploited as transparent electrodes for optoelectronic devices. The gas evolved was mostly aliphatic molecules, with very little to no presence of aromatics.
Given the difficulties associated with obtaining near-pure PP from waste, the incorporation of PP into other waste streams for recycling has drawn interest as well (some of the PE recycling strategies have already illustrated this approach; vide infra). The annual availability of gigaton quantities of biomass (primarily lignocellulosic) make this an attractive waste stream for valorization.[80-82 ] Simple pyrolysis of biomass waste alone generally leads to low-value final products. The addition of PP to biomass waste, however, can enhance the properties of the final products. Zhao et al.,[83 ] for example, mixed bamboo waste with PP and the copyrolysis of the two yielded bio oils that have potential as fuels. To accomplish this transformation, a zeolite catalyst (HZSM-5) was used while the bamboo:PP ratio and catalyst loading were varied. Even under optimal conditions, bamboo alone still gives quite a poor oil yield (30 wt%) and low oil quality. The bamboo-derived oil consisted primarily of aliphatic hydrocarbons.
When a 2:1 ratio of bamboo:PP was employed, they found that oil production could be more than doubled to 62 wt% with attendant improvement in oil quality. The bamboo/PP-derived oil consisted of more aromatic and naphthenic compounds. Such compositions are ideal feedstocks for jet fuel production due to the large amounts of heavier hydrocarbons produced. Lee et al.[84 ] further demonstrated the broader scope of such processes by showing that PP addition to high cellulose content agricultural waste from sources other than bamboo can likewise improve the production of oils in high yields and of more desirable composition than can be accomplished by pyrolysis of cellulose alone.
2.4 Current industrial efforts in plastic recycling
Given the contemporary importance of plastics recycling, there are significant industrial efforts to commercialize processes based on the previously discussed strategies to chemically recycle mixed waste streams comprising both hydrolysable plastics (primarily PET) and nonhydrolyzable plastics (such as PE, PP, and PS).
2.4.1 Industrial approaches to recycling PET and mixed polyester waste
There are many different companies active in the chemical recycling of PET and other polyesters on an industrial scale from real-world waste streams. Most of their recycling methods of PET employ variations of the depolymerization approaches discussed in the previous section with the aim of producing various monomers. Chemical recycling of PET has reached a more mature state due to the relative ease of its depolymerization as compared to polyolefins.
Depolymerization of polyesters to several different monomer feedstocks has been successfully commercialized. Carbios focuses on enzymatic hydrolysis of polyesters such as PET (Scheme 2) to yield TA. While the optimal enzyme varies depending on the particular polyester to be hydrolyzed, the enzymes employed by Carbios are all hydrolase enzymes produced by Thermobifida alba or Fusarium solana pisis. The use of T. alba, a thermophilic bacterial species, allows the hydrolysis to be carried out at higher temperatures and is a key to the success of this process.
Following enzyme-catalyzed hydrolysis, the product TA is purified and used to prepare virgin PET. The patented Carbios process accomplished depolymerization at 20–80°C and is operable over a wide pH range from 4 to 10.[85 ] The specific pH values are selected on the basis of the particular polyester to be depolymerized. The enzyme loading varies from 0.005 to 15 wt% and reaction times can vary from 5 to 72 hr.
Another company focusing on depolymerization of PET and polyesters is Gr3n. Their process focuses on less-easily processed polyester waste streams that include colored PET bottles and polyester textiles. The process they employ is their patented DEMETO (depolymerization by microwave technology) technology, which is able to cut reaction times down to 10 min.
In the first step of the patented process, the plastic waste is ground up and mixed with a solvent.[86 ] The solvent is typically ethylene glycol (Scheme 3) and an alkali base, such as NaOH, KOH, or LiOH. This heterogeneous mixture is then transferred to a reaction chamber where microwaves are used to heat the chamber to a temperature of 150–350°C with a pressure between 1 and 20 atm. After this treatment, the reaction mixture is filtered and the EG is distilled to be reused in the process. When PET waste is treated by the DEMETO process, TA is the major product and is readily obtained in pure form by simple precipitation, filtration, washing and drying. DEMETO also works as a method to recycle polyamides. In this case, the hydrolysis of amides is analogous to the hydrolysis of esters discussed in detail in the previous section. Amides, however, are somewhat less reactive to hydrolysis. For this reason, acidic media, such as HCl, H3PO4, or H2SO4, is used in the DEMETO processing of polyamides. Although DEMETO is a more energy-intensive process than the low-temperature Carbios process, the time required is much shorter.
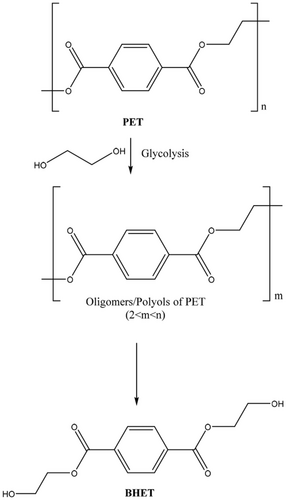
Whereas Carbios and Gr3n focus on production of TA from PET (Scheme 2), companies such as Ioniqa Technologies and Jeplan have developed commercial variations of the depolymerization of PET into BHET (Scheme 3). In Ioniqa's patented process, an ionic catalyst complex is able to degrade PET into BHET.[87 ] This ionic catalyst complex generally consists of a nanoparticle, such as magnetite or maghemite, that is magnetic in nature, a bridging group, such as triethoxysilylpropyl, and an ionic catalyst, such as (bmim)+ and FeCl4−. This catalyst is able to depolymerize PET waste at temperatures of 200°C with only a catalyst loading of 2 wt%. Yields for this process can be up to 93% with near quantitative conversion of the PET waste.
It is notable that Ioniqa's magnetic metal particles serve a dual role of catalyst and pigment adsorbent. Magnetic particles are also easily removed by magnetic catchers in the process line to prevent their incorporation in end products. In contrast, Jeplan uses separate catalyst and activated carbon to remove inks and dyes. This approach simplifies catalyst preparation but makes separation of the catalyst and adsorbent less straightforward that in Ioniqa's approach. Jeplan' process is successful for recycling mixed colored and clear PET bottles and fibers by heated the waste materials with BHET at 220°C for 1 hr.[88 ] This achieves what they term as a predecomposition product in which the PET is broken down into oligomers. Ethylene glycol and sodium methylate catalyst are added to the predecomposition solution and are heated at 200°C for 2 hr to completely decompose the PET to form BHET. The solution is cleaned with activated carbon in order to remove dyes and impurities. The BHET solution is then purified and obtained as a pure monomer in 98% yield, which is used to synthesize virgin PET.
Both the processed developed by Ioniqa Technologies and Jeplan demonstrate the additional considerations one must keep in mind when converting the knowledge learned from academic studies to practical handling of mixed waste streams. In the case of mixed PET waste processes, these considerations include recovering catalyst and removal of pigments and dyes, considerations mentioned much less frequently in academic investigations.
2.4.2 Industrial approaches to recycling mixed-composition textile waste
Mixed textile waste is a challenge for the recycling process because it most typically consists of hydrolysable synthetics such as polyesters and polyamides as well as cotton and other fibers. Commercially viable primary or secondary recycling of textiles is not developed due to the complexity of the constituents present in the material. Additionally, with such a high content of biomass, generally pyrolysis to form liquid fuels in unfavored due to the high oxygen content of biomass. Most recycling of textiles thus requires costly separation of the synthetic polymer from the natural fiber. Trash-2-Cash, Tyton, and Worn Again are three companies seeking to recycle textile waste.
Tyton focuses on recycling wasted textiles to regenerate the cellulose portion (from the cotton textiles) and convert the PET into TA and EG. They focus on the use of subcritical water as a media to break down the textile waste. In an exemplary application, they took mixed textile waste and placed it in a hydrothermal reactor at a temperature of 155°C and pressure of 120–150 psi with 5% (w/v) sodium hydroxide and 0.5% (w/v) of a phase transfer catalyst for 60 min.[89 ] The resulting mixture was taken and the TA was recrystallized, thus allowing it to be separated from the cellulosic pulp. The TA is used for making virgin PET, while the cellulosic pulp product is further washed and processed to decrease the molecular weight of constituent cellulose, thus allowing it to be used to make new textiles as well.
Trash-2-Cash focuses on cellulose regeneration by using an ionic liquid in which the cotton is dissolved and can be separated from the polyester. They focus on using their patented ionic liquid, Ioncell F, to selectively dissolve the cellulose portion in mixed textiles.[90 ] The ionic liquid generally consists of 1,5-diazabicyclo[4.3.0]non-5-enium acetate (DBNH-OAc), which is mixed with the textile waste for 1 hr at 80°C. The cellulose solution is then taken to a dry-jet wet spinning step in which cellulosic fibers are spun directly from the extractant. The undissolved polyester is taken and washed with the ionic liquid two more times to yield essentially pure PET resin. The ionic liquid and water are separated and fed back into the recycling process.
Worn again focuses on separating the PET resin from the cotton using a solvent-based system. This process focuses on the dissolution of the dyes in the textile waste followed by dissolution of the polyester. The solvents used in this process can vary, but 1,3-dimethyl-2-imidazolidinone (DMI) is a suitable solvent to dissolve both the dyes and the polyester resin.[91 ] In the first step of this process, the textile waste is mixed with DMI at a temperature between 90 and 100 °C for 10 min. This process selectively dissolves the dyes and not the polyester resin. The polyester resin is separated from the DMI by filtration, and the dye and DMI are separated from each other. The dye-free polyester resin is then dissolved in DMI at a temperature of 120–130°C for 2 hr. This mixture is then filtered to remove any solid impurities and the polyester resin is isolated by evaporation of the DMI solution. This allows for the recycling of the DMI solvent and the polyester resin.
Another company, Aquafil, focuses on reusing nylon 6, a polyamide that can undergo hydrolysis analogous to the processes used to hydrolyze PETs. The recovered nylon can be respun into their ECONYL yarn, which can then be fed back into textiles. Their patented process employs initial thermal decomposition of any polyurethane present in the material.[92 ] This is achieved by heating the waste at a temperature of 150–200°C for up to 24 hr. Temperature control allows selective decomposition of less thermally stable fibers while leaving the polyamide fibers intact. The next step of this process is to separate the polyamide fibers from the decomposed polyurethanes by washing in a polar solvent, such as ethanol, at a temperature of 5–78°C for up to 10 hr. At this point, the polyamide fibers are dried and purified and can be readily reused. The solvent is separated and purified from the degraded components and is reintroduced into the process.
The commercial processes described here for the treatment of mixed textile waste again demonstrate the additional strategies that must be implemented to deal with practically available waste streams. In the case of mixed textile streams, removal of dyes and pigments by either their differential solubility or thermal stability is one consideration. Another consideration is the separation of different types of material to produce one or more chemically distinct products. For textiles, this comes down to the separation of cellulose (cotton) and polyurethanes from polyesters. The foregoing examples illustrate methods to accomplish such separation by selective crystallization, variable solubility, or differential thermal stability.
2.4.3 Industrial approaches to recycling polyolefins
In a recent report by Closed Loop Partners, only one example of successful commercial recycling of PE was noted.[93 ] This may not be surprising given the lower reactivity and higher-temperature pyrolytic pathways that academic studies have revealed thus far for degrading PE, as discussed in the previous section. Despite these inherent challenges, BioCellection, Inc. has successfully recycled HDPE and LDPE through a patented process referred to as accelerated thermal oxidative decomposition (ATOD).[94 ] In ATOD, the polymer is broken down into oxygenated organic compounds to include succinic, glutaric, adipic, pimelic, suberic, and azelaic acid. To accomplish the oxidation, ATOD utilizes an oxidizing acid, such as HNO3, to treat mixed PE waste at 60–200°C for 30 min–30 hr. The resulting mixture is purified to yield the various organic acid compounds.
There is only one major company focused solely on utilizing PP as a feedstock. PureCycle Technologies has been successful in recycling PP to give a recycled PP product, whose mechanical properties are equivalent to those of virgin PP. The process is a purification method that can remove color, odor, and impurities from waste PP rather than relying on total chemical disintegration of the polymer. Their patented process focuses on using mixed postconsumer and postindustrial PP waste.[95 ] The PP waste is heated with a fluid solvent to a temperature of 80–220°C and at a pressure of 150–15,000 psig to produce reclaimed PP. The fluid solvent used does not dissolve the PP, but rather dissolves dyes, pigments, fragrance compounds, and other impurities that may be present in the waste PP. The resultant pure PP is dissolved in another solution and is then separated and precipitated out for use in any process typically requiring virgin PP.
Although the methods for chemical recycling of PS are not explicitly delineated in the previous section, the problems of low reactivity facing efforts to recycle PE and PP also affect PS, so the methods for its recycling are similar to those discussed for PE and PP. One unique practical barrier to PS recycling that is not faced in efforts to recycle other plastics is that much of the PS waste is in the form of Styrofoam. Styrofoam has a high volume for a given mass, making it considerably more costly to transport a given mass to a central recycling facility for processing.
Companies that focus on recycling PS include Pyrowave, Polystyert, and Agilyx. Agilyx converts PS into liquid styrene monomer using a pyrolysis technique similar to those discussed for pyrolysis of PE or PP. Their process involves initial melting and compacting of PS waste, which is then fed into the pyrolysis reactor.[96 ] The waste is then heated and gaseous products are released and condensed. Heavier hydrocarbons and oligomers can be fed back into the pyrolysis reactor for further cracking. The lighter hydrocarbon fractions and the styrene monomer are separated and isolated and can be used for further applications. Throughout the entire process, the maximum temperature reached is approximately 550°C, so it is rather energy intensive.
Pyrowave employs industrial microwave reactors to depolymerize PS to form monomers. Their process uses a pyrolysis reactor and a proprietary catalyst loaded 0.5–50% w/w. Using the catalyst allows them to affect transformation of the PS to monomers at a lower temperature (350–500°C) than in the Agilyx process.[97 ] The Pyrowave process then employs two sequential condensers, the first with a working temperature of 55–90°C, and the second with a working temperature of 2–10°C, facilitating efficient separation of target styrene monomer from other impurities. Yields for styrene monomer recovery can be up to 95% in purity needed for synthesis of virgin PS.
Whereas both Agilyx and Pyrowave processes rely primarily on thermal depolymerization techniques, Polystyvert has developed a process to purify PS. This approach is conceptually similar to that employed by PureCycle Technologies for reclaiming PP. The Polystyvert process[98 ] employs p-cymene to dissolve the PS waste. PS is quite soluble (up to 33% w/w) in p-cymene, minimizing the amount of solvent needed. Furthermore, p-cymene is a primary component of some naturally occurring essential oils, and so could conceivably be sustainably sourced. After dissolution of PS, insoluble materials are filtered out. The PS solution is then washed with a solvent in which PS is insoluble, typically heptane, hexane, or octane. This allows for the selective precipitation of PS, while other low molecular organics, including pigments and dyes, remain in solution. This process can be repeated with different precipitation solvents multiple times followed by drying at 120°C to give PS pellets appropriate for use in manufacturing new expanded PS and high-impact PS products. The p-cymene used in the process is recovered and continually used in the process.
While each of the aforementioned companies in this section has focused on one specific polyolefin, there are companies that have successfully commercialized processes for recycling mixed plastic waste. Given the complexity of separating organic compounds or purified polymers for even a single-component polyolefin waste stream, it is perhaps unsurprising that successful processes for valorizing mixed polyolefin waste streams to convert them into liquid oils that can be used as fuels or other commodity chemicals. This approach is in contrast to the focus on the regeneration of monomers or polymers to be fed back into PET or polyolefin production that typifies most of the efforts in commercial plastic recycling discussed so far.
Resynergi focuses on pyrolysis of variously comprised mixed plastic waste streams. Their process uses microwave energy to induce “fast pyrolysis” in which the plastic waste is heated to temperatures between 650 and 700°C and broken down into liquid fuel. Their patented process feeds waste into their pyrolysis chamber under an inert atmosphere of nitrogen or argon, with low pressures of 1–2 psig.[99 ] The pyrolysis chamber contains silicon carbide spheres and a screw-type mixer to ensure uniform heating during the pyrolysis process. The pyrolysis gases are then sent through a catalyst-filled cracking chamber to further decompose the plastic waste. The gases are then sent through subsequent condensers, which first condense heavy gases and waxes, then diesel gases and then lighter gases such as gasoline fuels. These fractions can then be used as fuels for a variety of applications.
Plastic2Oil employs a process similar to that employed by Resynergi. The Plastic2Oil process specifies that direct use of unsorted and unwashed plastic waste is achieved in their facility. The waste is first premelted at a temperature of 250–340°C, a temperature at which some of the lower molecular weight impurities are burned off or volatilized as well.[100 ] The premelted waste is then fed into a pyrolysis reactor held at 340–445°C, with residence times as short as 10 min. The pyrolysis gases are then taken to catalyst towers. These catalyst towers feature different temperature/catalyst zones to facilitate sequential reaction of target gases at each stage. The overall process yields approximately 87% liquid fuel products with the balance consisting of unspecified residue and syngas (a mixture of hydrogen and carbon monoxide gas that is an industrially important feedstock) in a ratio that is dependent on the particular nature of the mixed plastic waste used.
Plastic Energy is an emerging company employing a patented pyrolysis technique termed Thermal Anaerobic Conversion (TAC) in which plastic waste can be thermally decomposed into a mixture of various hydrocarbon oils that is referred to as TACOIL. TACOIL can then be used to make other plastics, or be used as a fuel. In the TAC process,[101 ] pellets or flakes of waste plastic are first extruded at ~300°C and then transferred into an oxygen-free pyrolysis chamber at 390–410°C, where they are agitated with a stirrer. The pyrolysis gases are sent through a condenser, in which heavier, long chain hydrocarbons are condensed and sent back into the pyrolysis reactor. This design feature eliminates the need for a catalytic reactor used in the Resynergi or Plastic2Oil processes to break down the larger/heavier hydrocarbons. The pyrolysis gases now consist of diesel, kerosene, light oil, and waxy components that are separated by distillation. Waxy components are fed back into the pyrolysis reactor and the diesel, kerosene, and light oil products can be sold as fuels.
GreenMantra Technologies is a company that utilizes proprietary catalysts to transform PE, PP, and PS into either liquid fuels or polymer additives. GreenMantra Technologies currently focuses on producing Ceranovus, an additive for PE or PP. The GreenMantra process involves a thermochemical process in which the plastic waste can consist of a mixture of PE and PP with some capabilities of handling small quantities of PS.[102 ] The plastic waste is pretreated by extrusion and then preheated through a proprietary cycle and then placed in the pyrolysis reactor in which the plastic waste undergoes depolymerization. This step can include catalysts such as zeolites or alumina based catalysts, in order to target specific end products. The gases are then sent to a cooling stage where the gases are condensed and then sent for purification. The purified products can be commercially viable without any further purification.
Cadel Deinking targets mixed waste comprising both polyolefins and PET. Rather than relying on thermal decomposition of these plastics, Cadel Deinking focuses on their purification and co-processing into processable pellets. The process consists of first washing the postconsumer and postindustrial plastic.[103 ] The separated polymer residue is then ground and placed into a tank that contains a mixture of surfactants (between 0.1 and 5 wt%) and has a pH of 11–13. The surfactant tank stage process is repeated a total of three times to ensure complete removal of inks and dyes. The material is then dried and can be processed into pellets for further use.
2.4.4 Industrial approaches to recycling mixed waste including SPI Code 3–7 plastics or other materials
Multilayer films and materials tend to include a more than just one polymer, precluding their recyclability by methods that rely on manual separation. For this reason, multilayer films tend to be difficult to recycle through primary and secondary recycling methods. Thus, most of the waste is thermally converted into other commodity chemicals. Companies such as Recycling Technologies and Enval focus on recycling mixed plastic waste 3–7 and multilayer films.
Recycling Technologies focuses on thermally cracking complex mixed plastic waste into gaseous and liquid products. This process involves initial separation of PET and HDPE from the mixed plastic waste components, in order to recycle them in a different process.[104 ] The rest of the mixed waste is shredded. The shredded waste is then dried until a water content of < 5 wt% is achieved. Dried waste is fed into a fluidized pyrolysis reactor at 400–600°C. Pyrolysis produces fuels comprising C5 to C100 hydrocarbons, with roughly 80% of the hydrocarbons consisting of C5 to C40. A fraction of the fuel from the pyrolysis is used to power the reactor.
Renewlogy has focused on commoditizing mixed plastics with SPI Code 3–7. One major benefit of their method is that it is a continuous process into which plastic waste is fed without ever having to cool and reheat the chamber, as is the case in batch reactors used by most recycling companies.[105 ] After some manual separation, the plastic waste consisting primarily of SPI Code 3–7 plastics is heated to 120–315°C to melt the material. Molten material is fed into the pyrolysis reactor with an auger. Preheating the material is another energy-saving aspect of this process that also helps maintain the reactor temperature so it does not fluctuate as it would by putting in colder plastic waste. Additionally, the auger is maintained at a steady rate in order to continuously feed the plastic into the reactor. All of these subtleties contribute to producing consistent products. The reactor temperature is maintained at 400–550°C, where gases are formed and sent through four condensers of varying temperatures. The first condenser removes heavy hydrocarbons and waxes that are fed back into the pyrolysis system. Up to 80% of the plastic is converted into fuels that can be used as feedstocks for other materials.
RES Polyflow also uses a continuous system and can recycle mixed plastics of SPI Codes 1–7 without the use of a catalyst. As this process can make use of all types of plastic, no manual separation is needed prior to feeding material into the reactor. Their patented process relies on multiple reactor zones for the sequential separation of commodities.[106 ] At each reactor zone, there is an exit source where the pyrolysis gases can be captured. The plastic waste is fed through each zone by either a conveyer belt or an auger with a fixed speed. Yields for this process can be up to 93% with the finished product having characteristics similar to that of crude oil. This material can then be processed in typical petroleum cracking systems.
Vadxx is another company that uses a continuous flow process. The products obtained are classified into four different types of fuel, EcoFuel-I, EcoFuel-II, EcoFuel-SNG, and EcoFuel-S. EcoFuel-I is used as diesel oil, whereas EcoFuel-II is used for gasoline, EcoFuel-S is a solid carbon-based fuel, and EcoFuel-SNG is the synthetic natural gas that is used by Vadxx to power the process. To separate these fuels from one another, seven different zones are employed.[107 ] The first zone is the feed, in this part of the process; the plastic waste is generally shredded and fed into the second zone. The second zone compresses the plastic waste and reducing the volume, this is most typically achieved by extrusion of the waste at ~100°C. Zone 3 melts the polymer waste at 100–300°C. At this point, the polymer waste melt is sent into the reactor, Zone 4, where it is heated at 300–365°C for up to 2 hr. This burns off most of the heteroatoms such as chlorine from PVC. The products from this stage are either gases, waxy solids, or char. The waxy solids are sent to zone five of this process, where pyrolysis occurs. At this point, the waxy solids are heated to 365–488°C for 30–90 min. The resulting gases are then taken to condensers where liquid fuels are purified. The solid char is taken to Zone 6 and heated to temperatures of 454–982°C in order to further crack this solid into gases. The gases can then be taken and sent to fractionation columns and purified. Zone 7 then cleans and purifies the char so that it can be used as a solid fuel source.
ReNew ELP focuses on using supercritical water as a medium to convert mixed plastic waste into chemical feedstocks for various applications. Cat-HTR (Catalytic Hydrothermal Reactor) is a patented hydrothermal process in which the breakdown of mixed plastic waste is achieved in up to 85% conversion to liquid oil. The process involves initial grinding of waste feedstock to produce slurry with water and oil.[108 ] The oil used can be paraffinic oil, crude oil, bio-oil, and so on. Depending on the initial feedstock, catalysts such as sodium hydroxide can be added. The slurry of waste is then fed into a reactor with a temperature of 250–350°C and a pressure of 100–350 psi for 10–25 min. After the allotted time the products are cooled to a temperature of 50–180°C followed by reducing the pressure to atmospheric pressure. The liquid oil obtained can be used for various fuel applications. One of the main reasons for the addition of oil to the process is that the use of sub/super-critical water generally restricts the concentration of organic matter that can be put into the slurry. Additionally, water requires more energy per unit volume to heat and can lead to charring of the feedstock waste. The addition of oil mitigates these issues.
Most of the companies that have demonstrated commercial processes for recycling mixed plastic waste have focused on the pyrolysis of waste plastics to give fuels. The temperature, feed rate, and residence time are all factors that can be adjusted to optimize the liquid products depending on the input of the waste plastic.
Some waste materials are composed of plastic laminated or otherwise integrated into metal housings. Enval has made inroads to recycling such materials with a particular focus on using microwaves to induce pyrolysis of aluminum laminates. In their patented process, they use a dual chamber reactor in order to sustain a continuous process.[109 ] Both chambers are interconnected and contain a bed of carbon black conduction source and a stirrer to fluidize the chamber. The chamber is heated at 500–600°C and the laminate is fed into Chamber 1. Gaseous products from the pyrolysis of the organic material leave both chambers, and the aluminum metal is fed out of an exit at the top of chamber 2. This process yields oil and aluminum metal in 98% purity that can be resmelted into virgin materials.
APK is another company that that is able to selectively purify polymers in mixed plastic waste from nonplastic components. Their Newcycling technology involves milling waste through a dry mechanical separation process to facilitate separation of plastic from textiles, sand, metal particles, and so forth.[110 ] The material is then taken and split into light and heavy fractions through air sifting. The lighter fractions typically consist of film particles, fibers, corks, and so forth. The heavy fraction mainly consists of hard plastics and heavy impurities. The heavy fraction is then placed into sink or float separation tanks in which the density of the media is altered to separate specific plastic resins. Impressively, this conceptually simple process can yield plastic resins with a yield and purity of > 97%. The plastic resins are then taken and dried through extrusion processes to form granulates, which can be used for further applications.
3 INDUSTRIAL ADVANCEMENTS IN MIXED SOLID MUNICIPAL WASTE
Pilot-scale facilities have begun to make headway in recycling full-spectrum MSW comprising plastic, textile, food, and other waste components. The complex nature of MSW has led companies that focus on this set of waste material to rely on thermal gasification.
Sierra Energy focuses on using MSW, biomass, construction, demolition, and industrial waste as feedstocks for gasification. In their patented FastOx gasification strategy, the waste material is converted into syngas. The MSW is shredded and dried to less than 50 wt% moisture and is fed into the reactor, which can have temperatures up to 2200°C. At this point, the waste is devolatilized and a stream of steam and oxygen is injected,[111 ] facilitating the high temperature conversion into syngas. From here, the crude syngas is sent to a cleaning chamber to be conditioned to meet requirements for its downstream use in other industries.
Enerkem strategically targets otherwise nonrecyclable, noncompostable MSW as a feedstock for its process. Their approach is again to produce a crude syngas through gasification, but they take the crude syngas and further convert it into fine renewable products such as methanol and fuel-grade ethanol.[112 ] For this purpose, MSW is pretreated with proprietary additives to “neutralize” impurities such as chlorine and sulfur. A fluidizing gas is sent into the system with the pretreated MSW and the material is heated to a temperature of 600–700°C to form the crude syngas. This crude syngas then needs to undergo various scrubbing and purification steps in order to be further used in other processes in a separate facility.
4 CONCLUSIONS
With many plastic recycling processes focusing on primary and secondary recycling, there is still a great disparity between the amount of plastic waste and how much is recycled. We must now turn to other processes that can be used in conjunction with current processes in order to alleviate the problems associated with the massive amounts of plastic waste being produced. As outlined here, there have been many academic studies that delineate general strategies for the chemical recycling of hydrolysable polymers like PET and nonhydrolyzable polymers like polyolefins. These studies suggest that chemical transformation of waste plastics into value-added chemicals can be a convenient avenue to supplement current recycling processes. Industrial efforts to commercialize chemical recycling processes for plastic waste illuminate more complex issues of separation and purification associated with real-world waste streams, from mixed plastic waste to multicomponent metal–plastic components to full spectrum municipal waste. Despite the complexity of the problem, by intelligently tailoring catalysts, solvents, temperature stages, residence times, and initial feedstock, impressive yields of desired products (monomers, gases, oils, and solids) have been achieved.
Continued efforts need to be focused on catalysts to improve the overall efficiency and lower the temperature to bring down energy demands of recycling processes. The current state of chemical recycling of plastic waste shows promise to become one of the main processes in which we can efficiently reduce the amount of waste in landfills.[113-121 ]
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (CHE-1708844).
Biographies

Timmy Thiounn earned his B.S. from the University of South Florida. He is currently a Ph.D. candidate in the research group of professor Rhett C. Smith at Clemson University. His research interests include sustainable and environmentally friendly polymers for durable applications.

Rhett C. Smith earned his B.S. from the University of Toledo and his Ph.D. from Case Western Reserve University, where he worked for John D. Protasiewicz. After that, he was a National Institute of Health Postdoctoral Fellow at Massachusetts Institute of Technology. In 2006, he joined the faculty at Clemson University, where his interests include organo-main group polymers for sustainable energy generation and construction applications.